Heterogeneity of non-alcoholic fatty liver disease:Implications for clinical practice and research activity
2021-12-06ParthaPalRajanPaluiSayantanRay
Partha Pal,Rajan Palui,Sayantan Ray
Partha Pal,Department of Medical Gastroenterology,Asian Institute of Gastroenterology,Hyderabad 500082,India
Rajan Palui,Department of Endocrinology,The Mission Hospital,Durgapur 713212,West Bengal,India
Sayantan Ray,Department of Endocrinology,Jagannath Gupta Institute of Medical Sciences and Hospital,Kolkata 700137,West Bengal,India
Sayantan Ray,Diabetes and Endocrinology,Apollo Clinic,Ballygunge,Kolkata 700019,West Bengal,India
Abstract Non-alcoholic fatty liver disease(NAFLD)is a heterogeneous condition with a wide spectrum of clinical presentations and natural history and disease severity.There is also substantial inter-individual variation and variable response to a different therapy.This heterogeneity of NAFLD is in turn influenced by various factors primarily demographic/dietary factors,metabolic status,gut microbiome,genetic predisposition together with epigenetic factors.The differential impact of these factors over a variable period of time influences the clinical phenotype and natural history.Failure to address heterogeneity partly explains the sub-optimal response to current and emerging therapies for fatty liver disease.Consequently,leading experts across the globe have recently suggested a change in nomenclature of NAFLD to metabolic-associated fatty liver disease(MAFLD)which can better reflect current knowledge of heterogeneity and does not exclude concomitant factors for fatty liver disease(e.g.alcohol,viral hepatitis,etc.).Precise identification of disease phenotypes is likely to facilitate clinical trial recruitment and expedite translational research for the development of novel and effective therapies for NAFLD/MAFLD.
Key Words:Non-alcoholic fatty liver disease;Metabolic-associated fatty liver disease;Heterogeneity;Phenotypes;nomenclature;Clinical trial;Effective therapies
INTRODUCTION
Non-alcoholic fatty liver disease(NAFLD)is increasing in both developed and developing countries,in parallel with the global obesity epidemic.Nevertheless,much is still unknown on the NAFLD phenotype.Moreover,since the term NAFLD was coined by Ludwiget al[1]in 1980,the nomenclature and diagnostic criteria have not been revisited.With a deeper understanding of the natural history of NAFLD,it has become gradually more obvious that this term is inherently complicated,chiefly due to the heterogeneity of NAFLD and principal driving factors between individuals.This heterogeneity in clinical presentation and the course of NAFLD is probably influenced by several factors which include age,gender,ethnicity,diet,alcohol consumption,genetic predisposition,microbiota,and metabolic milieu[2].The combined effect of the dynamic and complex systems-level interactions of these drivers is probably reflected in the phenotypic manifestations of NAFLD.Therefore,comprehensive phenotyping will translate into individual-level risk prediction and preventive strategies,and improvements in the design of clinical trials[2].The heterogeneity of NAFLD and the presence of multiple pathophysiological pathways intrinsic to its progression suggest that the nomenclature should be revised and NAFLD may be classified in a way that takes into account the various underlying processes[3].However,a change of name of any disease has considerable implications for both clinical practice as well as public health policy.Based on these evolving paradigms,this review will explore the factors contributing to NAFLD heterogeneity and its clinical and therapeutic implications.Besides,proposed changes in the current nomenclature and definition of NAFLD are discussed along with future perspectives.
HETEROGENEITY OF NAFLD:NEED FOR A NEW TERMINOLOGY
NAFLD represents an umbrella term with considerable heterogeneity among its subtypes.This is evidenced by variable disease severity and progression(disease phenotype)among patients with NAFLD[4].The disease phenotype in NAFLD is in turn influenced by primary drivers of the disease and dynamic interaction between various disease modifiers(age,sex,ethnicity,co-existing disease,diet,alcohol consumption,smoking,hormonal status,genetic and epigenetic factors,gut microbiota,and metabolic risk factors)[2].Although steatosis is highly prevalent,progression to steatohepatitis or other liver-related complications like cirrhosis and hepatocellular carcinoma(HCC)is highly unpredictable.The rate of fibrosis progression can also vary widely among patients.Moreover,there is growing evidence that HCC can develop in NAFLD without cirrhosis[5].
The suboptimal response rates of current investigational therapies(20%-40%)reflect a lack of consideration of heterogeneity of NAFLD[2,6].Hence,a structured dissection of the key pathogenetic pathway and precise disease sub-typing based on genetic background,metabolic profile and anthropometric parameters shall help predict individualized risk and provide effective treatment[2].The term NAFLD was coined in 1980 by Ludwiget al[1]and it was used to describe fatty liver disease without a history of significant alcohol intake.Although the prevalence of NAFLD has grown to epidemic proportions involving one-fourth of the population,the nomenclature and the diagnostic criteria have not been reevaluated[2].The term NAFLD does not consider the heterogeneity of the disease and hence does not reflect current knowledge.
Based on recent epidemiological studies,it has been increasingly recognized that there is no cut-off for safe drinking in so-called NAFLD as there is frequent coexistence of at-risk drinking and dysmetabolism[7].Moreover,accurate assessment of alcohol intake is often challenging especially in subpopulations like children and women due to cultural interdiction[8].To further confuse the issue,there is evidence that an altered gut microbiome can lead to excess production of endogenous alcohol in non-drinkers[9].Hence,the dichotomy between alcoholic liver disease and NAFLD should be abandoned.Until now,diagnosis of NAFLD was based on the exclusion of excess alcohol intake,concomitant viral hepatitis/other liver diseases,and secondary cause of fatty liver(e.g.drug-induced).With the increasing prevalence of NAFLD and the high prevalence of other liver diseases such as viral hepatitis particularly in countries like Middle East and north Africa,dual causes of liver disease should be considered[8].The current definition of metabolic-associated fatty liver disease(MAFLD)does not require the exclusion of the above,considering the co-existence of different pathology for fatty liver disease(Figure 1).However,it requires the presence of overweight/obesity,type 2 diabetes mellitus(T2DM),or 2 metabolic risk factors.The term “non” in “nonalcoholic fatty liver disease” trivializes a disease that has major hepatic,cardiovascular(CV),and oncological sequelae[2,10].Due to the “non”-rubric,it could be misinterpreted as something not serious and even encourage alcohol consumption.The term “alcohol” makes the nomenclature derogatory and thus stigmatizing the condition blaming the patient for their condition[2].This has profound implications on recognition of the disease as a major public health problem and resource allocation by regulatory authorities to intercept this potentially deadly disease.
Due to the aforementioned reasons,the term MAFLD was proposed by Lonardo and Carulli 16 years back[11].However,NAFLD nomenclature remained unchanged until now.For the same reasons,Polyzos and Mantzoros[12]have proposed the term dysmetabolism associated fatty liver disease(DAFLD).Recently two consensus guidelines have proposed a change in the nomenclature of NAFLD to MAFLD and have redefined the condition based on the presence of hepatic steatosis and metabolic risk factors[2,13](Figure 2).The impact of such change was reflected in the identification of patients with hepatic steatosis with a higher risk of disease progression in a cross-sectional study of more than 13000 patients based on data from the third National Health and Nutrition Examination Surveys of the United States[14].Another study from Hong Kong has shown that MAFLD definition reduces the incidence of fatty liver disease by 25%[more so in patients with low body mass index(BMI)],while the prevalence remains unchanged.Patients with a fatty liver disease not fulfilling the criteria of MAFLD were unlikely to have significant liver disease.
