Chelation therapy in liver diseases of childhood:Current status and response
2021-12-06JayendraSeetharamanMoinakSenSarma
Jayendra Seetharaman,Moinak Sen Sarma
Jayendra Seetharaman,Moinak Sen Sarma,Department of Pediatric Gastroenterology,Sanjay Gandhi Post-graduate Institute of Medical Sciences,Lucknow 226014,Uttar Pradesh,India
Abstract Chelation is the mainstay of therapy in certain pediatric liver diseases.Copper and iron related disorders require chelation.Wilson’s disease(WD),one of the common causes of cirrhosis in children is treated primarily with copper chelating agents like D-penicillamine and trientine.D-Penicillamine though widely used due its high efficacy in hepatic WD is fraught with frequent adverse effects resulting discontinuation.Trientine,an alternative drug has comparable efficacy in hepatic WD but has lower frequency of adverse effects.The role of ammonium tetra-thiomolybdate is presently experimental in hepatic WD.Indian childhood cirrhosis is related to excessive copper ingestion,rarely seen in present era.DPenicillamine is effective in the early part of this disease with reversal of clinical status.Iron chelators are commonly used in secondary hemochromatosis of liver in hemolytic anemias.There are strict chelation protocols during bone marrow transplant.The role of iron chelation in neonatal hemochromatosis is presently not in vogue due to its poor efficacy and availability of other modalities of therapy.Hereditary hemochromatosis is rare in children and the use of iron chelators in this condition is limited.
Key Words:Wilson’s disease;D-Penicillamine;Trientine;Indian childhood cirrhosis;Deferoxamine;Deferasirox;Hemochromatosis
INTRODUCTION
Chelation is a process in which a synthetic compound is administered to remove an excess mineral or heavy metal from the body.There are various liver diseases that are caused by excess deposition of various heavy metals such as copper,iron and arsenic.Some of these are genetic-metabolic,others are due to environmental exposure.In the landmarks of chelation therapy in hepatology,Walshe documented cupriuresis after administering dimethyl cysteine(penicillamine)in Wilson’s disease(WD)in 1956[1].Chelation was thereafter used in non-Wilsonian liver diseases.In the subsequent years newer chelators such as trientine and ammonium tetra thiomolybdate were identified for WD.From the 1970s,transfusion-related liver siderosis of hemolytic anemias was revolutionized by the use of deferoxamine[2].The use of iron chelators was attempted in gestational alloimmune liver disease and hereditary hemochromatosis.This review explores the rationale and outcome of chelation therapy in various pediatric liver diseases.
MECHANISM OF CHELATION
Metal ion(M)complexes with cheating agent(L)through an equilibrium reaction to form metal-ligand complex(ML)or chelate.The concentration of the chelate in the solution is directly proportional to the concentration of metal ion[M]and the ligand[L].
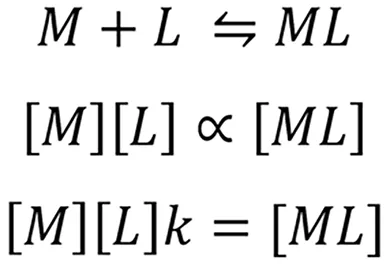
Where k is the effective stability constant.Value k denotes the affinity of the chelating agent.High k values suggest high affinity of the chelating agent.The value of k depends on the nature of the chelating agent,temperature,pH of the solution[3].Thein-vivomilieu is not similar to thein-vitrochemical reaction.The presence of weak acids in the body fluids like glutamate,sulfate,citrate,amino acids,albumin,macroglobulinetc.affect the chelation.These are called biological ligands.Chelating agent binds to the biological ligands and the effective concentration in the body fluid is lowered.Hence the equation becomes.
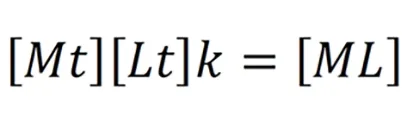
Effective chelation occurs when concentration of M and/or L is high,when affinity of the chelator(k)is high or when the concentration of the chelate[ML]is low.The metal ion concentration[M]in the body depends on the severity of the disease.For example,in a WD presenting as acute liver failure,serum copper(Cu)levels are usually very high.The concentration of chelating agent[L]is increased by increasing the dosing and/or frequency as tolerated by the patient.For the chelation to progress,urinary excretion of chelate[ML]is very important as it effectively reduces the concentration[3].Ideal chelating agents must have good oral absorption,acceptable bioavailability,high affinity to metal ions,low toxicity at appropriate plasma concentration,undergo rapid elimination or detoxification after combining with metal ions and more importantly should be available in affordable price[5].
CHELATION IN WD
WD is an autosomal recessive disorder caused by mutation of ATP7B gene that encodes for a protein P-type ATPase which transports copper into trans Golgi network and for biliary excretion of copper.In lysosomes,copper is incorporated into ceruloplasmin.In WD,due to defect in ATPase transport protein,ceruloplasmin formation is defective and biliary excretion of copper is impaired[6,7].This causes excess accumulation of intracellular copper subsequently increasing the levels in blood causing accumulation in extra-hepatic organs(Figure 1).

