Isometric exercise promotes arteriogenesis in rats after myocardial infarction
2021-12-04XintongZhangYuZhengCanruGengJuntaoGuanLuWangXiuZhangYihuiChengJiananLiXiaoLu
Xintong Zhang, Yu Zheng, Canru Geng, Juntao Guan, Lu Wang, Xiu Zhang, Yihui Cheng,Jian'an Li,✉, Xiao Lu,✉
1Department of Rehabilitation Medicine, the First Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu 210029, China;
2Department of Rehabilitation Medicine, the Affiliated Suzhou Hospital of Nanjing Medical University, Suzhou, Jiangsu 215000, China.
Abstract Isometric exercise (IE) is a promising intervention of noninvasive revascularization in patients with acute myocardial infarction (AMI). This study aimed to investigate the impact and mechanisms of IE training on arteriogenesis in AMI. Male Sprague-Dawley rats were randomly assigned into the sham-operation group (SO),myocardial infarction (MI) group, and 13 IE subgroups treated according to training intensity, frequency,duration, or monocyte chemoattractant protein-1 (MCP-1), or/and fibroblast growth factor-2 (FGF-2) inhibitors for eight weeks. Our results demonstrated that the IE group achieved superior improvement compared with the MI group in terms of left ventricular ejection fraction (LVEF), myocardial infarction size (MIS), arterial density(AD), monocytes (MNCs), smooth muscle cells (SMCs), endothelial cells (ECs), relative collateral blood flow(RCBF), MCP-1, and FGF-2 at the endpoint. Positive correlations between MCP-1 and MNCs, MNCs and FGF-2,FGF-2 and SMCs, SMCs and AD, as well as AD and RCBF were observed. This study demonstrated that with MI of 100% load 20 times daily for eight weeks, the arteriogenesis was improved, which may be attributed to the recruitment of MNCs and SMCs in remote ischemic myocardium caused by increases in MCP-1 and FGF-2 expression.
Keywords: isometric exercise training, arteriogenesis, acute myocardial infarction, monocyte chemoattractant protein-1, fibroblast growth factor-2
Introduction
Acute myocardial ischemia (AMI) is mainly caused by interrupted coronary blood flow[1]. The acute phase of myocardial ischemia (MI) features a high mortality risk, while ventricular remodeling and heart failure may occur in the chronic phase[2]. In AMI,inappropriate blood pumping resulted from cardiac dysfunction fails to meet the regular circulatory demand[3]. The treatment goal of AMI is to restore collateral blood flow of the ischemic myocardium and reduce infarct size. Although the development of fibrinolytic agents and primary percutaneous coronary intervention (PCI) has significantly improved the prognosis of AMI over the past decades, its mortality remains unchanged[4]. Furthermore, pharmaceutical and invasive treatment may not be applicable for all patients considering their poor systemic status or various contraindications. Therefore, innovative strategies are warranted for compromising myocardial ischemia.
To date, evidence suggested that exercise training is a non-invasive approach that can benefit patients with AMI[5]. And isometric exercise (IE), which features isometric contraction of skeletal muscles, is a promising intervention despite controversy over its security[6]. IE training was believed to increase blood pressure to some extent and lead to unstable hemodynamics and malignant arrhythmia, which may cause cardiovascular and cerebrovascular accidents,such as aneurysmal rupture, especially in elderly and hypertensive individuals[7]. However, recent evidence reveals the therapeutic potential of IE in restoring coronary blood flow through peripheral biological effects-induced central response. Jianget aldemonstrated that IE improved blood perfusion in the ischemic myocardium[8]. Linet alalso found that isometric handgrip exercise promoted remote collateral recruitment and growth in patients with coronary artery disease[9]. Nevertheless, the underlying mechanisms have not been well studied.