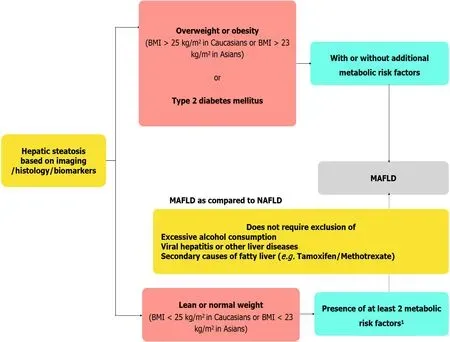
Figure 1 Proposed diagnostic criteria of metabolic associated fatty liver disease and key differences with non-alcoholic fatty liver disease definition.1Metabolic risk factors include(1)Waist circumference ≥ 102/88 cm in Caucasian men and women(≥ 90/80 cm for Asian men and women);(2)Blood pressure ≥ 130/85 mmHg or on drug treatment;(3)Triglyceride levels ≥ 150 mg/dL(≥ 1.70 mmol/L)or on drug treatment;(4)Plasma high density lipoprotein[HDL < 40 mg/dL(< 1.0 mmol/L)for men and < 50 mg/dL(< 1.3 mmol/L)]for women or on drug treatment;(5)Pre-diabetes[i.e.,fasting glucose levels 100 to 125 mg/dL(5.6 to 6.9 mmol/L),or 2-h post-load glucose levels 140 to 199 mg/dL(7.8 to 11.0 mmoL)or HbA1c 5.7% to 6.4%(39 to 47 mmol/moL)];(6)Homeostasis model assessment of insulin resistance score ≥ 2.5;and(7)Plasma high-sensitivity C-reactive protein level > 2 mg/L.BMI:Body mass index;MAFLD:Metabolic-associated fatty liver disease;NAFLD:Non-alcoholic fatty liver disease.

Figure 2 Key drivers of metabolic-associated fatty liver disease,resulting in disease heterogeneity and its clinical implications.Genetic predisposition,metabolic health,and environmental factors influence molecular and phenotypical heterogeneity of metabolic-associated fatty liver disease leading to various disease subtypes,variable disease progression,and response to therapy.MAFLD:Metabolic-associated fatty liver disease;NAFLD:Non-alcoholic fatty liver disease.
However,the future implications of change in the nomenclature are still unknown.Hence,Younossiet al[15],on behalf of the American Association for the Study of Liver Disease[15]have cautioned about the impact of premature change in terminology to MAFLD.While there are still existing challenges in widespread disease awareness,identification of treatment endpoints,and biomarkers for risk stratification,changing terminology may negatively impact the field[15].Moreover,international consensus involving all scientific societies,regulatory bodies,pharmacological industry,and patient organizations is required before a change in terminology.No matter what is the terminology for fatty liver disease,it is clear that it is a heterogeneous disease with varying manifestations.
NAFLD AND CARDIOVASCULAR RISK
Patients with NAFLD are more likely to have morbidity and mortality from cardiovascular disease(CVD).Currently proposed term MAFLD is closely linked to DM,dyslipidemia,hypertension,systemic inflammation which are known to increase CVD risk.A higher risk of CVD and CVD associated events have been noted in epidemiological and observational studies in NAFLD[16,17].NAFLD not only damages the coronary arteries(atherosclerosis and ischemic heart disease),but also the other cardiac structures like myocardium(heart failure),cardiac valves(aortic stenosis,mitral annular calcification),and conduction system(atrial fibrillation,conduction defects)[18].CV disease in NAFLD can be subclinical(coronary and courted atherosclerosis)or clinical(myocardial infarction,stroke).Pathophysiological factors include dyslipidemia,oxidative stress,systemic inflammation,endothelial dysfunction,and a pro-thrombotic state leading to structural and functional cardiac changes including arterial stiffness,atherogenic plaque formation,and coronary calcification[19].Among genetic factors related to NAFLD,MBOAT7 may promote venous thromboembolism whereas Transmembrane 6 superfamily 2(TM6SF2)appears to be protective and PNPLA3 seems not to be associated with the risk of CVD.Other pathogenetic mechanisms of NAFLD such as environmental factors(diet,obesity,etc.),gut microbiota(through the gut liver axis and altered intestinal permeability),and epigenetic alterations also influence the CV risk[16].
Lifestyle modification and weight loss help in primary and secondary prevention of CVD in NAFLD.Aspirin and statins may be considered for primary and secondary prevention in individuals with NAFLD who are at high risk of CVD.Newer antidiabetic medications such as SGLT2 inhibitors and GLP-1 receptor agonists are known to reduce CV events in T2DM and may be useful in this regard.Additional data are required on CV risk modification by farnesoid X receptor(FXR)agonists such as obeticholic acid.Future studies will likely address the predictive factors responsible for elevated CVD risk in NAFLD as there is a lack of targeted pharmacological therapy.Hence,CV endpoints should be included in clinical trials in NAFLD/MAFLD[16,19].
FACTORS FOR HETEROGENEITY
Age
The prevalence,risk of hepatic/extra-hepatic complications,and all-cause mortality of NAFLD increase with age.This is due to multiple factors like reduction in hepatic blood flow/volume,decrease in bile acid synthesis,altered cholesterol metabolism,increase in oxidative respiration due to decrease in mitochondria numbers,cellular aging,increased exposure to disease drivers over a prolonged period,and progressive increase in insulin resistance(IR)due to change in body composition(sarcopenia,abdominal and visceral adiposity with ectopic fat deposition)[20-23].
Gender and menopause effect
The prevalence of NAFLD and degree of hepatic fibrosis are lower in pre-menopausal women compared to men and postmenopausal women with better overall survival rates in the former[24].Changes in body fat distribution(abdominal obesity after menopause),differences in metabolic risk factors,sexual dimorphism of key metabolic pathways(lipid metabolism,insulin signaling,and inflammation),and differences in hepatic gene expression of various metabolic pathways(e.g.FXR,liver X receptor)are likely mechanisms for the difference[25-27].The prevalence of NAFLD and fibrosis risk is lower in postmenopausal women on hormone replacement therapy(HRT)compared to those who are not on HRT[28].The extent of hepatic fibrosis increases with the prolonged duration of estrogen deficiency in postmenopausal women[29].Hence,risk stratification in NAFLD should be based on gender and menopausal status.
Ethnicity
The prevalence of NAFLD and risk of nonalcoholic steatohepatitis(NASH)are seen in decreasing order of frequency in Hispanics,non-Hispanic whites,and African Americans[30].It is important to note that the risk of fibrosis did not vary based on ethnicity.The plausible explanations for such racial disparity are differences in genetic predisposition,metabolic traits(IR and body fat distribution),environmental factors(dietary habits like increased carbohydrate consumption,physical inactivity,and cultural factors).For example,the frequency of risk alleles of Patatin-like phospholipase domain-containing protein 3(PNPLA3)gene in Hispanics,non-Hispanic whites,and African-Americans are 49%,23%,and 17% respectively[31].Importantly,Asian individuals tend to accumulate liver fat at lower BMI,have a higher degree of inflammation,and have a possibly higher risk of fibrosis compared to other ethnicities[32,33].PNPLA3 rs738409 risk allele frequency is more common in East Asians compared to Caucasians[34].