Figure 1 Pathophysiology of Wilson’s disease.Due to mutation in ATP 7Bgene,P type ATPase is defective and copper is not incorporated in ceruloplasmin.Free copper increases in blood and is deposited in liver and extrahepatic sites(brain,kidneys,bones,cornea,RBC).
Chelating drugs
D-Penicillamine(3,3-dimethylcysteine)is the most commonly used medication for WD worldwide.The L-isomer of this drug is not advised for treatment due to its neurotoxicity.The chelation property of DPA is due to the presence of thiol(-SH),which is responsible for its high affinity towards divalent metal ions such as copper.The mechanism of action of D-Penicillamine(DPA)is by inducing cuprieuresis,inducing hepatic metallothionine synthesis,reducing fibrosis(by preventing collagen formation).DPA also has an anti-inflammatory property[8].It is rapidly absorbed in proximal intestine but only 40%-70% are absorbed[9].The peak plasma concentration occurs after 1-3 h after ingestion.It circulates in the plasma predominantly by binding to albumin(80%),while the rest of the compound is present as free or disulphide forms.DPA is metabolized in the liver by conjugation with sulfide or by methylation(phase II reaction)and excreted in urine with almost 80% being eliminated within 10 h of ingestion.After discontinuation of therapy,the drug is eliminated in about 3-6 d[10].Food,antacids,iron and zinc preparations reduce the bioavailability by almost 50%.Plasma concentration reduces significantly when the drug is taken with food[11].It is recommended to give the drug either 1- hour before or 2- h after food.The drug is given in the dose of 20 mg/kg per day(up to 1500 mg)rounded to nearest 250 mg in 2-4 divided doses and can be maintained at 1000 mg/d once the disease is in remission[12].As DPA causes pyridoxine deficiency,pyridoxine should be supplemented at 25-50 mg/d.In case of neurological WD,to prevent paradoxical neurological worsening,the drug is started at low dose(125-250 mg)and slowly increased(125-250 mg every week)to reach the desired dose by 4-6 wk[13].
Trientine(triethylenetetramine)is an alternative chelating agent in WD.It is a derivative of spermine and putrescine and binds to copper in the ratio 1:1 to form a stable complex,which is eliminated in the urine.Trientine dihydrochloride is the oral ingestible form requiring storage at 2-8 degree Celsius to maintain stability.10% of the trientine is absorbed in the proximal small intestine and achieves its peak concentration 1.5-4 h after ingestion.Trientine is extensively metabolized in tissues by acetylation but the enzyme responsible for it is not identified.1% of ingested trientine and 8% trientine metabolite acetyltrien,appears in the urine.Plasma concentration of the trientine significantly reduces when given with food due to its affinity to dietary copper in the lumen thereby compromising the removal of tissue copper and the other reason could be due to the physiological polyamines secreted during food intake inhibits effective trientine absorption[14].Trientine is not to be given with iron as it forms toxic complexes.The dose recommended is 20 mg/kg per day with the maximum of 1500 mg/d rounded to nearest 250 mg(300 mg capsules in North America)and maintenance dose of 1000 mg/d.Similar to DPA,trientine also should be ingested 1 h before or 2 h after food intake[12,15].The decoppering efficacy of any chelating agent is evident from the effective stability constant(k)which denotes copper affinity.The comparison of k-value of DPA(2.38 × 10-16)and trientine(1.74 × 10-16)suggests the decoppering efficacy of DPA is much higher than trientine[16].
Efficacy of chelation
Improvement in symptoms and biochemical parameters in WD takes around 2-6 mo in hepatic forms whereas in isolated neurological forms it may take up to 12-24 mo[12].DPA in WD children shows an efficacy of almost 70%-90%[17-20].The response depends on whether it is hepatic or neurological form and severity of the disease at presentation.Long term of follow up of WD(median duration- 15.1 years)studied by Bruhaet al[19]showed the response to DPA to hepatic forms is 82% compared to 69% for neurological forms.One of the largest series of WD patients(n= 327)from Euro Wilson consortium,showed hepatic forms had 91% response compared to only 68% in neurological forms after a median follow up duration of 13.3 years[20].In most series,trientine is used as a second line either due to poor response or due to toxicity to DPA.Hence,there are no head-to-head randomized trials comparing the efficacy of DPA and trientine.Overall efficacy of trientine is reported to be 80%-92%[21,22].Retrospective analysis of efficacy of the two drugs by Hölscheret al[23]showed response in hepatic forms with DPA was 92% compared to 84% response with trientine after a median follow up duration of 13.3 years.In neurological forms,DPA fares significantly better(68%)than trientine(48%,P =0.008)[23].In Euro Wilson consortium,the response of both the DPA and trientine were comparable when used as a first line in both hepatic(90.7%vs92.6%,P =0.98)and neurological forms(67.5%vs55%,P =0.76).However when used as a second line therapy,trientinevsDPA showed similar response in hepatic form(75%vs68.9%,P =0.76)but better response in neurological form(51%vs23.1%,P =0.01)[20].
