Ovalicin attenuates atopic dermatitis symptoms by inhibiting IL-31 signaling and intracellular calcium influx
2021-12-04SungHyunHwangYeseulYangYejiJeongYongbaekKim
Sung-Hyun Hwang, Yeseul Yang, Yeji Jeong, Yongbaek Kim
1Laboratory of Clinical Pathology, College of Veterinary Medicine, Seoul National University, Seoul 08826, Republic of Korea;
2The Brain Korea 21 Future Veterinary Medicine Leading Education and Research Center, College of Veterinary Medicine, Seoul National University, Seoul 08826, Republic of Korea;
3Research Institute for Veterinary Science, College of Veterinary Medicine, Seoul National University, Seoul 08826,Republic of Korea.
Abstract Atopic dermatitis (AD) is a common skin disorder difficult to be treated with medication. This study investigated the potential of ovalicin extracted from Cordyceps militaris for the treatment of AD using in vitro and in vivo models. We found that, in canine macrophage cell line DH82, lipopolysaccharide (LPS) upregulated the expression of genes associated with inflammation and pruritic responses through activating calcium and interleukin-31 (IL-31) signaling, and the upregulation could be suppressed by ovalicin, with an effect significantly stronger than dexamethasone. Ovalicin also reduced the expression of IL-31 downstream genes, including JAK2(Janus kinase 2), TRPV1 (transient receptor potential vanilloid receptor-1), and HRH2 (histamine receptor H2).Ovalicin significantly alleviated the allergic symptoms in the AD mouse model. Histologically, the number of macrophages and mast cells infiltrated in the dermis was significantly reduced by ovalicin treatment. In the skin tissue of AD mice, reduction of IL-31 receptor was observed in the ovalicin treated group compared to the group without ovalicin treatment. To our knowledge, this is the first study to elucidate the anti-atopic mechanism of ovalicin, which could be an alternative to steroidal drugs commonly used for AD treatment.
Keywords: ovalicin, IL-31, pruritus, inflammation, calcium, atopic dermatitis
Introduction
Atopic dermatitis (AD) is characterized by pruritus,a cardinal symptom accompanying dermatologic changes to worsen the quality of life[1]. In AD patients,scratches make the skin barrier vulnerable to infection[2–3]. Antipruritic agents with anti-inflammatory effects are beneficial for AD patients. Steroidal drugs are widely used to relieve pruritus, but have severe side effects, such as petechiae, telangiectasia, muscle atrophy, and liver damage[4–6]. Efforts to find their alternatives have been explored.
Ovalicin, extracted from an entomopathogenic fungusMetarhizium anisopliaeinCordyceps militaris,is an active ingredient of antidandruff and antibacterial agents[7]. Ovalicin is purified by filtration,extraction with ethyl acetate, vacuum evaporation, and elution in florisil-packed chromatography[8]. Recent studies have shown that ovalicin relieves AD symptoms in mouse models; however, the molecular mechanism underlying its antipruritic effects is unclear.
In AD patients, skin tissue has deviated immune reactions to T helper 2 (Th2) cells. When exposed to an allergen, interleukin (IL)-31 produced from Th2 cells conjugates with its receptor[9]. In turn, the IL-31 communicates with the dorsal root ganglion and induces a pruritic response[10]. Comparative analysis of the pruritic response in wild-type and IL-31 knockout mice shows a lower frequency of scratching behavior inIl31knockout mice, while the inflammatory responses are comparable[11]. A higher level of IL-31 and its dimeric receptors, IL-31RA and oncostatin M receptor (OSMR), is detected in AD patients compared to healthy individuals[12]. Transient receptor potential cation channel 1 (TRPV1) is upregulated by histamineviaphospholipase A2 activation, and subsequently induces scratching behavior[13]. TRPV1 also induces the calcium influx for histamine release.However, the function of TRPV1 in IL-31 signaling is unclear.
In this study, we investigated the effects of ovalicin on DH82 cells and in 2,4-dinitrochlorobenzene(DNCB)-induced AD mouse model, and found that ovalicin may function as a potent antipruritic agent by repressing IL-31 signaling.
Materials and methods
Cell culture
The canine macrophage cell line, DH82, was kindly provided by Dr. Yun sang Cho (Animal and Plant Quarantine Agency, Republic of Korea). DH82 cells were cultured in Roswell Park Memorial Institute Medium (Gibco-Life Technology, USA) containing 100 μg/mL of penicillin/streptomycin (Gibco),1 mmol/L of sodium pyruvate (Sigma-Aldrich, USA),10 mmol/L of 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (Sigma-Aldrich), 10 mmol/L of sodium carbonate (Sigma-Aldrich), and 10% fetal bovine serum (Corning, USA).