Diet and gut microbiota
It is well known that a Western diet with high fat and fruit content leads to a higher incidence of NAFLD.On the other hand,the adoption of the Mediterranean diet is associated with decreased liver fat content and CV risk[35].Gut microbial composition changes rapidly according to changing dietary patterns.The effect of diet in fatty liver disease is difficult to differentiate from those due to diet-induced change in gut microbial composition[36].Gut microbiome composition can identify individuals with a higher risk of NAFLD progression[37].The gut microbiome and its metabolites influence bile acid metabolism,which in turn influences lipid,choline,and glucose metabolism.Alteration in gut microbial composition and intestinal permeability in NAFLD leads to the circulation of bacterial metabolites such as lipopolysaccharide which is in turn sensed by hepatic Toll-like receptors which induce activation of hepatic pro-inflammatory cells and stellate cells leading to inflammation and fibrosis progression[38,39].Apart from dietary factors,genetic makeup and ethnicity influence gut microbiome composition[40,41].
Metabolic health
Obesevs lean NASH:Although intra-hepatic fat content is closely influenced by obesity,45% of the obese are said to be metabolically healthy as they don’t have any components of metabolic syndrome(MetS)[42].It is not clear whether these individuals have a lower risk of CV complications compared to normal-weight,metabolically healthy individuals[43].On the other hand,30% of normal-weight individuals have MetS and higher cardiometabolic risk.This is because the distribution and nature of fat are more important than the amount of fat in predicting metabolic risk[2].Visceral fat is associated with higher metabolic risk compared to peripheral and subcutaneous fat.Fat distribution is influenced by ethnicity(higher visceral adiposity in Asians)and genetic makeup[44].5%-45% of NAFLD(20% among Europeans)are also lean NAFLD as defined by the presence of hepatic steatosis with normal BMI in the absence of significant alcohol intake[45].Lean NAFLD has distinct genetic predisposition,metabolic and microbial profiles.Increased prevalence of TM6SF2 risk allele,increased bile acids/Farnesoid receptor activity due to intact metabolic adaptation,and gut microbial profile which facilitates liver fat generation have been seen in lean NAFLD.Individuals with lean NALFD have a better metabolic profile compared to their obese counterparts[46].The data on the natural history of disease progression in lean NAFLD have shown variable outcomes.Distinct pathways of liver fat accumulation are being recognized.In type 1/metabolic NAFLD,calorie excess due to dietary intake and physical inactivity leads to increased hepatic fatty acid supply by peripheral lipolysis and hepatic lipogenesis[4].This is associated with IR and other components of MetS thus leading to increased cardiometabolic risk.The accumulated liver fat is composed of monounsaturated triacylglycerols and free fatty acids enriched with ceramides.In type 2/PLNPLA3 NAFLD(with rs738409 risk allele),there is increased intra-hepatic lipogenesis and impaired lipolysis leading to steatosis[47].The fat composition is predominantly polyunsaturated triacylglycerols.This is not associated with IR and adverse cardiometabolic outcomes although the risk of NASH and HCC is increased.Increasingly various metabolomic signatures leading to hepatic steatosis are being recognized based on RNA-sequencing analysis study[48].Identification of the key pathway for hepatic steatosis by genetic and molecular profiling may thus help in predicting the risk of progression,cardio-metabolic,and treatment outcomes.
Genetics and epigenetics
Among the multiple variant genes associated with NAFLD identified on genome-wide association studies,few common variants(PNPLA3,TM6SF2,GCKR,MBOAT7,HSD17B13)are worth mentioning which have divergent metabolic effects[49].PNPLA3 and TM6SF2 variants increase the risk of NAFLD and advanced fibrosis[50,51].PLPLA3,TM6SF2,and GCKR variants are associated with T2DM[52].MBOAT7 and HSD17B13 variants do not affect serum lipid or glucose levels and do not increase cardiometabolic risk[53,54].These variants explain only a minority of NAFLD.That is why it is important to consider the effect of other variants,gene-environment interactions(described with thePNPLA3gene),and epigenetics.Epigenetic alterations of key regulators of metabolic,inflammatory,and fibrotic pathways represent a bridge between variant genes and the environment in NAFLD.Micro-RNAs such as miRNA-122,miRNA-192,and miRNA-34a are unregulated in NAFLD[55].miRNA-34A also correlates with disease activity.The role of long non-coding RNAs(lncRNAs)in NAFLD is limited requiring further elucidation[56].Reversible alteration of methylation signatures of key regulatory pathways is seen in NAFLD which reverses following weight reduction surgery[57].Methylation signatures can help identify patients with advanced fibrosis[e.g.hyper-methylation of peroxisome proliferatoractivated receptor gamma(PPARγ)][58].Epigenetic alterations can alter the expression of PNPLA3 explaining the gene-environment link[59].There is increasing evidence that maternal high fat diet leads to epigenetic alterations in fetal liver and increasing the possibility of NAFLD in adolescence in the offspring[60,61].Higher maternal BMI is associated with hypermethylation of the PPARγ coactivator 1(PGC1)gene which regulates energy metabolism in the newborn[62].
Familial risk
Twin studies,prospective and retrospective family studies have shown heritable factors in hepatic steatosis and fibrosis.In a prospective study,the risk of advanced fibrosis in first-degree relatives of patients with NAFLD-cirrhosis was 18% which is significantly higher than the general population risk[63,64].Hence family history also should be considered while doing risk stratification of NAFLD patients.
Alcohol intake
The effect of alcohol use in fatty liver disease has a dose-dependent response which synergistically increases in the presence of metabolic risk factors[65].This is contrary to the earlier belief that alcohol consumption has a “J” shaped effect on fatty liver disease progression with a beneficial effect on light to moderate use and deleterious effect on excessive use[66].Hence,it is being increasingly revealed that there is no safe cutoff of alcohol consumption in fatty liver disease.
CLINICAL IMPLICATIONS OF NAFLD HETEROGENEITY
NAFLD sub-classification
The heterogeneity in NAFLD due to its multifactorial etiology,pathophysiological diversity,genetic polymorphisms,and on the other side,the ultimate unifying fate of steatosis and its progression,made NAFLD more like an umbrella disease with multiple subtypes.The proposed change of nomenclature as MAFLD,will not truly represent the full spectrum of the disease pathophysiology and thus this overgeneralized new nomenclature has been criticized.Singhet al[3]had proposed the ‘MEGA-D’ classification representing the ‘Mega-diversity’ of the NAFLD.They had proposed five sub-types of the disease,each representing a major pathophysiological hypothesis behind each subtype.The subtypes are as follows:M-Metabolic syndrome,E-Environmental stressor,G-Genetic Factor,A-Bile Acid dysregulation,and D-Gut dysbiosis related NAFLD.Moreover,it is also suggested to consider fatty liver disease as an umbrella term to include the whole spectrum of cryptogenic to classic to alcoholassociated fatty liver disease.Till any consensus-driven widely accepted terminology and sub-classification of NAFLD comes into place,it is prudent to consider fatty liver disease as common outcome pathology with different etiological triggers.
Alteration of lipid metabolism is one of the major pathophysiological factors behind the development and progression of NAFLD.Lipidomics based sub-classification of patients with NAFLD had been proposed which depends upon the signature patterns of alteration in the fatty acid homeostasis pathway[67].‘M-subtype’ is characterized by increased hepatic fatty acid uptake and reduced hepatic glutathione and S-adenosine methionine(SAM)content.On the other hand,the ‘non-M subtype’ occurs due to increased de novo hepatic lipogenesis and is characterized by normal hepatic SAM levels.Gut microbiota composition-based sub-classification of NAFLD had also been proposed.However,till now no studies had been able to reveal any signature gut microbiota profile suitable for phenotypical classification of NAFLD patients.