Previous studies have demonstrated that monocyte chemoattractant protein-1 (MCP-1) and fibroblast growth factor-2 (FGF-2) serve as signal transducers to activate monocytes (MNCs) and smooth muscle cells(SMCs)[10–11]. It has been reported that mice deficient in MCP-1 develop severe defects in monocyte recruitment in response to immunological signaling[12].Significantly decreased migration of primary mouse MNCs is observed after MCP-1 antagonist treatment[13]. Stimulation of SMCs with FGF-2 triggers an approximately 50-fold increase in DNA synthesis compared to that of controls[14]. FGF2 significantly increases SMC proliferation in ballooninjured porcine carotid arteries in thein vitroartery perfusion culture model[15]. Interestingly, injured endothelial cells (ECs) stimulates FGF-2 expression,which then induces proliferation and migration of SMCs[16]. These cytokines facilitate the growth of functional collateral arteries from pre-existing arterioarteriolar anastomoses, and in turn, increase blood flow. However, the causal relationships between IE training and arteriogenesis have not been well studied.
In this study, we showed that arteriogenesis could be improved following a long-term of IE training after AMI and investigated the mechanisms from the molecular and cellular up to the tissue level. We also demonstrated that the optimal protocol including intensity, duration, and frequency of IE training achieved superior training effects.
Materials and methods
Animal model
Male Sprague-Dawley rats (8 weeks, 220 to 240 g)were housed under climate-controlled conditions with a 12-hour light-dark cycle. All protocols were approved by the Animal Use and Care Committee of Nanjing Medical University, and followed the Guide for the Care and Use of Laboratory Animals[17].
MI was induced with ligating the left anterior descending artery (LAD)[18]. Animals were anesthetized with 2% isoflurane and ventilation was delivered by a small animal respirator. Left-sided thoracotomy was performed paralleled to the sternum at the third intercostal space. After the heart was exposed, the pericardium was opened and then the LAD ligated. Sham animals only underwent thoracotomy without ligating the LAD. Regional myocardial infarction was identified with electrocardiography (ECG)[19](Supplementary Fig. 1,available online). Following surgical modeling, the animals were intramuscularly administered with penicillin sodium (80 U) to prevent infection. All the rats had access to food and waterad libitumfor one week and were then divided into the different experiment subgroups using the random number table method (Supplementary Fig. 2, available online).
Isometric exercise training
IE training was carried out with the animals vertically gripping on a fence and initiated on the third postoperative day. Resistance was loaded by fastening a small sandbag to the animal's tail. The sandbag was weighted to allow the rat to grip on the fence for 1 minute[20]. The training protocol consisted of 20 cycles of 1-minute IE and rest, and was performed 5 days/week for a total of 8 weeks. The weight of the sandbag was reevaluated every two weeks[21](Supplementary Fig. 3, available online).
Echocardiography
Echocardiography was performed with a Vevo 3100 system (VisualSonics, Canada) at the endpoint,and 5% and 1.5% isoflurane were utilized for anesthesia induction and maintenance, respectively.Data analysis was performed with VevoStrain as described previously[22]. The parasternal long-axis and short-axis views of the heart were examined in the M mode at the papillary muscle level, and left ventricular ejection fraction (LVEF) data were obtained.
Masson's trichrome staining
All rats were euthanized at the endpoint. The myocardial samples were collected and fixed in 4%formalin for 48 hours at 4 °C, and dehydrated with graded ethanol. After dehydration, the samples were embedded in paraffin and cut into 5-μm thick sections.Masson's trichrome staining was performed and imaging was fulfilled by microscope. Image J was used to assess myocardial infarction size (MIS) with the formula as follows: MIS(%)=(fibrosis area/total left ventricle area)×100%.
Microspheres for relative collateral blood flow
Microspheres (Dye-Trak; Triton Technology,3×106/set, USA) in eosin were administered into the left atrium before LAD ligation during MI surgery. At the endpoint, microspheres in violet were administered into the same location before myocardium sample collection. Each sample was weighted, cut, and placed in 15 mL polyethylene centrifuge tubes at ambient. After 2 weeks of incubation, 6 mL KOH (1 mol/L) was added, and the samples were kept overnight at 60 °C with periodical shaking to digest the issues. After staining,microsphere extraction was performed with Degens'method[23]. Absorbance was obtained from spectrophotometry (Ultrospec 2000, Pharmacia Biotech, USA), and microspheres were counted based on absorbance at the wavelength corresponding to the dye color. Coronary blood flow (CBF) and coronary collateral blood flow (CCBF) were estimated by microsphere amounts pre- and post-occlusion,respectively. To exclude the effect of individual differences, relative collateral blood flow (RCBF) was finally estimated by the following formula:RCBF(%)=(CCBF/CBF)×100%[24].