Reagents
Purified ovalicin was provided by Mycoplus Inc.(Republic of Korea) and diluted as described previously[8]. Ovalicin was dissolved in complete medium. DH82 cells were treated with 10 μmol/L ruxolitinib (Ruxo; Selleckchem, USA), a Janus kinase(JAK) inhibitor, for 24 hours. Intracellular calcium was chelated using 10 μmol/L BAPTA-AM (Calbiochem, USA) for 24 hours.
Cell viability assay
DH82 cells (1×104) were treated with ovalicin,lipopolysaccharide (LPS; Sigma-Aldrich), and/or dexamethasone (Dexa; Sigma-Aldrich) for 3 days.After treatment, cells were incubated with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (Sigma-Aldrich) for 2 hours at 37 °C. Then,formazan was dissolved by dimethyl sulfoxide and 2-propanol mixture in a 1:9 ratio, and absorbance was measured at a wavelength of 570 nm using a microplate reader (BioTek Epoch, Spain).
Real-time RT-PCR
Total RNA was extracted using Trizol reagent(Ambion, USA), followed by quantitation using an Epoch microplate spectrophotometer (BioTek Epoch).Next, complementary DNA (cDNA) was synthesized using 500 ng of total mRNA and a commercial cDNA synthesis kit (Enzynomics, Republic of Korea). The SYBR Green reverse transcriptase PCR (RT-PCR) kit(Enzynomics) was used to determine the expression levels ofIL4,IFNG,IL31RA,OSMR,TRPV1, andHRH2mRNA encoding IL-4, interferon-γ (IFN-γ), IL-31RA, OSMR, TRPV1, and histamine receptor H2(HRH2), respectively. All primers are listed inSupplementary Table 1(available online). Data were analyzed using the 2−ΔΔCtmethod[14].
Measurement of reactive oxygen species
Intracellular reactive oxygen species (ROS) were measured using 2,7-dichlorodihydrofluorescein diacetate (H2DCFDA; Molecular Probes, Invitrogen,USA) according to the manufacturer's instructions.Briefly, DH82 cells were harvested and treated with 2.5 μmol/L of H2DCFDA and incubated for 30minutes at 37 °C in the dark. Subsequently, the cells were washed thrice with phosphate-buffered saline(PBS) and immediately analyzed by flow cytometry using FACSVerse (Becton Dickinson, USA).
Measurement of IL-31 in cell culture medium by enzyme-linked immunosorbent assay
The cell culture medium was collected and centrifuged at 3000gfor 3 minutes to eliminate cell debris. The IL-31 level was measured using an enzyme-linked immunosorbent assay kit(MyBioSource, USA) according to the manufacturer's instructions. Briefly, standards and samples were plated in each well with a balanced solution and a conjugate solution. After washing thrice, a substrate solution was added and incubated for 15 minutes at 37 °C. Next, a stop solution was added, and the optical density was measured at a wavelength of 450 nm using the Epoch microplate spectrophotometer(BioTek Epoch). The expression level of IL-31 was calculated according to a standard curve.
Western blotting assay
Proteins of DH82 cells were extracted using Ez RIPA buffer (Atto, Japan) and quantitated it using Bradford assay (Bio-Rad Laboratories, USA). Next,10 μg of the whole lysate in each sample was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a nitrocellulose membrane (Amersham, GE Healthcare,USA). Blocking buffer (5% skim milk in PBS with 0.1% Tween 20) was added for 1 hour at room temperature. Primary antibodies, including total-STAT3 (Cell Signaling, USA), p-STAT3 (Cell Signaling), JAK2 (Cell Signaling), inducible nitric oxide synthase (iNOS; Santa Cruz Biotechnology,USA), cyclooxygenase-2 (COX2; Cell Signaling),nuclear factor kappa B (NF-κB; Santa Cruz Biotechnology), β-actin (Santa Cruz Biotechnology), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Santa Cruz Biotechnology), were added in a dilution of 1:1000 and incubated for 16hours at 4 °C. Then secondary anti-rabbit or antimouse antibody conjugated with horseradish peroxidase (Santa Cruz Biotechnology) was added,and bands were detected using the Luminata Forte HRP substrate (Millipore, USA).
Calcium measurement
A total of 1×105DH82 cells were seeded in a confocal dish (SPL, Republic of Korea) and treated with 5 μmol/L Fluo-4AM (Molecular Probes,Invitrogen, Spain), followed by incubation for 30 minutes in 37 °C in the dark. Nuclei were stained by NucBlue Live Cell Stain (Life Technologies, USA),and fluorescence was detected using fluorescence microscopy (Thermo Fisher Scientific, USA) after gentle washing thrice with PBS.