Automated algorithm-driven cluster sub-classification,based on demographic factors(age,gender,ethnicity),clinical and laboratory findings[68],had been evaluated in a cohort of 13290 NAFLD patients in the United States.The whole cohort had been divided into 5 subtypes and evaluated for disease outcomes including survival rates.In subtype 1,there were mostly female Hispanics with mild metabolic comorbidities with minimal fibrosis,but on the other hand subtype 2 had mostly patients with MetS with signs of developing liver dysfunction.Subtype 3 was a mostly young and healthy population with mild disease and minimal abnormalities.Subtype 4 patients were predominantly elderly male Caucasians who had more severe disease at baseline with features of fibrosis and also showed features of progression to cirrhosis stage.Subtype 5 patients were the oldest with more severe cirrhosis and associated with significant co-morbidities.Among the disease outcome,subtype 5 was at the highest risk mortality and subtype 4 had the highest risk of cirrhosis and HCC.Although this type of cluster-based subtyping of the disease needs to be validated clinically it can help to identify relevant disease subtypes in future studies.
In a gene expression study by Hoanget al[48],the disease progression score of individual genes had been evaluated and it showed a strong correlation with histological manifestations of disease severity.In this study,the authors proposed NAS(gene-level NAFLD activity score)and gene-level fibrosis stage(gFib)scores.These score-based subtypes of NAFLD not only can assess the risk of disease progression but also can predict the response to therapy.This molecular-based cluster classification either can be the forerunner of different clinical subtypes of NAFLD or can represent different phases of a dynamic spectrum of the disease.
Though genetic,clinical cluster,and pathophysiological based sub-classification of NAFLD had been proposed as discussed above,none of them are universally accepted.Moreover,detailed literature is mainly limited to disease phenotypes depending upon demographic factors,obesity,and clinical outcomes.
Inter-individual variation
Demography(Asianvs Western countries):The prevalence of NAFLD is now showing an increasing trend in Asian countries.A meta-analysis done in 2016[69]showed a higher prevalence in Asia(27.4%)than North America(24%)or European Union(23.7%).In a recent meta-analysis[70],the prevalence in Asia was found to have increased further(29.62%)and a secular trend of the rising prevalence in the last few decades had been reported.The increase in prevalence in Asia is likely due to an increase in obesity,sedentary lifestyle,changing westernized eating habits,and various socio-economic factors[71].The prevalence in the rural area was significantly lower than in the urban areas,suggesting the detrimental effect of urbanization on obesity and the consequent NAFLD[72].In both Asian and western countries,the prevalence increases with age.Prevalence is higher in males as well as among elderly women indicating protective effects of estrogen in females in the reproductive age group.Apart from the increased prevalence of metabolically unhealthy obesity and excessive visceral obesity,alteration of gut microbiota and bile acid profiles has also been postulated as possible contributing factors behind the development of steatosis[40].Among the genetic factors,PNPLA3 polymorphism(rs738409)had been strongly associated with hepatic steatosis in both western and eastern studies[31].However,a higher prevalence of PNPLA3 risk allele had been reported in Asia than in African or European countries[73,74].Genetic polymorphisms of other genes likeTM6SF2,AGTR1,HSD17B13,andGCKRgenes had also been linked with increased susceptibility of NAFLD in Asian subjects[54,75-77].Sarcopenia and hypovitaminosis D also was associated with NAFLD development[78,79].One of the major differences in Asian countries from their western counterpart is the increased prevalence of lean NAFLD(discussed later)in the former.Though the overall prevalence of NAFLD is almost similar in eastern and western countries,however,the rate of complications is still lesser in Asian countries.In a retrospective study from Japan with a median follow-up of 5.8 years,only 0.25% of patients developed HCC with an annual incidence of 0.043%[80].In contrast to western countries,NAFLD still contributes only to a minor proportion of liver-related complications requiring liver transplantation in Asia.In a Japanese nationwide survey,only 2.1% of patients with cirrhosis had NASH and almost two-thirds of the patients had viral hepatitis[81].The indolent course of NAFLD in Asian countries is likely due to relatively short disease duration in the majority of the patients in this part of the world.As there is a considerable lag in economic growth and consequent obesity epidemic in Asian countries,the rise in NAFLD and its complications are likely to follow the western trend in the coming years.Moreover,the relatively higher chance of co-existence of viral hepatitis and NAFLD in Asian countries increases the risk of hepatic complications further[82].
Ethnicity:Irrespective of ethnic variability,a trend of overall increased prevalence of NAFLD had been seen globally.In the world,Middle East had the highest prevalence of NAFLD,and in Africa;it is the lowest[69].Studies from the United States reported that Hispanics had shown the highest risk of NAFLD and on the other hand,the risk is much less in the Alaskan Native.Among Asian ethnicity,the prevalence is highest among Indonesian and lowest in Japanese[70].Interestingly,people of South Asian origin who are living in the United Kingdom,also showed higher risk[83].In a recent meta-analysis,which evaluated ethnic heterogeneity of NAFLD in the United States,both higher overall prevalence of NAFLD and risk of progression to NASH had been reported in Hispanics and the risks were lowest among Blacks[30].Although there was no significant difference in patients with fibrosis among different ethnicities.The reasons behind the ethnic variation are multifactorial.A significantly high risk of NAFLD among American Japanese than the native Japanese suggests the impact of socio-economic development and differences in lifestyles in the pathogenesis[70].Specific western dietary patterns in different ethnicities,like consumption of red meat and hydrogenated fat,had also been associated with an increased risk of fibrosis[84].Intake of saturated fatty acids increases and on the other hand,consumption of omega 3 fatty acid-rich food reduces the risk of steatosis.Genetic factors can explain the heterogeneity of NAFLD across different ethnicities.Among genetic variants of thePNPLA3gene,rs738409 increases the risk of NAFLD in Hispanics and Southeast Asians[85].On the other hand,the increased prevalence of protective polymorphism of the samePNPLA3gene(rs6006460)can explain the reduced risk of NAFLD among African Americans[31].The rs738409 variant had been also associated with an increased risk of progression to NASH and hepatic fibrosis[86,87].However,in a study from Malaysia,though the frequency ofPNPLA3risk allele was higher among Chinese individuals but the prevalence of NAFLD was much less in them in comparison to Malay and Indian participants[87].This paradox can be explained by the involvement of multiple candidate genes in disease pathophysiology among different ethnicities.With the advent of Genome Wise Association studies,the role of predisposing polymorphisms of other candidate genes likeTM6SF2andGCKRgene had been explored further.The rs58542926 variants of theTM6SF2gene were significantly associated with intra-hepatic fat(triglyceride)accumulation in White and African-American but not among Hispanic individuals[88].Different polymorphisms in theAGTR1gene were protective among Indians but not in Chinese and Malay subjects[75].Recently,polygenic gene scores had been developed to evaluate the cumulative effects of multiple candidate genes in the development and progression of NAFLD[89].Further studies are needed in the future to explore the complex interaction of different genetic polymorphisms which can explain disease heterogeneity across different ethnic populations.