Adverse effects of copper chelators
Adverse effects of DPA are always a major concern with up to 30% of the patients develop one or more adverse effects(Table 1)[20,24,25].Adverse effect can be early onset(less than 3 wk of therapy)or late(more than 3 wk to up to 2-3 years of initiation of therapy).Early adverse effects like fever,rash,arthralgia,lymphadenopathy,pancytopenia are predominantly immune mediated[26].Nephropathy,the most common late adverse effect of DPA is seen in 5%-30%.Presentations include proteinuria,glomerulonephritis,nephrotic syndrome less commonly as Good Pasture’s syndrome[27-29].More than 90% of the nephropathy occurs within 12 mo of therapy.High doses of DPA,decompensated liver disease,intrinsic renal diseases or presence of HLA-B8/DR3 are probable risk factors of nephropathy[30].Eighty percent are membranous glomerulonephritis on renal biopsy.In a study by Hallet al[27]of 33 patients with DPA nephropathy,one-third each showed resolution at 6,12 and 18 mo respectively,after drug discontinuation.There are no clear recommendations as to whether the drug can be rechallenged after resolution of nephropathy.However,in such situations,it is prudent to continue the patient on an alternative drug such as trientine or zinc.DPA related myelotoxicity occur in up to 7% patients undergoing chelation with DPA[31-33].Two types of myelotoxicity are known to occur,idiosyncratic(usually with in 1 year of therapy)or dose dependent(more than after 1 year therapy)[34].Though,there are no definite guidelines for monitoring and treatment of myelotoxicity,European society of Pediatric Gastroenterology Hepatology and Nutrition(ESPGHAN)suggests weekly blood counts initially,1-3 mo till remission and 3-6 monthly thereafter[35].If two or more values of total leukocyte count less than 3.5 × 103per cubic mm,drug is to be discontinued.Bone marrow examination and reticulocyte counts differentiates this condition if concomitant hypersplenism is present[36,37].Blood products,colony stimulating factor and anti-thymocyte globulin may improve the counts.Usual time of spontaneous recovery is 4-12 wk.Rarely hematopoietic stem cell transplantation may be required in refractory and prolonged cases.Once bone marrow toxicity has ensued,the drug should not be re-challenged.Adverse effects of DPA related to skin may be due to either acute hypersensitivityreaction presenting as morbilliform rash,urticaria,degenerative dermatoses(cutis laxa or elastosis perforans serpingosa)or an autoimmune phenomenon(pemphigus,scleroderma or lichen planus[38].Rare musclar adverse effects of DPA include myasthenia(1%-2%)and ptosis.Anti- nicotinic acetyl choline receptor or Anti- MuSK(Anti- Muscle Specific tyrosine Kinase)is present in up to 70%[39].Systemic lupus erythematosus can occur within 6-12 mo after the onset of DPA therapy presenting as pleurisy,arthritis,rash with or without presence of anti-nuclear antibody[40].Deutscheret al[41]noted 3 out of 50 WD children with elevated transaminases within 6 wk of DPA therapy who resolved subsequently following discontinuation.Trientine also present with similar adverse effects as DPA like nausea,vomiting,arthralgia,myalgia,leukopenia,elevation in anti-nuclear antibody(ANA),nephropathy but adverse effects requiring discontinuation of trientine is significantly lower compared to DPA[20].

Table 1 Adverse effects of copper chelating drugs
In hepatic WD,paradoxical neurological worsening occurs commonly within 6 mo of therapy,in patients with an underlying overt or occult neuropsychiatric feature.Paradoxical neurological worsening occurs even when dosing and compliance is good[42].It occurs due to the sudden release of Cu from the liver following chelation therapy causing oxidative brain injury.Overall incidence of paradoxical neurological worsening ranges from 7%-26%.Those with previous known neurological WD,the incidence of worsening is up to 75%[19,24,25].Both DPA and TA have shown to cause neurological worsening.In series from Euro Wilson consortium,paradoxical neurological worsening occurred significantly more with TA compared to DPA[20].Litwinet al[13]studied natural history of 143 WD(70 Neuro/Neurohepatic WD and 73 hepatic WD),of whom 23% neurological cohort and none of the hepatic cohort developed early neurological worsening on chelation.In this series,median time of onset of neurological worsening was 2.3 mo.Fifty-three percent were completely reversible and 13% were partially reversible on drug discontinuation with median time of reversibility of 9.2 mo[13].Prior neurological involvement,lesions in brain stem or thalamus and concomitant anti-dopaminergic drugs had higher chances of neurological worsening.Treatment consists of drug discontinuation and addition of zinc for a transition period.Chelators can be restarted in lower doses with gradual increment once the symptoms improve[13].