Immunohistochemical staining
The myocardial samples were fixed in 10%buffered formalin overnight, dehydrated, and embedded in paraffin; the 5-μm tissue paraffin sections were used for immunohistochemical staining in accordance with following procedures. Paraffin sections were incubated with the antibodies including CD68 (1:200, Abcam, USA), CD31 (1:200, Abcam),and α-SMA (1:200, Abcam). Anti-rabbit or antimouse peroxidase-conjugated secondary antibodies(1:50, Santa Cruz Biotechnology, USA) were applied,and the DAB Substrate (Dako, Germany) was used for coloration. Counterstaining was performed with hematoxylin (Sigma, USA). The mean number of positive cells of ECs, MNCs, and SMCs were represented using mean integrated optical density in five random fields in the dye areas by light microscopy (200×) for each animal. Observers blinded to treatment conducted all evaluations and measurements. All measurements were carried out using ImageJ software (NIH, USA).
An arteriole was defined as a vessel with an internal diameter of 11 to 150 μm, and surrounded by at least one layer of SMCs. Arterial density (AD) (no./mm2)was derived from five random fields in dye areas by light microscopy (200×).
Western blotting analysis
Tissue protein was extracted using the Radio Immunoprecipitation Assay (RIPA) lysis buffer.Equal amounts of protein were electrophoresed on 10% SDS-polyacrylamide gel and then transferred onto polyvinylidene difluoride membranes. The membranes were blocked in 5% fat-free milk with 0.1% Tween-20 for 1 hour at room temperature,followed by incubation with different primary antibodies against MCP-1 (Santa Cruz Biotechnology), FGF-2 (Boster Biological Technology, USA), or β-actin (1:1000, Sigma)overnight at 4 °C. After washing the membranes three times, the blots were incubated with fluorescent-based anti-rabbit IgG secondary antibody (1:1000,Fermentas, Lithuania) for 1 hour at room temperature.Image J was utilized to quantify the relative protein expression levels after densitometric scanning of the exposed films.
Statistical analysis
SPSS 23.0 (IBM Inc., USA) was utilized for data analysis. Data are presented as mean±SD when skewed normal distribution was observed. The data,including LVEF, MIS, AD, RCBF, MNC number,SMC and EC level, and MCP-1 and FGF-2 expression, were analyzed with one-way ANOVA to compare the difference among groups. LSD test and Games-Howell test were used for the comparison of homogeneous variance and uneven variance respectively. Pearson's correlation test was performed to identify the correlations of MCP-1 protein expression and MNC number, MNC number and FGF-2 protein expression, FGF-2 protein expression and SMC number, SMC number , as well as AD and RCBF. For all analyses, statistical significance was set atP<0.05.
Results
IE training improved cardiac function and increased AD and relative collateral blood flow in MI rats
To determine the effect of IE training on arteriogenesis following MI, rats were randomly assigned into the sham-operated group (SO, received thoracotomy only,n=6), MI group (received MI modeling only,n=6), and IE training group (IE,received MI modeling and IE training 20 times/day with 100% load for eight weeks,n=6). LVEF was reduced significantly in the MI group compared to the SO group; by contrast, this effect was rescued in rats of the IE training group (Fig. 1A). Meanwhile,Masson's trichrome staining showed remarkably reduced fibrotic area and smaller MIS in the IE training group compared to the MI group (Fig. 1B).RCBF was significantly elevated in the MI group compared to the SO group, while higher values were further observed in the IE training group in comparison with the MI group (Fig. 1C). AD in the IE group was significantly higher than that in the MI and SO groups (Fig. 1D). The results revealed that IE training could improve cardiac function and promote ischemic myocardial collateral circulation blood flow in AMI.

Fig. 1 IE training improved LVEF, MIS, AD, RCBF in AMI rats. A: Representative images of echocardiography of rats. B: Fibrosis levels and myocardial infarction size with Masson's trichrome staining. Scale bar: 100 μm. C: Total relative collateral blood flows with microspheres. D: Total artery densities in the ischemic myocardium with immunohistochemical staining. Data are presented as mean±SD.n=6 for each group. One-way ANOVA was used to analyze the significance between groups. *P<0.05. SO: sham-operation group; MI:myocardial infarction group; IE: isometric exercise group; LVEF: left ventricular ejection fraction; MIS: myocardial infarction size; RCBF:relative collateral blood flow; AD: arterial density; MCP-1: monocyte chemoattractant protein-1; FGF-2: fibroblast growth factor-2.