Age(Children and adolescents):With the increasing prevalence of pediatric obesity,the prevalence of NAFLD in children and adolescents is ever rising.The pooled prevalence of pediatric NAFLD in general population and obesity clinic were 7.6%(95%CI:5.5%-10.3%)and 34.2%(95%CI:27.8%-41.2%)respectively[90].The factors which can influence the intrauterine metabolic milieu of the developing fetus,like maternal obesity and diabetes,had been postulated to increase the future risk of NAFLD[91,92].Increased consumption of fructose-rich beverages,processed food,saturated fat along with decreased intake of dietary fibers(westernized dietary habits)had been strongly associated with the development of NAFLD in children[93].On the other hand,breastfeeding was protective against the development of NAFLD[94].The genes which had been shown to increase the risk of pediatric NAFLD are similar to the adults.Genetic variants ofPNPLA3(rs738409),TM6SF2(rs58542926),andGCKRgene had been shown to increase the susceptibility of development of NAFLD in pediatric patients[31,88].Though histological diagnosis of NAFLD remains ideal,diagnosis by imaging(ultrasound/MRI)is the most practical one in the pediatric population.As the prevalence of obesity in children is ever-increasing,the chance of co-existence of other secondary causes of hepatic steatosis should also be carefully evaluated before confirming the diagnosis of NAFLD.Histological pattern in pediatric NAFLD(periportal distribution-Type 2 NASH)differs from that of their adult counter-part(pericentral distribution-Type 1 NASH)[95].Both fibrosis and steatosis are mainly present in the periportal region in type 2 NASH and are seen more in younger children.Moreover,the classical ‘ballooning’ change is also seen less frequently in children.On the other hand,type 1 NASH of the adult pattern can be seen in the older adolescent age group[96].There is a paucity of longitudinal studies evaluating the natural history of pediatric NAFLD.Around 10%-25% of patients had advanced fibrosis and almost half of the patients had NASH at the time of diagnosis[97].Though the incidence of HCC in the pediatric age group is extremely rare,a large number of pediatric patients with NAFLD are at increased risk of developing HCC in early adulthood.Weight loss and lifestyle changes were effective in the reversal of steatosis in pediatric patients[98].
BMI(lean/non-obese NAFLD):Lean and non-obese NAFLD is defined as NAFLD in a person with BMI < 25 kg/m2(< 23 for Asian subjects)and < 30 kg/m2(< 25 for Asian subjects)respectively.In a meta-analysis that included 93 studies from 24 countries,the prevalence of lean and non-obese NAFLD in the general population was reported as 5.1% and 12.1% respectively[99].Globally,the prevalence of non-obese NAFLD among the whole NAFLD group was 40% and in countries like India,it is as high as 47%,indicating that a large proportion of fatty liver disease is now developing in the non-obese population.Though non-obese NAFLD initially was more common in Asian countries,now almost similar prevalence of NAFLD is being reported from the western part of the world(United States 43.2%).Globally the prevalence of lean/nonobese NAFLD is showing an increasing trend over the last 3 decades[100].Though Shiet al[101]had reported a lower prevalence of hypertension,hyperuricemia,and fasting blood glucose in lean/non-obese NAFLD patients compared to obese NAFLD,these lean patients are not necessarily metabolically healthy.Rather lean NAFLD patients are more likely to have visceral obesity,metabolic syndrome,dyslipidemia,hypertension,and DM as co-morbidities than the lean controls[101].The pathophysiological basis of the development of NAFLD in lean/non-obese individuals is complex and multi-factorial.Increased prevalence of the PNPLA3 G allele had been found in lean NAFLD patients[102].Other genetic factors like TM6SF2(T)[46],cholesteryl ester transfer protein,and interferon lambda 3(IFNL3)/IFNL4(C)had also been found to increase the risk of lean/non-obese NAFLD[103,104].On the other hand,possible roles of distinct gut microbiota,bile acid profile[46,105],increased lysine,tyrosine,lysophosphatidylcholines,and phosphatidylcholines,had also been implicated in the development of NAFLD among lean individuals[106].The progression of NAFLD in the lean population can be conceptualized as a state of gradual attenuation of metabolic adaptation.Pathophysiologically,this can be divided into 3 stages- stage of susceptibility,stage of adaptation,and stage of failure[107].Studies evaluating the true natural history of lean NAFLD are sparse in the literature.In the largest meta-analysis Yeet al[99]reported that among lean/non-obese NAFLD patients,NASH and fibrosis(> stage 2)were present in 39% and 29% of patients respectively,which was lesser than the prevalence among obese NAFLD population.However,liver-related mortality was reported as almost twice in lean/non-obese NAFLD patients than in the obese NAFLD group.In another study with a mean longitudinal follow-up of almost 20 years,lean NAFLD patients did not show any significantly increased risk of overall mortality but the risk of progression to severe hepatic diseases was significantly higher(HR 2.69)than the obese NAFLD population[108].Like obese NAFLD,lifestyle modification in the form of dietary modifications and increased physical activity remains the main therapeutic approach in lean NAFLD patients[109].
Variable natural history
Classic and dynamic model:Previously,the natural history of NAFLD had been conceptualized as a disease spectrum that follows a linear model of disease progression.This classic model hypothesized that there is a gradual progression of the disease from NAFL to NASH to cirrhosis and HCC.However,this progressive worsening of the disease does not occur in all of the patients of NAFLD and significant heterogeneity in the natural history of NAFLD had been observed.Recent literature had identified that not all the patients with NAFLD follow this ‘classic linear model’ of natural history.A study by Paiset al[110],which systemically evaluated serial liver biopsy in NAFLD patients,had shown that 60% of NAFL patients had progressed to NASH and around 25% of patients of NAFL had directly progressed to the fibrotic stage.Various factors like DM,obesity,old age,and a higher degree of baseline abnormality were identified as possible risk factors for disease progression.In another longitudinal follow-up study by McPhersonet al[111],no significant difference in the rate of fibrosis progression between NAFL and NASH patients was found.In an excellent systematic review by Singhet al[112],serial liver biopsy data of 411 biopsyproven NAFLD from 11 cohort studies were analyzed.They had also re-emphasized that both NAFL and NASH can progress to the fibrotic stage.However,it takes much longer(14 years)time to progress one fibrosis stage in NAFL than in NASH(7 years).The annual fibrosis progression rate was slower in NAFL(0.07 stage)than in NASH(0.14 stage).Moreover,NAFL and NASH had a comparable rate of CV mortality(OR 0.9)though all-cause and liver-related mortality are higher in NASH[113].To summarize,NAFL can progress both to the NASH and fibrosis stage directly and on the other hand,NASH can also regress to NAFL or progress to the fibrotic stage.Thus,in the ‘dynamic model’ of NAFLD,it has been conceptualized that in early NAFLD,there is dynamic cycling between NAFL and NASH[114](Figure 3).
Slow and rapid progressor:In the same meta-analysis discussed above,Singhet al[112]also had identified significant heterogeneity among disease progression in NAFLD.They reported 2 subtypes of NAFLD patients according to fibrosis progression rate- rapid and slow progressor.The rapid progressors were around 20% of the NAFLD group who progressed rapidly from baseline(stage 0 fibrosis)to advanced(stage 3 or 4 fibrosis).On the other hand,the majority of NAFLD patients are slow progressors who only progressed 1 or 2 stage fibrosis in a similar time frame.Older age,low ASL:Alanine aminotransferase(ALT)ratio,co-morbidities like diabetes mellitus or hypertension,and genetic polymorphisms are probable risk factors for rapid progressors[103,115](Figure 3).