Assessment of adequacy of chelation:Clinical parameters
Currently there is no fool-proof,gold standard yardstick to assess chelation adequacy.All have fallacies in assessment and hence multiple parameters are considered.Chelation adequacy can be assessed firstly by assessing compliance to drug intake.Compliance is assessed by having a pill count,self-reporting by patients themselves or by checking empty blister packs during follow up outpatient visits[43].There are various scales being developed assessing medication adherence(MAQ:Medication adherence questionnaire,MARS:Medication adherence Rating scale)but none have been validated in children[44].More objective way of assessing compliance is by measuring drug levels but it is not routinely available under clinical setting.Secondly,follow up of clinical parameters assess the adequacy of chelation like improvement in jaundice,ascites,encephalopathy which usually take 2-6 mo post therapy.Resolution of neurological symptoms may take longer than 2-3 years[12].The resolution of Kayser-Fleischer ring on de-coppering therapy has considerable controversies to the same.Studies have heterogeneity in their assessment and reports.It appears to be independent on type of presentation(neurologicvshepatic),stage of disease(presymptomaticvssymptomatic)and choice of chelator and compliance.Initial reports showed,Kayser-Fleischer(KF)ring disappearance in 81% of the patients(completely in 41% and incompletely in 59%),more in pre-symptomatic stage(60%)than those in symptomatic phase with ongoing therapy(2%)over 22 years of follow-up on DPA(90%)and zinc or trientine(10%).Conversely one-third of asymptomatic patients the rings did not reabsorb even after therapy of > 10 years.In this study,the fading of KF rings seemed to be independent of the stage of the disease and effectiveness of the decopperizing treatment[45].In a study by Fenuet al[46]where 66% were hepatic and 31% were neuro-hepatic(90% on DPA ± zinc therapy),partial or total KF ring resolution was observed in 28%,deterioration in 6% and static in the rest of the cohort over 1-3 years of therapy.Other smaller cohorts report reduction of KF ring in neuropsychiatric manifestation or disappearance over 10 years on maintenance zinc and molybdate therapy in pediatric hepatic WD[47,48].KF rings may reappear with non-compliance,and occasionally even with successful maintenance therapy[49].
Liver status can be appropriately assessed by Pediatric end-stage liver disease or Child-Turcotte-Pugh score.Biochemical parameters like serum albumin,total bilirubin and prothrombin time normalizes by 6 mo but liver enzymes might take longer[12].In the author’s experience it takes 9-12 mo for complete normalization of Liver function tests in majority of the cases[50].In patients who have additional neurological involvement,neurological response is monitored by indices such as Global assessment scale(GAS)[51].Even with neurological WD with significant MRI changes,50% show improvement with long term chelation[52].
She walked straight along the road in front of her, without knowing very well where she was going or what was to become of her, for she had never been shown how to work, and all she had learnt consisted of a few household rules, and receipts of dishes which her mother had taught her long ago
Assessment of adequacy of chelation:Biochemical parameters
Presently the most widely acceptable way to assess adequacy of chelation is by 24-h urine copper and non-ceruloplasmin copper.Twenty-four hours urine copper(UCu)increases immediately following chelation and takes around 12-18 mo to reach a stable level[53].European Association for the Study of the Liver(EASL)and American Association for the Study of Liver diseases(AALSD)recommends targeting 24-h urine copper between 200-500 mg/d for adequate chelation[12,15].Values > 500 mg/d suggest under chelation as lot of unchelated copper is remaining in the body.Values < 200 mg/d may be either due to over chelation or poor compliance(Table 2).This can be differentiated by non-ceruloplasmin copper(NCC)levels calculated by the formula(serum copper(mg/L)- 0.3 x serum ceruloplasmin(mg/L)[54].NCC has a few fallacies.Firstly,almost 20% of NCC are negative values,seen mostly when immunoassay method was used to measure ceruloplasmin as it measures both holoceruloplasmin and apoceruloplasmin.NCC calculation becomes inappropriate when inactive apoceruloplasmin is included.Secondly,there are variabilities in reference ranges in ceruloplasmin values between various laboratories across the world creating disparities in NCC cut-offs[55].According to EASL guidelines,NCC > 15 mg/dL suggest poor compliance and < 5 mg/dL suggest over chelation.Additionally,24-h urine copper after 48-h cessation of therapy has been recommended by EASL.Values > 100 mg/d is suggestive of under chelation or poor compliance while values < 100 mg/d suggest adequate treatment[15].

Table 2 Twenty-four hours urine copper and non-ceruloplasmin copper in various stages of Wilson’s disease treatment
A novel and upcoming modality to assess chelation is the use of exchangeable copper.Exchangeable copper is the fraction of copper bound to albumin,peptide and amino acids which are easily chelated by chelating agents.It denotes a direct estimation of non-ceruloplasmin copper(NCC)[56].On WD with chelation for long time,exchangeable copper values tend to reduce comparable to non-Wilson children.In a pilot study by the authors,the role of exchangeable copper was assessed in a cohort of 96 children with hepatic WD.Exchangeable copper was significantly higher in newly diagnosed WD compared to WD on chelation for more than 1 year(3 ± 7 μmol/Lvs0.9 ± 0.6 μmol/L,P =0.03).Exchangeable copper values were lower in stable liver disease compared to unstable liver disease(0.86 ± 0.5mmol/Lvs1.3 ± 0.6 mmol/L,P =0.01).Exchangeable copper values showed excellent correlation with non-ceruloplasmin copper(r= 0.92,P< 0.001).Predictive model incorporating exchangeable copper into standard monitoring tools improved the yield of disease control assessment by 21%[57].