DNCB-induced atopic dermatitis mouse model
All animal experiments were approved by the Institutional Animal Care and Use Committee at Seoul National University, Republic of Korea (SNU-180327-1-2). Female BALB/C mice aged 5 weeks were purchased from Jungang Lab Animal Inc.(Republic of Korea) and shaved their back skin. To establish the AD model, 1% of DNCB solution was painted twice on the shaved back skin, and after 2 days, 0.1% DNCB solution was applied. The DNCB-treated AD mice were administered 1 and 10 μg/mL of ovalicin daily for 5 days. As a negative control, 10 μg/mL of ovalicin was painted on the shaved back skin of DNCB-untreated mice. After day 5, the dermatitis lesion score was measured from 0 to 4 based on the degree of hemorrhage, scarring,edema, and erosion[15].
Histological analysis
Skin tissues from mice were collected and fixed in 10% neutral buffered formalin solution. The fixed tissues were routinely processed, embedded in paraffin, and cut into 3-μm-thick slices. Sections on glass slides were prepared and stained with hematoxylin and eosin (H&E) and toluidine blue (TB). Histopathological analysis was performed by a veterinary pathologist according to published criteria[16].
Immunohistochemistry
IL-31 expression in paraffin-embedded skin tissues was assessed by immunohistochemistry (IHC)[16].Briefly, the prepared glass slides were deparaffinized by incubating in xylene solution, followed by hydration using serial alcohols, antigen retrieval using 1× citrate buffer (Sigma-Aldrich), and blocking using 2.5% normal horse serum (Vector Labs, USA). After washing with PBS, the slides were incubated overnight with ionized calcium-binding adaptor molecule 1 (IBA1) (Fujifilm Wako Chemicals, USA)and IL-31 receptor antibody (Santa Cruz Biotechnology) containing 2.5% normal horse serum and then incubated with HRP-conjugated horse antirabbit secondary antibody (Vector Labs) for 2 hours.After treatment with 3,3'-diaminobenzidine solution(Vector Labs), the slides were counterstained using hematoxylin, dehydrated in serial alcohols, and mounted using a xylene-based solution.
Statistical analysis
Statistical significance was determined using oneway analysis of variance (ANOVA) and Tukey's test(pairwise) with GraphPad Prism 5 software (USA).P<0.05 was considered statistically significant.
Results
Optimization of ovalicin and LPS concentrations
To determine the nontoxic concentration of ovalicin, DH82 cells were treated with 1, 10, 20, 40,100, and 200 μg/mL of ovalicin for 24, 48, and 72 hours, respectively, and the cell viability was assessed.The results showed that DH82 cell viability decreased dose-dependently after ovalicin treatment (Fig. 1A).At 24 and 48 hours, more than 90% of cells survived after treatment with 1 and 10 μg/mL of ovalicin(Fig. 1A). Therefore, 2, 4, and 10 μg/mL of ovalicin were used for the subsequent experiments. DH82 cells were treated with LPS for 1 day, followed by evaluation of cell viability andIL4mRNA expression.After treatment with 500 ng/mL of LPS, cell viability was comparable to that after PBS treatment, whileIL4mRNA expression significantly increased (Fig. 1BandC). DH82 cells were treated with 50, 100, 200,500, and 1000 μmol/L Dexa for 1 day, and 25 μmol/L of Dexa was selected for further experiments based on the cell viability assay results (Fig. 1D).
Ovalicin mitigates the effects of LPS treatment on DH82 cells
Pretreatment with LPS augments the proliferation after few days by NF-κB signaling-induced stimulation,leading to disorder of immune response[17–18]. To examine if ovalicin suppressed the LPS-induced stimulation in macrophages, DH82 cells were challenged with 2, 4, and 8 μg/mL ovalicin or Dexa for 1 day after pretreatment with 500 ng/mL LPS for 1 day, and the viability was assessed. LPS pretreatment increased cell viability compared to LPS-untreated cells, but ovalicin treatment decreased cell viability dose-dependently (Fig. 2A). Intriguingly, the decrease in cell viability after 10 μg/mL ovalicin treatment was more than that after 25 μmol/L Dexa treatment. LPSinduced upregulation ofIL4andIFNGmRNA expression was significantly attenuated after ovalicin treatment (Fig. 2BandC). In addition, the inhibition of their mRNA levels after 10 μg/mL ovalicin treatment was more than that after 25 μmol/L Dexa treatment.