HCC:With the progressive increase in the prevalence of NAFLD worldwide,the risk of HCC and liver-related mortality are likely to rise as a consequence.Viral hepatitisrelated HCC usually occurs in the background of the advanced stage of cirrhosis.Though classically HCC usually occurs in the advanced stage of cirrhosis in the NAFLD spectrum,this is not true for all the cases of NAFLD-related HCC[116].Rather one of the most common causes of chronic liver disease-related HCC without evidence of cirrhosis is NAFLD[5].Leunget al[117]had reported 15% percent of NAFLD-related HCC as non-cirrhotic and they usually had larger hepatic tumor diameter at diagnosis.In a retrospective analysis,Mohamadet al[118]also reported that HCC in NAFLD patients without cirrhosis are likely to present in the older age group with a larger tumor size with a high recurrence rate in comparison to those with cirrhosis(Figure 3).
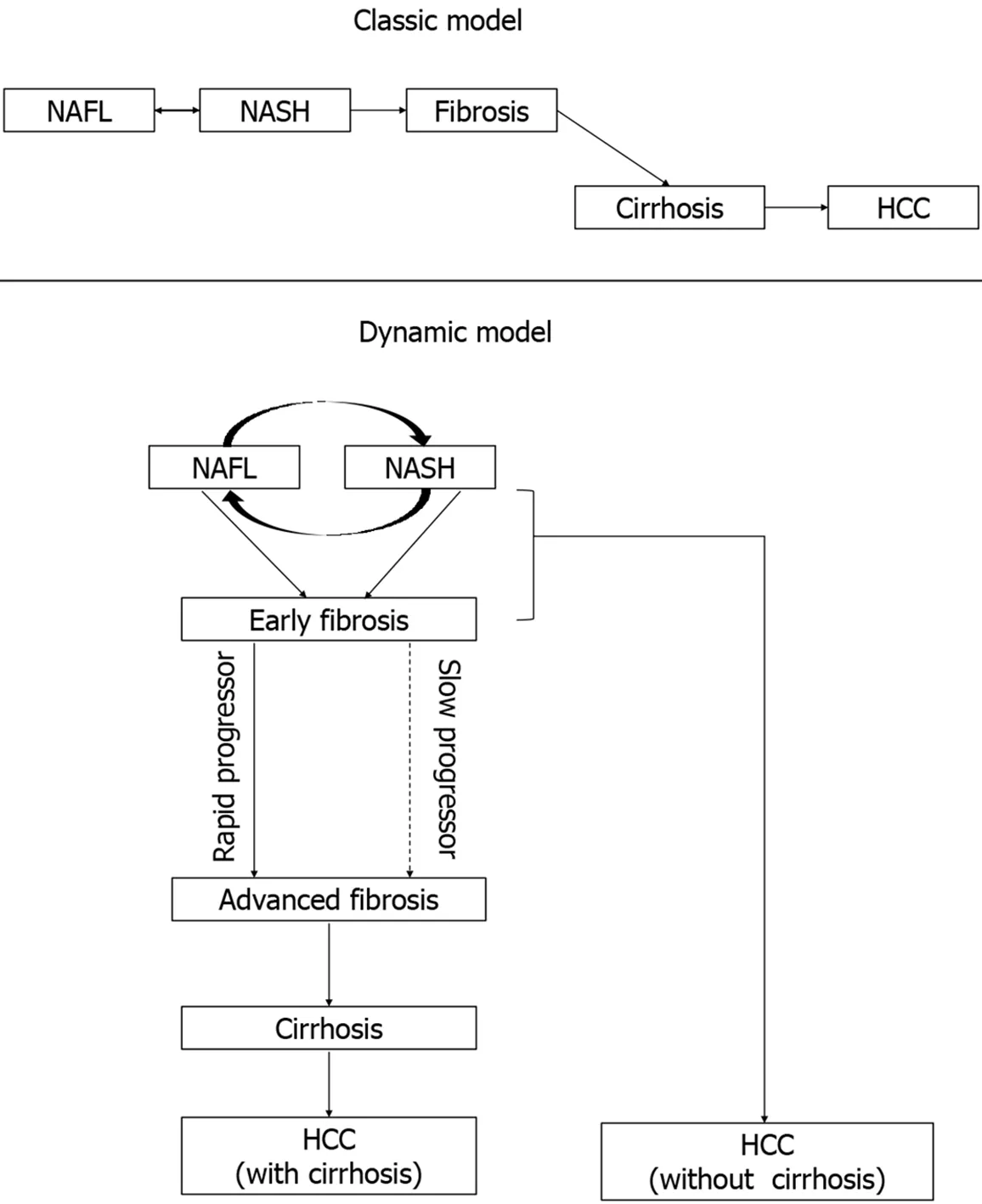
Figure 3 Natural history of non-alcoholic fatty liver disease(classic and dynamic model).HCC:Hepatocellular carcinoma;NAFL:Nonalcoholic fatty liver;NASH:Nonalcoholic steatohepatitis.
THERAPEUTIC AND RESEARCH IMPLICATIONS
NAFLD progression and prognostication
Many factors may influence the progression of NAFLD to the more advanced stage but are not routinely or easily assessed in day-to-day practice(e.g.,genotype,gut microbiome,mitochondrial function,immunological response)[119].Consequently,we need to consider the natural history studies to help provide clinical,biochemical,and histological variables that can be utilized to decipher which patients will develop severe disease with worse outcomes.With regard to clinical features,a paired biopsy study by McPhersonet al[111]underscores the impact of IR with 80% of patients with NAFL and progression of fibrosis developing diabetes by the time of follow-up biopsy compared with 25% of nonprogressors.Other studies have also shown that weight gain and worsening IR are associated with fibrosis progression in NAFLD[110].Data for biochemical predictors are somewhat deficient.However,a study found that in patients with biopsy-proven NASH and compensated cirrhosis;lower levels of serum cholesterol,ALT,and platelets are independently associated with hepatic complications and higher aspartate aminotransferase(AST)/ALT ratio with overall mortality[120].In NAFLD,baseline histology can provide a good prognostic value.According to a systemic review and meta-analysis of paired-biopsy studies,a third of individuals with NAFLD will have progression of fibrosis with a mean progression rate of 0.14 stagesperannum for NASH,corresponding to one stage of fibrosis progression over a median of 7.1 years[112].Nevertheless,many epidemiological studies have deemphasized the presence of NASH and confirmed the presence and degree of fibrosis as the most important histologic predictor of liver-related morbidity and mortality[121,122].
It is now widely accepted that the severity of fibrosis is the only significant predictor of outcomes in NAFLD.The histological differentiation between NAFL and NASH is unlikely to predict fibrosis progression and carries very little prognostic value.Thus,it is better to consider the diagnosis of patients with advanced fibrosis(F3 and F4)because this stage is a predictor for hepatic and extrahepatic morbidity and mortality[123].This strategy identifies those with liver disease sufficient to call for specific interventions to prevent complications of cirrhosis and the development of HCC.People with NAFL or NASH with early F0-F2 don’t need to be considered as having liver disease necessitating intervention owing to the low risk of liver-related complications.In these persons,metabolic risk factors like diabetes should be addressed to optimize CV outcomes,with likely benefits on liver disease[123].As progressive fibrosis indicates a poor prognosis with unfavorable CV and adverse hepatic outcomes,the approach should now focus on the risk stratification of patients and identify those needing liver-specific intervention.