Comparison of single vs dual chelation:Which is better in hepatic WD?
Strictly zinc is not considered as a systemic chelator.Oral zinc(Zn)induces metallothionine in enterocyte.Metallothionine is an endogenous chelator that has high affinity to copper.Hence induced metallothionine combines with luminal Cu,preventing its entry into circulation.This Cu is removed through feces when enterocyte is shed.Znalso induces hepatic metallothionine[58].Hence,Zn is used in pre-symptomatic WD,stable well chelated WD on maintenance therapy,severe neurological WD.It is also used as a last resort in those with DPA or trientine intolerance.In severe hepatic disease,many centers consider giving a trial of dual chelation DPA and zinc for rapid chelation and quick stabilization.In a study conducted by the authors,65 children with > 9 mo chelation were followed up for long term outcome.Majority had advanced disease at presentation.83% of children were treated with DPA monotherapy and 17% treated with DPA and zinc combination.Trientine was started in 4 children due to DPA toxicity.77% of children responded to DPA monotherapy even when the disease is severe at presentation and 50% responded when DPA and zinc combination was started.The overall response to oral chelation is 71%[50].Hence,DPA should be the first line of therapy for any hepatic WD and zinc is added in those who failed to show optimal response with DPA in desperate circumstances with the hope of rapid synergistic chelation and quicker liver recuperation[50].Though there are no comparative trials of dual or single chelation therapy,there are limited case series that have used DPA or trientine with zinc for WD presenting with ascites,coagulopathy and encephalopathy[59-61].Though the efficacy of dual therapy in these studies were 91%-100%,sample sizes were small.Systematic review of 17 studies that assessed the efficacy of dual therapy(DPA/ Trientine with zinc)showed pooled efficacy rate(60.4%,95%CI:55.8-65.0)compared to DPA(73.7%,95%CI:65.1-85.4)and trientine monotherapy(82.6%,95%CI:75.4-89.5).Adverse effects following monotherapy is also lesser with either DPA or trientine compared to combination therapy[62].Another retrospective study assessed 30 of 313 patients on dual chelator therapy,showed long term discontinuation and non-adherence was higher as compared to monotherapy(P =0.006).Combination therapy,may fare better in neurological WD compared to exclusive hepatic forms[63].Compliance and adequate spacing with chelating agent need careful consideration in the treatment schedule.If consumed together,chelator can combine with zinc in the lumen and effective absorption of both the medication gets reduced.Animal studies have shown that hepatic zinc stores is also significantly reduced during decoppering[64].Hence,when chelator is combined with zinc,a proportion of chelator is used up in removing the body zinc thereby compromising the efficacy.
Efficacy of ammonium tetra thiomolybdate
Ammonium tetra thiomolybdate is a strong decoppering agent used in limited trials.It prevents intestinal absorption of copper if given with meals but also reduces serum copper when given in between meals.Ammonium tetra thiomolybdate(ATM)is predominantly advised for neurological forms due to it low risk of neurological worsening[65].In the comparative study of ATM with trientine in neurological WD,paradoxical neurological worsening is significantly lower with ATM(4%)compared to trientine(26.1%,P =0.01)[66].At larger doses,ATM can form toxic insoluble complex that gets deposited in liver causing hepatoxicity[67].Hence the role of ATM in hepatic WD is precarious.Up to 10% of patients receiving ATM might develop bone marrow toxicity also[68].Bis-choline tetra thiomolybdate(WTX101)is an investigational derivative of ATM being studied recently in neurological WD with better stability and lower toxicity[69].Twenty-four weeks treatment of the drug caused improvement in 71% of neurological WD.Seven percent developed leukopenia and almost 39% developed elevated liver enzymes post therapy[69].Robust experience in exclusive hepatic WD is not yet available.
CHELATION IN INDIAN CHILDHOOD CIRRHOSIS
Indian childhood cirrhosis is commonly seen in children between 6 mo and 5 years of age in Indian subcontinent with its peak incidence seen during 1970-1990[70].Presently this entity seems to be waning in the Indian subcontinent.Predominant etiology advocated was excessive copper ingestion with use of copper utensils[71].There was also a possibility of genetic predisposition affecting copper metabolism[70].Clinical features consist of nonspecific symptoms to start with like fever,lethargy,easy fatiguability,palpable liver with leafy edges in stage I,splenomegaly and ascites in stage II and jaundice,coagulopathy and encephalopathy in stage III.Histopathological examination of liver shows diffuse hepatocyte necrosis,presence of Mallory bodies and granular orcein staining.Treatment monitoring is by liver function tests(LFT),serum copper and in many studies,by repeat hepatic copper and liver histology,while on treatment.Mortality is almost 60% in stage II but reaching almost 90% in stage III[72].In the study by Bavdekaret al[73]65 children with Indian childhood cirrhosis(ICC)on treatment with DPA were followed up for the mean duration of 3.5 years,showed response in 60% of the children in pre-icteric phase compared to only 6% response(P< 0.01)in icteric phase(Table 3).Another study in ICC children who received DPA or DPA with steroids showed 50% survival as compared to10% in placebo group(P =0.002)[74].In a pediatric study,DPA therapy has showed better response compared to DPA with intravenous immunoglobulin(P =0.018)[75].Chelation may improve symptoms if given early as prognosis is poor in advanced disease despite treatment[75].