Ovalicin inhibits IL-31 expression and its downstream signaling
LPS treatment of DH82 cells increased IL-31 expression compared to LPS-untreated cells, but ovalicin treatment significantly inhibited IL-31 expression more than Dexa treatment did (Fig. 3A). In addition, ovalicin treatment of LPS-pretreated DH82 cells decreased the mRNA expression of IL-31 dimeric receptors,IL31RAandOSMR(Fig. 3BandC). Compared to Dexa treatment, the mRNA expression levels of these receptors were significantly decreased after 4 and 10 μg/mL ovalicin treatment.Notably, ovalicin treatment decreased the expression ofIL31RAmore markedly than that ofOSMR.Furthermore, downstream molecules of IL-31 signaling, JAK2 and p-STAT3, were increased after LPS treatment compared to LPS-untreated cells,which was inhibited by ovalicin treatment dosedependently (Fig. 3D).

Fig. 1 Optimization of ovalicin and LPS concentrations. A: Viability of DH82 cells treated with 0–200 μg/mL of ovalicin for up to 3 days. Data are presented as mean±SEM (n=8). B: Viability of DH82 cells treated with 0–2000 ng/mL of LPS for 1 day. Data are presented as mean±SEM (n=8). C: Transcription level of IL4 in DH82 cells treated with 0–2000 ng/mL of LPS for 1 day. Data are presented as mean±SEM (n=4). **P<0.01 and ***P<0.001 vs. untreated DH82 cells. D: Viability of DH82 cells treated with 0–1000 μmol/L Dexa for 1 day.Data are presented as mean±SEM (n=8). Statistical analysis was conducted by ANOVA and Tukey's test (pairwise) for comparison with samples. PBS: phosphate-buffered saline; LPS: lipopolysaccharide; Dexa: dexamethasone.
Ovalicin suppresses the expression of pruritusrelated genes by inhibiting calcium influx
To examine the expression of pruritus-related genes,TRPV1andHRH2, we treated LPS-pretreated DH82 cells with ovalicin. Ovalicin treatment significantly inhibited the LPS pretreatment-induced increased mRNA expression of these genes, and the decrease was more evident than that after Dexa treatment (Fig. 4AandB). Ruxo, a selective JAK2 inhibitor, decreased the mRNA expression ofTRPV1andHRH2in LPSpretreated DH82 cells, but the inhibitory effect was lower than that after ovalicin treatment.
LPS treatment enhanced the calcium influx compared to LPS-untreated cells in DH82 cells, which was attenuated by treatment with 10 μg/mL of ovalicin (Fig. 4C). The effect of ovalicin in LPSpretreated cells was stronger than that of either Dexa or Ruxo treatment, and Ruxo inhibited calcium influx more effectively than Dexa did (Fig. 4C). To determine the effect of calcium influx on IL-31 signaling and pruritus-related genes, we chelated intracellular calcium by BAPTA-AM treatment. In DH82 cells, intracellular calcium was significantly chelated by 10 μmol/L BAPTA-AM treatment for 1 day (Supplementary Fig. 1, available online).Treatment of LPS-pretreated cells with 10 μg/mL of ovalicin inhibited the mRNA expression ofIL31RA,TRPV1, andHRH2more significantly than Ruxo treatment did (Fig. 4D). DH82 cells treated with 10 μmol/L BAPTA-AM were used as a positive control for calcium chelation.
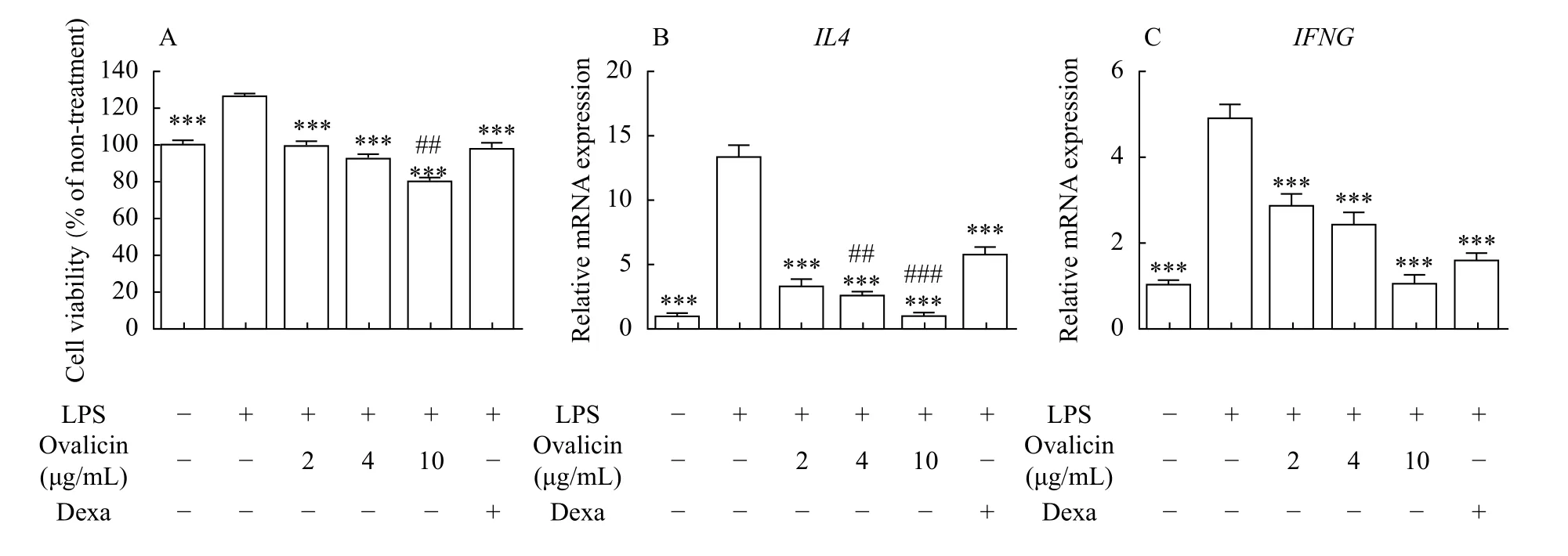