Non-invasive tests of hepatic fibrosis
As the severity of fibrosis is the major driver for the long-term prognosis of NAFLD patients,it is,therefore,critical to identify patients at higher risk of advanced fibrosis to optimize their management[124].Although required to detect patients with NASH and early fibrosis,liver biopsy is an invasive procedure.Patient acceptability is low,and it is not desirable to perform liver biopsy repetitively to assess disease progression and response to treatment.Moreover,as only a small proportion of the patients would develop liver-related complications,performing non-invasive tests(NITs)as the primary assessment is preferable[125].This section focuses on the confounding factors that can affect the performance and accuracy of NITs of liver fibrosis in patients with NAFLD.
Impact of confounding factors
Non-invasive fibrosis scores are usually used to detect or exclude advanced fibrosis in individuals with NAFLD.A few studies purposely looked at reasons for imprecise prediction by these scores.In a multicentric European study in subjects with biopsyproven NAFLD,the AST-to-ALT ratio,NAFLD fibrosis score(NFS)and Fibrosis-4(FIB-4)index performed poorly for the detection of significant fibrosis in persons aged 35 years or below[126].The specificity of the FIB-4 index and NFS reduced to unacceptable levels in those aged 65 years and older in the same study.This reason is that age is a component of both the fibrosis scores.The performance of NITs and the used transient elastography(TE)liver stiffness cutoffs in different ethnic populations and special subpopulations such as individuals with diabetes and obesity also need to be taken into account.For example,depending on the ethnicity,the diagnostic accuracy of the NITs may be altered.Compared to Western populations,South Asians develop more metabolic complications at lower body mass indices.The accuracy of the NFS,AST-to-platelet ratio index,FIB-4,AST/ALT ratio,and BARD score is found to be lower in the South Asian population in comparison with the Caucasian population[127].In addition,the NFS has a lower sensitivity in individuals of South Asian descent,as the majority had a lower BMI and were younger than Caucasian counterparts with a comparable disease stage,and therefore had a lower score[125].Serum markers of liver fibrosis and possible confounding factors are summarized in Table 1.

Table 1 Non-invasive tests of hepatic fibrosis and potential confounding factors
With regards to imaging modalities that estimate liver stiffness as a potential surrogate of hepatic fibrosis,vibration-controlled transient elastography(VCTE)has been widely validated against liver histology[128]and shows correlation with clinical outcomes in longitudinal studies[129].However,there are a number of factors to be considered while using this modality.Pathologies that increase liver stiffness can lead to a false-positive diagnosis of advanced fibrosis.Besides,high BMI and severe hepatic steatosis have been reported to increase the false positive rate of VCTE[130].A recent study suggests that when using the XL probe in obese patients,steatosis does not augment liver stiffness independent of fibrosis[128].Magnetic resonance elastography(MRE)can surmount many of these barriers,except for iron overload and acute inflammation;nonetheless,restricted availability at most centers and cost are the limiting factors.MRE has higher applicability and accuracy than VCTE when compared head-to-head[131].
While it is expected that blood-based parameters or imaging modalities will replace liver biopsy for the diagnosis in people who would benefit from treatment,equally it indicates that validation of any future marker should be done in more specifically defined cohorts.A recent International Consensus Panel suggested that the factors that shape the NAFLD heterogeneity should be taken into account when devising riskstratification scores and algorithms[2].Caution should be exercised by clinicians during the interpretation of test results when the tests are applied in patients with potential confounding factors.
Considerations for best practice
Early detection of advanced fibrosis is essential in the efforts to halt the NASH progression.Therefore,screening is vital to ensure that patients,mainly those with advanced F3-F4,are identified and linked to care before they develop end-stage liver disease.With the development of reliable NITs to identify patients with advanced fibrosis,there is now potential to put management strategies earlier in place[132].Clinicians need to be more proactive in detecting patients with advanced fibrosis due to NASH.Figure 4 shows a diagnostic algorithm that targets screening of patients with characteristics of MetS who are at risk of progressive fibrosis.This is in accordance with guideline recommendations to screen this high-risk group[133].This pathway includes sequential use of NITs(preferably a serum biomarker and an imaging technique)and can decrease secondary and tertiary referral rates and achieve larger cost savings.
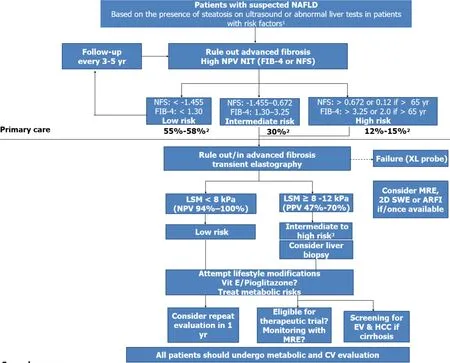
Figure 4 A suggested algorithm for the use of non-invasive tests for risk stratification of patients with suspected non-alcoholic fatty liver disease in clinical practice.1Obesity,type 2 diabetes,or metabolic syndrome;2Estimated prevalence for low,intermediate,and high risks groups;3Patented serum biomarkers(FibroTest,Fibrometer,or ELF)could be considered in patients with intermediate-risk.ARFI:Acoustic radiation force imaging;LSM:Liver stiffness measurement;MRE:Magnetic resonance elastography;NPV:Negative predictive value;PPV:Positive predictive value;SWE:Shear wave elastography.
In the Asia-Pacific region,quite a few studies have assessed the cross-sectional accuracy of non-invasive surrogates of liver biopsy among NAFLD patients[134,135].It has been suggested that the serum tests and physical tools when used in combinations can yield more reliable data than that provided by either method alone[136].Nevertheless,concerns are there regarding the definition of threshold values in Asian patients and Asia-Pacific Working Party stated that “at the present time,the clinical use of such tools to avoid liver biopsy remains undefined”[137].
Newsomeet al[138]recently published the FibroScan-AST(FAST)score for the noninvasive identification of patients with significant fibrosis(≥ F2)and a NAFLD activity score(NAS)of ≥ 4 to detect those at increased risk of disease progression.This could reduce unnecessary liver biopsies in patients unlikely to have significant disease.The incorporation of VCTE values in the score enhanced the diagnostic performance.This prospective study was validated in multiple global cohorts from North America,Europe,and Asia.Discrimination was considerably higher for the FAST score when compared with FIB-4 and NFS.Now,further research on the performance of the FAST score is required to transition the use of such predictive models to clinical practice.The diagnostic accuracy of the sequential combination of FIB-4 and VCTE had been evaluated recently in an individual participant data meta-analysis that included 5735 patients.Depending upon the different cut-offs used,this combined algorithm can diagnose cirrhosis with a specificity of 95%-98%,obviating the need for liver biopsy[139].
Identification of novel therapeutic targets
As the burden of NAFLD has become increasingly evident,so also have hurdles to developing effective therapeutic points of action.The development of progressive steatohepatitis is connected to excess metabolic substrate delivery to the liver that,in turn,induces cell stress,which can activate inflammatory and apoptotic signaling.Eventually,inflammation triggers a fibrogenic response that can lead to cirrhosis in the end[140].This simplified model facilitates the evaluation of precise mechanisms underlying each of these factors and targeting them for treatment.Table 2 summarizes proposed ‘druggable’ pathophysiologic targets in NAFLD[141-153].