CHELATION IN NON-WILSONIAN COPPER RELATED DISORDERS
Non-Wilsonian copper related diseases termed by Bakeret al[76]as copper associated childhood cirrhosis includes ICC from India and ICC-like illness from western countries.This ICC like illnesses is otherwise called idiopathic copper toxicosis.Type I copper associated childhood cirrhosis(CACC)resembles ICC,with an early onset of disease and related to increased copper intake.Type II CACC has onset later than 4 years of age and possibly has an autosomal recessive inheritance without an obvious increase in copper intake[77].Although there are few case reports of ICC- like illnesses,meagre number of reports use chelation therapy probably due to its conflicting results.One child from Bangladeshi origin,presented with jaundice,anorexia,weight loss at 7 years,with normal serum ceruloplasmin,and elevated hepatic copper 2319 mg/g.Improvement in symptoms and decrease in liver copper(35 mg/g)was noted after 19 mo of DPA therapy(Table 3)[77].In contrast,a 10 year old Italian child with ascites and hepatomegaly,normal ceruloplasmin levels and liver copper of 1970 mg/g did not show any improvement clinically and biochemically even after 2 years of DPA[78].Largest cohort of endemic Tyrolean infantile cirrhosis studied by Mulleret al[79]showed both genetics and copper contamination were responsible for the disease.However there is paucity of chelation therapy experience in this condition.
IRON CHELATION IN GESTATIONAL ALLOIMMUNE LIVER DISEASES
In Gestational alloimmune liver disease alloimmunization of fetal liver antigen occurs in maternal blood resulting in IgG fetal liver antibody causing complement activation in fetal liver and significant impairment in hepcidin production(Figure 2)[80].This causes iron storage in various organs like liver,heart,gonads,pancreasetc.Gestational alloimmune liver disease(GALD)causes liver failure as a result of hemochromatosis in newborn period and has high mortality if not intervened earlier.The liver injury causes reduced production of hepcidin resulting in uncontrolled iron absorption through placenta.This excess iron might further aggravate liver injury and also result in extra-hepatic iron deposition[81,82].There have been few studies of GALD being treated with iron chelators(intravenous deferoxamine)and antioxidants with no clearcut benefit.In the series by Flynnet al[83]five infants with neonatal hemochromatosis received intravenous deferoxamine but only one survived without liver transplantation.In the study by Rodrigueset al[84]10 infants received iron chelation but only one survived without transplantation.In another series by Sigurdssonet al[85]six infants with neonatal hemochromatosis received supportive measures whereas eight infants received combination of deferoxamine and antioxidants.Two out of six whoreceived supportive measures survived compared to only one who received chelation.It is not clear if the small proportion of response to chelation is due to efficacy of the drug in already advanced disease or due to natural history.In the recent years,it now clear that intravenous immunoglobulin has a superior role than chelation therapy in GALD.
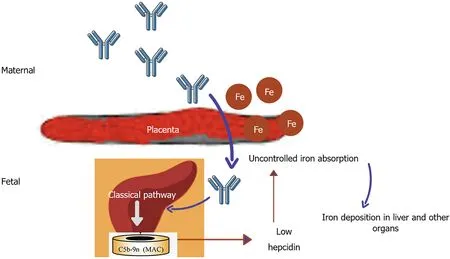
Figure 2 Pathogenesis of gestational alloimmune liver disease.Alloimmunization of fetal liver antigen by maternal blood produces IgG antibody passively transferred through the placenta to cause fetal liver injury by complement activation.Liver injury reduces the hepatic synthesis of hepcidin resulting in uncontrolled placental iron absorption.Excess iron is deposited in liver,pancreas,heart,gonads,etc.