Fig. 2 Effects of ovalicin on LPS-pretreated DH82 cells. A: Viability of DH82 cells. DH82 cells were treated with 500 ng/mL LPS for 1 day, and the medium was replaced with that containing ovalicin and/or 25 μmol/L Dexa for 1 day. Error bars indicate SEM (n=8). ***P<0.001 vs. LPS-pretreated DH82 cells. ##P<0.01 vs. LPS-pretreated DH82 cells with Dexa. B and C: Transcription levels of IL4 and IFNG in DH82 cells. LPS-pretreated (500 ng/mL) DH82 cells were cultured in a medium containing ovalicin and/or 25 μmol/L Dexa for 1 day. Error bars indicate SEM (n=4). ***P<0.001 vs. LPS-pretreated DH82 cells. ##P<0.01 and ###P<0.001 vs. LPS-pretreated DH82 cells with Dexa. Statistical analysis was conducted by ANOVA and Tukey's test (pairwise) for comparison with samples. LPS: lipopolysaccharide; Dexa:dexamethasone.
Ovalicin shows anti-inflammatory effects in LPSpretreated DH82 cells
The expression of inflammatory markers, including iNOS, COX2, and NF-κB, was enhanced after LPS treatment, which was inhibited by ovalicin treatment(Fig. 5A). Although Dexa treatment decreased the expression of these genes in LPS-pretreated cells, the degree of decrease was less than that by ovalicin treatment (Fig. 5B). Likewise, the mean fluorescence intensity of ROS in LPS-pretreated cells was mitigated after ovalicin treatment dose-dependently(Fig. 5C).
Ovalicin relieves pruritic lesions through inhibited IL-31 signaling in the DNCB-induced AD mouse model
Histological analysis of skin tissue in the DNCBinduced AD mouse model showed epidermal detachment and inflammation with loss of hair follicles. Ovalicin treatment relieved pruritic lesions through enhanced re-epithelialization and granulation tissue formation (Fig. 6A). In control mice, ovalicin treatment did not cause any morphologic change in the skin. IL-31 is also produced from macrophages by stimulation and conjugates with its receptor on mast cells to release histamine, leading to scratching behavior[11,19]. To evaluate the infiltrated macrophages in the mouse skin, we performed IHC staining for IBA1. Compared to the DNCB-untreated control mice, the macrophages positive for IBA1 were easily detected in the dermis of DNCB-induced AD mice.And the number of IBA1 positive macrophages was dramatically decreased by ovalicin treatment dosedependently (Supplementary Fig. 2A, available online). TB staining of skin tissue showed that ovalicin treatment significantly decreases the number of infiltrated mast cells in the DNCB-induced AD mouse model (Fig. 6B). Lesion scores compiling the extent of hemorrhage, scarring, edema, and erosion were comparable in DNCB-untreated control mice regardless of ovalicin treatment. In contrast, lesion scores in DNCB-treated AD mice were significantly higher compared to DNCB-untreated control mice,and the scores were significantly lower in the ovalicintreated group (Fig. 6C). Additionally, albumin and total protein were higher in DNCB-treated AD mice than in DNCB-untreated control mice, which was reversed after ovalicin treatment (Table 1). Likewise,treatment of DNCB-treated AD mice with ovalicin enhanced the weight and mitigated percentage of neutrophil, monocyte, and eosinophil compared to non-treatment (Supplementary Fig. 2BandC,available online).