Table 2 Liver-targeted therapies in development for the treatment of nonalcoholic fatty liver disease
Quite a few of the recently carried out phase 2 and 3 studies failed to reproduce the encouraging antifibrotic or NASH-resolving effects observed in animal models.Reasons for this discrepancy between preclinical models and clinical settings are likely diverse.Most importantly,no model can ever assess compounds in the actual physiological settings of heterogeneous human populations.This aspect may become further relevant if mechanisms are not entirely translatable between two different species[154].Additionally,none of the available NASH models used for preclinical trials adequately represents all the human disease aspects from the macroscopic to the molecular level.Moreover,only a few models reflect linked extrahepatic diseases(such as atherosclerosis,obesity,or IR).Finally,a higher heterogeneity in humans in relation to genetics,the gut microbiota,gender,and existing comorbidities leads to even more complications.It is,therefore,critical to recognize the drawbacks of preclinical models to improve clinical trial outcomes in drug development.
There is significant interindividual variability in the NAFLD susceptibility and for progression to liver-related complications[49].It is becoming more and more apparent that there is substantial heterogeneity in the molecular and cellular processespropelling the disease from one patient to the next.This understanding raises the possibility of matching specific therapeutic strategies to the particular disease drivers in a given patient.The development of such personalized approaches and the detection of subpopulations with distinctive disease drivers will need a combination of phenotypic,genetic,and molecular data[140].Furthermore,genetic insights present a powerful approach to deduce and prioritize candidate drugs.Such selection can avoid numerous drawbacks while defining likely benefits[155].However,drug discovery based on genetics is still in its infancy,and this area will present its challenges.NAFLD is associated with several metabolic disturbances.As many circadian clock-controlled genes are fundamental in the metabolic processes of the body,it is not unexpected that some of these genes can be potential therapeutic targets[156].Thus,by considering the circadian cycling of their targets,new drugs for NAFLD can be administered in a way that optimizes the benefits and minimizes the side effects.
Impact on clinical trials and endpoints
Given the rising disease burden associated with NAFLD,the development of outcome measures to assess the at-risk population and validate clinically relevant study endpoints is vital.Nevertheless,the natural history of NAFLD is highly variable,often nonlinear in progression.In addition,NAFLD itself is a heterogeneous disease that is shaped by the dynamic interaction between genetic predisposition,environmental factors,and several modifiable risk factors[157].This pathogenetic background provides numerous potential targets for therapeutic intervention,however,this same complexity limits defining clear,measurable,and objective clinical endpoints[158].Considering these factors,surrogate endpoints,which can be used to predict outcomes on clinically relevant endpoints,are expected to be beneficial in most patients.Furthermore,NAFLD is a slowly progressive disease,with a gap of many years between onset and development of “hard” clinical outcomes,such as liver-related and all-cause mortality.As stated earlier,the fibrosis stage is the most important predictor of liver-related outcomes.Unfortunately,the progression of fibrosis itself is also slow,with a median of 7.1 years in subjects with NASH[112].Thus,selecting meaningful clinical endpoints has been a major challenge in drug development and validation.At present,before enrolling patients into NASH clinical trials,identifying which patients with NAFLD have NASH,particularly those with advanced fibrosis,is one of the major stumbling blocks.Once these at-risk patients have been selected,monitoring for fibrosis regression in individuals with advanced fibrosis appears to be the optimal endpoint in clinical trials and should supplant NASH-based endpoints[158].Surrogate measures of liver-related outcomes also seem reliable.Although important,to assess for all-cause mortality(primarily CV death)and liver-related mortality will require longer-term follow-up.
Liver biopsy is essentially prone to sampling error and interobserver variability;its invasive nature also makes it a barrier for large clinical trials.Given these limitations,the development of accurate,robust,and reproducible noninvasive surrogate endpoints which may ultimately replace biopsy in trials are eagerly sought in NAFLD research[159].Algorithms such as NFS and FIB-4 may be useful tools for prescreening,in order to enrich the patient group with an appropriate spectrum of NASH and fibrosis for enrollment.Noninvasive imaging methods such as VCTE and MRE are likely to play a future role but presently lack the ability to differentiate between closely related fibrosis stages[160].
To summarize,a combination of the slow nature of disease progression in NAFLD,heterogeneity of therapeutic targets,and inherent limitations of serial liver biopsy to evaluate effects of intervention have considerably hampered clinical trial design as well as the development of new and effective therapies[158].Thus,the standard trial design that does not consider the disease heterogeneity may not be the best approach for learning this complex disease.Future clinical trials need to target patients with specific characteristics(gender,hormonal status,genetic susceptibility,metabolic and microbiota signatures,and the presence or absence of comorbidities)once the connections between these characteristics and the therapeutic targets are clearly understood[2].
FUTURE PERSPECTIVES
With increasing recognition of heterogeneous molecular and genetic drivers of NAFLD,there is a possibility of precision medicine based on the identification of specific drivers of the disease.An integrated model of NAFLD development based on genetic,molecular,histology,“omics” based data(transcriptome,metabolite,proteome,microbiome),and disease phenotype to identify disease subpopulations is required for such personalized approaches[140].Critical data on molecular heterogeneity and its relation to clinical outcomes of NAFLD to going to explore new horizons in the management of this global pandemic[161].A better understanding of bidirectional and dynamic disease progression and regression(e.g.fibrosis),the influence of behavioral factors,and establishing a correlation with end-organ damage is warranted.Prospective follow-up data on the evolution of pediatric NAFLD into adulthood shall shed light on pediatric disease evolution[162].Identification and validation of non-invasive methods of disease assessment and biomarkers will accelerate the development of pharmacotherapy and testing of combination therapies.Seamless phase II-IV trial designs,virtual placebo cohort analysis,master clinical trials testing multiple agents and multiple disease types,use of effectiveness trials in realworld settings,and patient-reported outcomes would revolutionize clinical trials for NAFLD.Precise terminology,characterization of disease heterogeneity(both molecular and clinical),novel translational models to identify new therapeutic target,and thus better designed clinical trials would help reduce the burden of the disease[2].
CONCLUSION
The impact of the upsurge in NAFLD patients and a rising proportion with advanced disease will be reflected in higher rates of hepatic and extrahepatic morbidity and mortality,which will continue to burden the health care system heavily.On the other hand,a lack of enough consideration of heterogeneity in risk profiles and responsiveness to treatment posing impediments that hampers progress to effective treatments.It is anticipated that a more robust understanding of pathophysiology will result in better characterization and subphenotyping of the disease and its drivers.In turn,this understanding of disease variability may help the introduction of appropriate noninvasive biomarkers for each subtype,thus promoting more individualized interventions.In this regard,any discussions on the update of nomenclature or more appropriate terminology are in the right direction.However,the proposed redefining of the disease should increase the prioritization of research activity on NAFLD to fill current knowledge gaps and find new tools to overcome the challenges.It appears to be important to place NAFLD/MAFLD/DAFLD under the same umbrella with significant comorbidities and approach NAFLD/MAFLD/DAFLD holistically rather than facing NAFLD as a separate entity.Future studies are likely to provide us the necessary prerequisites for designing more appropriate clinical trials to identify finely tailored diagnostic and treatment strategies for our patients.
杂志排行
World Journal of Hepatology的其它文章
- Incidence of umbilical vein catheter-associated thrombosis of the portal system:A systematic review and meta-analysis
- Role of endoscopic ultrasound in the field of hepatology:Recent advances and future trends
- Porta-caval fibrous connections—the lesser-known structure of intrahepatic connective-tissue framework:A unified view of liver extracellular matrix
- Promising diagnostic biomarkers of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis:From clinical proteomics to microbiome
- Fatty acid metabolism and acyl-CoA synthetases in the liver-gut axis
- Liver involvement in inflammatory bowel disease:What should the clinician know?