IRON CHELATION IN HEREDITARY HEMOCHROMATOSIS
Hemochromatosis is due to iron accumulation in various organs with secondary causes being commoner in children than hereditary hemochromatosis.Secondary causes of hemochromatosis are commonly related to repeated transfusions in hemolytic anemia especially thalassemia major.In normal individuals,increased plasma iron induces the genes like HFE,TFR2 and HJV.This causes release in hepcidin,binding with ferroportin in enterocytes and macrophages,reducing iron absorption.Hereditary hemochromatosis(HH),most commonly due to mutation in HFE,cause impaired production of hepcidin making checkpoint for iron absorption defective[86].Animal studies showed excessive fat intake causes impaired hepcidin production and increased transferrin receptor 1 and divalent metal transporter 1 Levels by altering mRNA expression.Hence,increased iron absorption and iron related liver injury may be responsible for development of non-alcoholic steatohepatitis[87].Hereditary hemochromatosis(HH)is extremely rare in children.Excess iron in the serum causes liver cirrhosis,skin pigmentation,pancreatic insufficiency,cardiac dysfunction and hypothyroidism[88].Iron chelation forms the mainstay of therapy in transfusion related siderosis in various hemolytic anemias in children.In a few studies,iron chelators have been implicated in treatment of HH also.Deferoxamine is parenteral iron chelator,given either as subcutaneous or intravenous infusion(20-50 mg/kg per day)over 8-24 h.Adverse effects seen are local reaction in injection site,hearing abnormalities,bone abnormalitiesetc.Deferasirox is an oral chelator with a similar efficacy as deferoxamine in removing hepatic iron but prone for its gastrointestinal side effects.Deferiprone,also an oral chelator is prone for gastrointestinal side effects and agranulocytosis and is highly effective in removing cardiac iron compared to other chelators(Table 4)[89].Phataket al[90]from Italy studied multiple doses of deferoxamine in HH,showed 10 mg/kg is the dose with optimal response and lower side effects.Nagleret al[91]analyzed 2 patients treated for 6 mo and 10 mo respectively who showed significant reduction in serum ferritin in the follow up.EASL and AASLD guidelines on HH recommend phlebotomy as the treatment of choice in HH[92,93].Chelation may be considered in HH when phlebotomy is not tolerated due to severe congestive cardiac failure,anemia and in case of difficult venous access.
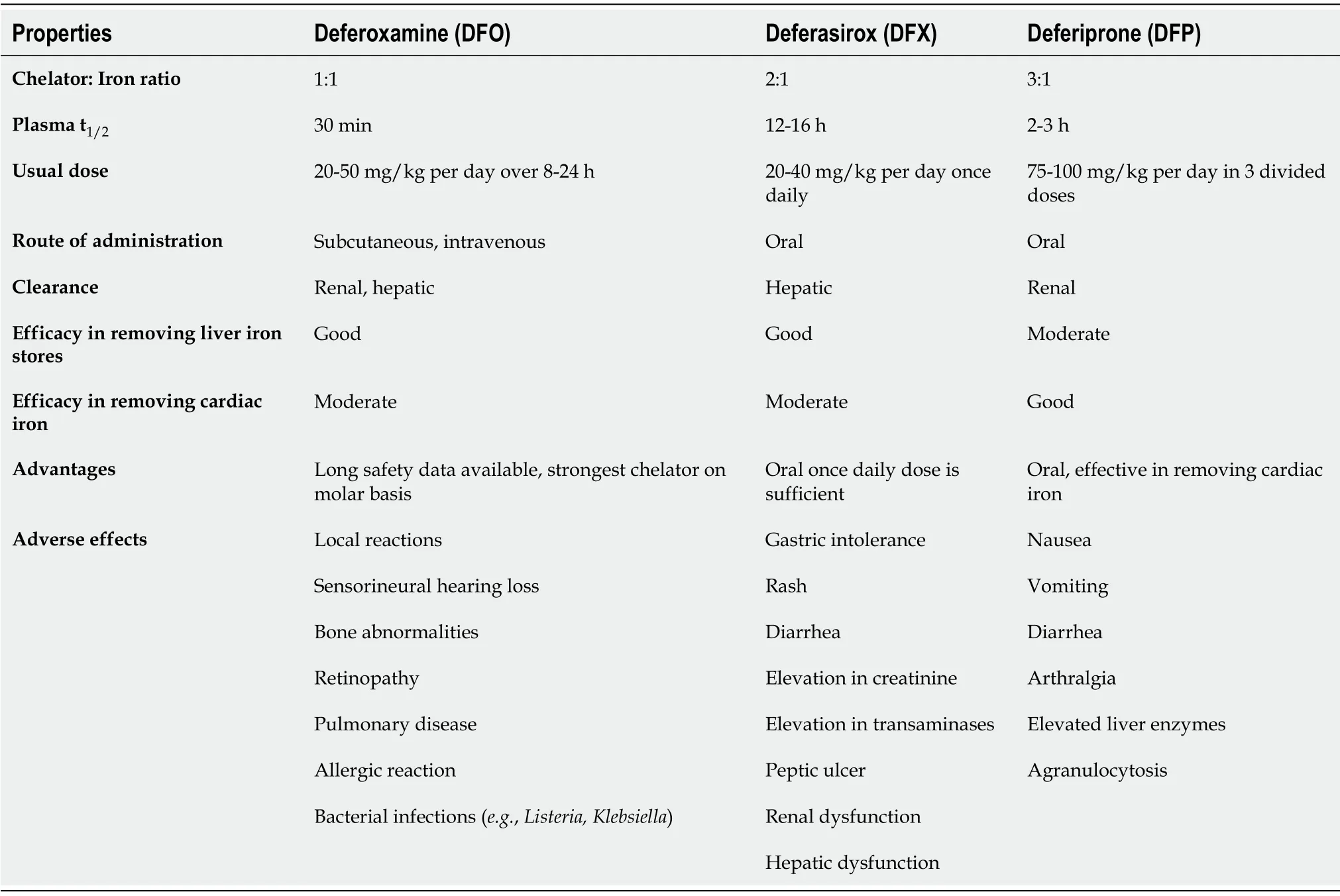
Table 4 Properties of iron-chelators
IRON CHELATION IN SECONDARY HEMOCHROMATOSIS
In children,secondary hemochromatosis is more common than HH and is usually caused by transfusion related iron overload seen in chronic hemolytic anemia especially beta thalassemia[94].Each milliliter of packed RBC adds 1mg of iron to the body stores.Iron is usually bound to transferrin in plasma.However when the iron load increases,transferrin sites saturate and excess iron spills as labile plasma iron causing free radical injury to heart,liver and endocrine organs[95].Multiple transfusion causes liver injury by various mechanisms such as siderosis causing hepatitis eventually progressing to fibrosis and cirrhosis.Hepatic foci of hemopoiesis and transfusion related hepatitis B and C infection are also seen[96].