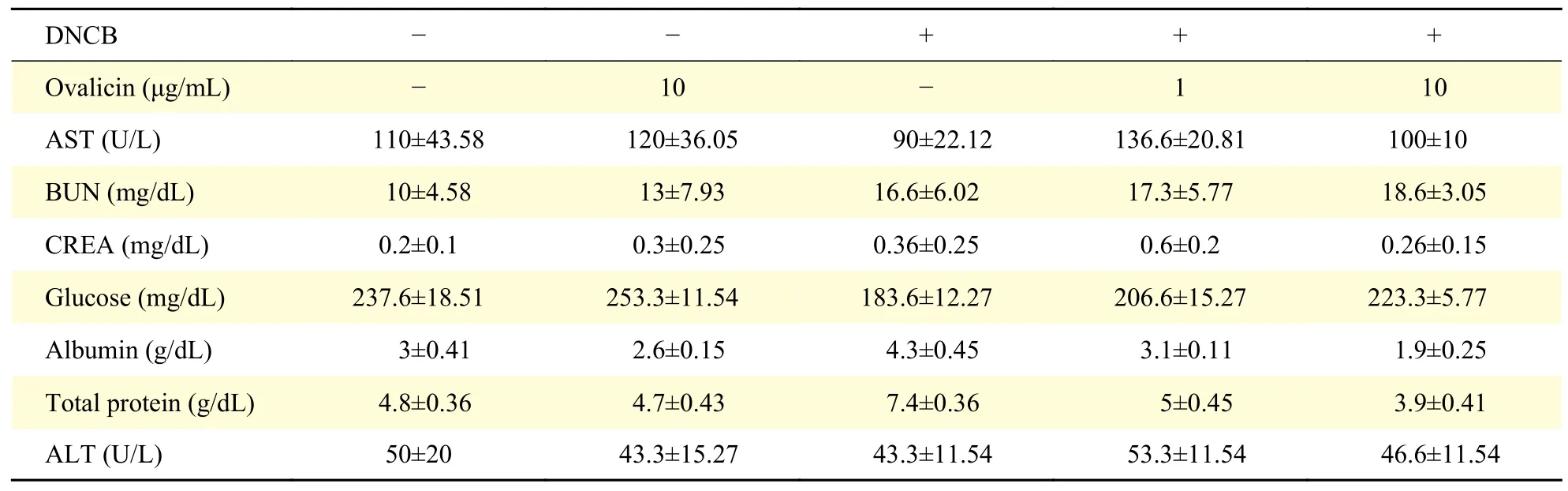
Table 1 Analysis of serum chemistry parameters

Fig. 3 Effect of ovalicin on the expression of IL-31 and its downstream molecules. A: Concentration of IL-31 in the supernatant of DH82 cells treated with 500 ng/mL LPS, ovalicin, and/or 25 μmol/L Dexa. Error bars indicate SEM (n=4). ***P<0.001 vs. LPS-pretreated DH82 cells. ##P<0.01 vs. LPS-pretreated DH82 cells with Dexa. B and C: Transcription levels of IL31RA and OSMR in DH82 cells treated with 500 ng/mL LPS, ovalicin, and/or 25 μmol/L Dexa. Error bars indicate SEM (n=4). **P<0.01 and ***P<0.001 vs. LPS-pretreated DH82 cells. ##P<0.01 vs. LPS-pretreated DH82 cells with Dexa. D: Western blotting assay of JAK2, and p-STAT3 in DH82 cells treated with 500 ng/mL LPS, ovalicin, and/or 25 μmol/L Dexa. Statistical analysis was conducted by ANOVA and Tukey's test (pairwise) for comparison with samples. LPS: lipopolysaccharide; Dexa: dexamethasone.
In DNCB-treated AD mice, ovalicin treatment decreased the mRNA expression ofTRPV1andHRH2compared to AD mice without ovalicin treatment(Fig. 6D). Compared to DNCB-untreated control mice, higher IL-31 expression was detected in the serum of DNCB-treated AD mice, and this increase was suppressed by ovalicin treatment dosedependently (Fig. 6E). Analogously, IHC staining of skin tissue revealed significantly higher expression of IL-31R in DNCB-treated AD mice than in DNCBuntreated control mice, which was decreased after ovalicin treatment (Fig. 6F).

Fig. 4 Effect of ovalicin on pruritus-related genes and intracellular calcium influx. A and B: Transcription levels of TRPV1 and HRH2 in DH82 cells treated with 500 ng/mL LPS, ovalicin, 25 μmol/L Dexa, and/or 10 μmol/L Ruxo. Error bars indicate SEM (n=4). ***P<0.001 vs. LPS-pretreated DH82 cells. ##P<0.01 and ###P<0.001 vs. LPS-pretreated DH82 cells with Dexa. $P<0.05 and $$$P<0.001 vs. LPS-pretreated DH82 cells with Ruxo. C: Representative images of Fluo-4AM and DAPI staining in DH82 cells treated with 500 ng/mL LPS, 10 μg/mL of ovalicin, 25 μmol/L Dexa, and/or 10 μmol/L Ruxo. Scale bar, 200 μm. D: Transcription levels of IL31RA, OSMR, HRH2, and TRPV1 in DH82 cells treated with 500 ng/mL LPS, 10 μg/mL of ovalicin, 10 μmol/L BAPTA-AM, and 10 μmol/L Ruxo. Error bars indicate SEM(n=4). ***P<0.001 vs. LPS-pretreated DH82 cells. Statistical analysis was conducted by ANOVA and Tukey's test (pairwise) for comparison with samples. LPS: lipopolysaccharide; Dexa: dexamethasone; Ruxo: ruxolitinib.

Fig. 5 Anti-inflammatory effect of ovalicin on LPS-pretreated DH82 cells. A: Expression of iNOS, COX2, NF-κB, and GAPDH in DH82 cells treated with 500 ng/mL LPS, ovalicin, and/or 25 μmol/L Dexa. B: Densitometric analysis of iNOS, NF-κB, and COX2 expression, using ImageJ, which was normalized by the value of GAPDH expression. Error bars indicate SEM (n=3). ***P<0.001 vs. LPSpretreated DH82 cells. #P<0.05, ##P<0.01, and ###P< 0.001 vs. LPS-pretreated DH82 cells with Dexa. C: Representative histogram showing H2DCFDA staining in DH82 cells treated with 500 ng/mL LPS, ovalicin, and/or 25 μmol/L Dexa. Error bars indicate SEM (n=3). **P<0.01 and ***P<0.001 vs. LPS-pretreated DH82 cells. Statistical analysis was conducted by ANOVA and Tukey's test (pairwise) for comparison with samples. LPS: lipopolysaccharide; Dexa: dexamethasone.

Fig. 6 Effect of ovalicin on histopathologic lesions and IL-31 signaling in the DNCB-induced AD mouse model. A and B:Representative images of skin tissue stained with H&E and TB in DNCB-untreated control and 0.1% DNCB solution-treated AD mice with/without ovalicin treatment. Scale bar: 200 μm. C: Mean lesion scores in the skin of DNCB-untreated control and 0.1% DNCB solutiontreated AD mice with/without ovalicin treatment. The lesion scores of the mouse skin tissue were from 0 to 3 on the basis of the extent of hemorrhage, scarring, edema, and erosion. Error bars indicate SEM (n=5). **P<0.01 and ***P<0.001. D: Transcription levels of TRPV1 and HRH2 in skin tissue from DNCB-untreated control and 0.1% DNCB solution-treated AD mice with/without ovalicin treatment. Error bars indicate SEM (n=5). **P<0.01 and ***P<0.001 vs. DNCB-untreated control mice without ovalicin treatment. E: Concentration of IL-31 in serum of DNCB-untreated control and 0.1% DNCB solution-treated AD mice with/without ovalicin treatment. Error bars indicate SEM(n=5). ***P<0.001 vs. DNCB-untreated control mice without ovalicin treatment. F: Representative immunohistochemical staining for IL-31R in skin tissue of DNCB-untreated control and 0.1% DNCB solution-treated AD mice with/without ovalicin treatment. Scale bar, 200 μm.DNCB: 2,4-dinitrochlorobenzene; H&E: hematoxylin and eosin. Statistical analysis was conducted by ANOVA and Tukey's test (pairwise)for comparison with samples. AD: atopic dermatitis; DNCB: 2,4-dinitrochlorobenzene.
Discussion
AD is an inflammatory skin disease and commonly occurs under sensitization to environmental allergens,such as grass, pollen, dust, and mites. AD patients have a quality of life worsened by pruritus, chronic inflammation, and secondary viral and bacterial infections[10]. In AD pathogenesis, macrophages play a crucial role and recognize the pathogen-associated molecular patterns by Toll-like receptors (TLRs).Activated macrophages produce various chemokines,such as IFN, IL-1, and IL-4, leading to recruitment of circulating monocytes and lymphocytes to skin lesions[20]. In addition, M2 polarization of macrophages by Th2 immunity stimulates IL-31 secretion in the dermis, culminating in pruritus[21]. However, the molecular mechanism of macrophages in AD pathogenesis, especially in the development of pruritus and inflammation, is unclear. In this study, we demonstrated that ovalicin shows antipruritic and antiinflammatory effects by inhibiting IL-31 signaling and calcium influx in macrophages and the DNCBinduced AD mouse model.