Iron overload related liver injury can be assessed by various modalities.Serum ferritin is easily available and an inexpensive method to assess iron overload but its utility is limited in the presence of infection and inflammation.Liver iron concentration > 15 mg/g dry weight of liver is associated with significant mortality and morbidity[97].The superconducting quantum interface device(SQUID)measures liver iron stores non-invasively but the SQUID scanners are not available in many centersworldwide[98].Magnetic resonance imaging estimates liver iron by R2 and R2* techniques and it correlates well with liver iron concentration attained from biopsy.Magnetic resonance imaging(MRI)has now become the primary monitoring tool for both liver and cardiac iron[99].
Liver injury due to iron overload was common in children in pre-chelation era.Liver biopsies obtained in 80 children with beta thalassemia during splenectomy showed cirrhosis in 40% of children > 11 years with risk of cirrhosis increasing with age.60% of the children showed hypoalbuminemia and 70% showed elevated transaminases[96].Iron-chelators are well established treatment modality to prevent iron overload related liver injury.In a retrospective study by Mairaet al[100]deferasirox for a duration of 4 ± 1.5 years showed significant improvement in liver stiffness measurement by transient elastography(7.4 ± 3.2 kPavs6.6 ± 3.2 kPa,P =0.017)and liver iron concentration(LIC)(4.81 ± 3.82 mg/gvs3.65 ± 3.45 mg/g,P =0.001).Thus,iron chelation not only prevents progression of liver injury but also reverses inflammation and fibrosis.In the multicentric cross-sectional study from Italy,924 beta-thalassemia patients were evaluated for iron overload assessment and management.The study showed serum ferritin had an excellent correlation with liver iron concentration.Deferasirox(38.3%)was most preferred chelator,especially in children because of its safety and easy administration[101].Deferiprone was less commonly used when transaminases were elevated due to its concern of hepatic fibrosis[97].Combination of two chelators were used whenever serum ferritin > 2500 ng/mL or MRI R2* values < 20 ms.Guidelines suggest that LIC assessment should be done at 1-2 yearly intervals[102].Iron over load needs to be monitored and treated pre- and post-alloimmune hematopoietic stem cell transplantation(HSCT)for hemolytic anemia.Pre-transplant serum ferritin > 1000 ng/mL is associated with increased risk of post-transplant complications such as chronic liver disease,graftvshost disease(GVHD),sinusoidal obstruction syndrome and infection[103,104].Hence it is mandatory to rapidly reduce ferritin levels before HSCT.Gruppo Italiano Trapianto di Midollo Osseo(GITMO)study group recommends switching to intravenous deferoxamine for rapid lowering of serum ferritin pre-transplant.From 6 mo post-transplant,iron overload is to be assessed by serum ferritin and MRI R2*.If LIC in MRI > 7 mg/g phlebotomy is preferred,but when LIC > 15 mg/g phlebotomy along with iron chelators are required to prevent complications[105].
CONCLUSION
Copper chelation by D-penicillamine and trientine forms the mainstay of treatment in childhood WD.Appropriate dosing,compliance to medications and scheduled monitoring with liver function tests,24-h urine copper and non- ceruloplasmin copper are required for better control of the disease.D-penicillamine is a promising treatment for Indian childhood cirrhosis especially in early stages.The role in other non-Wilsonian copper diseases is doubtful.The use of iron chelator in Gestational alloimmune liver disease is waning due to its poor efficacy.Iron chelator may be considered as an alternative therapy in hereditary hemochromatosis when the primary treatment fails or not feasible but in case of secondary hemochromatosis chelation forms the main treatment.
杂志排行
World Journal of Hepatology的其它文章
- Incidence of umbilical vein catheter-associated thrombosis of the portal system:A systematic review and meta-analysis
- Role of endoscopic ultrasound in the field of hepatology:Recent advances and future trends
- Porta-caval fibrous connections—the lesser-known structure of intrahepatic connective-tissue framework:A unified view of liver extracellular matrix
- Promising diagnostic biomarkers of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis:From clinical proteomics to microbiome
- Fatty acid metabolism and acyl-CoA synthetases in the liver-gut axis
- Liver involvement in inflammatory bowel disease:What should the clinician know?