To relieve pruritus in AD patients, various drugs,including steroidal drugs, have been developed, but their clinical applications are restricted because of severe side effects. Corticosteroids inhibit the release of cytokines by the activation of glucocorticoid receptors and decrease local inflammation and pruritus[22]. However, their usage in case of generalized skin diseases is not recommended because of cutaneous side effects such as atrophy, acne, and telangiectasia[23]. Dexa is widely used for the alleviation of pruritus in AD patients, but it induces topical burning, itching, and tingling[24]. Topical calcineurin inhibitors and antihistamine drugs inhibit pruritus in AD patients by T-cell inactivation, but these drugs also cause side effects, including localized burning and stinging[25–26]. In contrast, herbal medicines efficiently improve AD symptoms and exert fewer side effects[27]. This study showed that ovalicin alleviates AD symptoms in a DNCB-induced AD mouse model by inhibiting IL-31 signaling and the inflammatory response more than Dexa does.
Ovalicin is a herbal medicine extracted fromC.militarisand is commonly used to control schizonts and parasite growth[7,28]. In compound 48/80-induced AD mice model, purified ovalicin alleviated AD symptoms[8], but the underlying molecular mechanism is unclear. In this study, we showed that ovalicin inhibits IL-31-signaling with decreased expression of pruritus-related genes, includingTRPV1andHRH2, in LPS-pretreated macrophages. TRPV1 plays a critical role in IL-31-induced scratching behavior and augments the intracellular calcium influx[12,29]. To the best of our knowledge, this is the first study to demonstrate that ovalicin inhibits calcium influx by inhibiting TRPV1, resulting in the suppression of IL-31 signaling in macrophages. The antipruritic efficacy of ovalicin is stronger than that of Dexa. Likewise, in our DNCB-induced AD mouse model, ovalicin treatment mitigated pruritic symptoms and decreased mast cell infiltration through attenuating IL-31-signaling.However, the effect of ovalicin on other immune players is unclear, which warrants further studies.
Scratching behavior due to pruritus induces severe inflammation, potentially leading to infection, cancer,neurologic, and endocrine disorders[30]. As a positive loop, severe inflammation aggravates pruritus through excessive production of Th2-type cytokines, including IL-31, IL-4, and IL-13[2]. Therefore, an anti-pruritic drug for AD treatment is essential for attenuating inflammation. Conjugation of TLRs with LPS plays a crucial role in the inflammatory response and activates transcription factors, such as NF-κB, interferon regulatory factors, and activation protein 1[31]. In addition, IL-31 administration in mice enhances the inflammation with recruitment of monocytes and lymphocytes, resulting in thickening of the skin barrier[32]. Our data showed that ovalicin treatment decreases inflammatory molecules, such as ROS,iNOS, COX2, and NF-κB, in LPS-pretreated DH82 cells more than Dexa treatment does. Analogously,inflammatory lesions in the DNCB-induced AD mouse model were significantly relieved by ovalicin treatment.
This study illustrated that purified ovalicin significantly improves pruritic symptoms of AD by inhibiting IL-31 signaling with inhibition of downstream genes in LPS-pretreated macrophages and the DNCB-induced AD mouse model. Our data indicate that ovalicin has better efficacy in the management of AD symptoms than Dexa and can be developed as an alternative treatment. Further characterization of the ovalicin efficacy in AD patients is warranted. However, the systemic toxicological research of ovalicin treatment in animal models is limited and warrants further study.
Acknowledgments
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (Grant No.2020R1A2C1010215) and the Brain Korea 21 Future Veterinary Medicine Leading Education and Research Center, College of Veterinary Medicine, Seoul National University.
杂志排行
THE JOURNAL OF BIOMEDICAL RESEARCH的其它文章
- Application of a continuous respiratory sound monitoring system in thoracic surgery
- Protective effects on acute hypoxic-ischemic brain damage in mfat-1 transgenic mice by alleviating neuroinflammation
- Target specificity of selective bioactive compounds in blocking α-dystroglycan receptor to suppress Lassa virus infection: an in silico approach
- Isometric exercise promotes arteriogenesis in rats after myocardial infarction
- Construction of miRNA-mRNA network reveals crucial miRNAs and genes in acute myocardial infarction
- Paclitaxel-induced stress granules increase LINE-1 mRNA stability to promote drug resistance in breast cancer cells