IE training increased proliferation of endothelial cells, monocytes, and smooth muscle cells in ischemic myocardium
To evaluate the numbers of ECs, MNCs, and SMCs in ischemic myocardium, we performed immunohistochemical staining with the antibodies against CD31(marker for ECs), CD68 (marker for MNCs), and α-SMA (marker for SMCs). As shown inFig. 2, the number of ECs, MNCs, and SMCs were significantly increased in the IE training group compared to the other two groups (allP<0.05). These results demonstrated that IE training could enhance the proliferation of ECs, MNCs, and SMCs in ischemic myocardium, which played a key role in arteriogenesis progress.
MCP-1 and FGF-2 might be involved in IEmediated improvement of arteriogenesis following MI
To investigate whether MCP-1 and FGF-2 were involved in IE-mediated improvement of arteriogenesis following MI, we detected the expression levels of MCP-1 and FGF-2 in myocardial samples and analyzed their correlations to MNCs, SMCs, AD,and RCBF. The results of Western blotting analysis showed that MCP-1 and FGF-2 levels were increased significantly in the IE training group as compared to those in the SO and MI groups (Fig. 3AandB).Further, statistically significant correlations were observed between MCP-1 protein level and MNCs(r=0.830,P<0.001), MNCs and FGF-2 protein level(r=0.865,P<0.001), SMCs and FGF-2 protein level(r=0.706,P=0.001), SMCs and AD (r=0.685,P=0.002), and AD and RCBF (r=0.892,P<0.001)(Fig. 3C–G).
These results suggested IE training might improve collateral circulation blood flow by increasing AD in the ischemic myocardium. Meanwhile, MCP-1 and FGF-2 might function in IE-mediated arteriogenesis by activating MNCs and SMCs.
MCP-1 or/and FGF-2 inhibitor decreased RCBF,AD, and the numbers of MNCs and SMCs in MI rats after IE training
RCBF and AD were significantly elevated in the MI group compared to those in the SO group while higher values were further observed in the IE training group compared to the MI, MCP-1 inhibitor group (Inhi-MCP, received MI modeling, IE training, and intragastric administration of leflunomide [MCP-1 inhibitor; 10 mg/{kg·day}],n=6), FGF-2 inhibitor group (Inhi-FGF, received MI modeling, IE training,and intragastric administration of formononetin[FGFR-1 inhibitor; 100 mg/{kg·day}],n=6), and MCP-1 and FGF-2 inhibitor groups (Inhi-MCP/FGF,received MI modeling, IE training, and intragastric administration of leflunomide [10 mg/{kg·day}] and formononetin [100 mg/{kg·day}],n=6). RCBF and AD in the Inhi-MCP/FGF group were lower than those in the Inhi-MCP and Inhi-FGF groups (Fig. 4AandB).
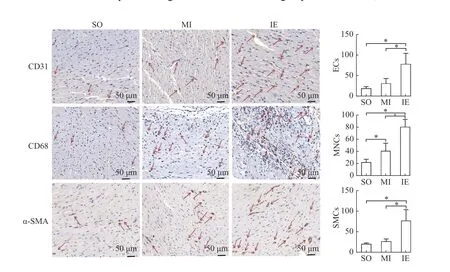
Fig. 2 IE training increased the amount of MNCs, SMCs and ECs in AMI rats. Representative images of immunohistochemical staining of ischemic myocardium. Immunohistochemical staining was performed with the indicated antibodies, CD31 (marker for ECs),CD68 (marker for MNCs), and α-SMA (marker for SMCs). Scale bar: 50 μm. The number of positive cells (red arrows) of ECs, MNCs, and SMCs were represented by integrated optical density. Data are presented as mean±SD. n=6 for each group. One-way ANOVA was used to analyze the significance between groups, *P<0.05. SO: sham-operation group; MI: myocardial infarction group; IE: isometric exercise group;ECs: endothelial cells; MNCs: monocytes; SMCs: smooth muscle cells.
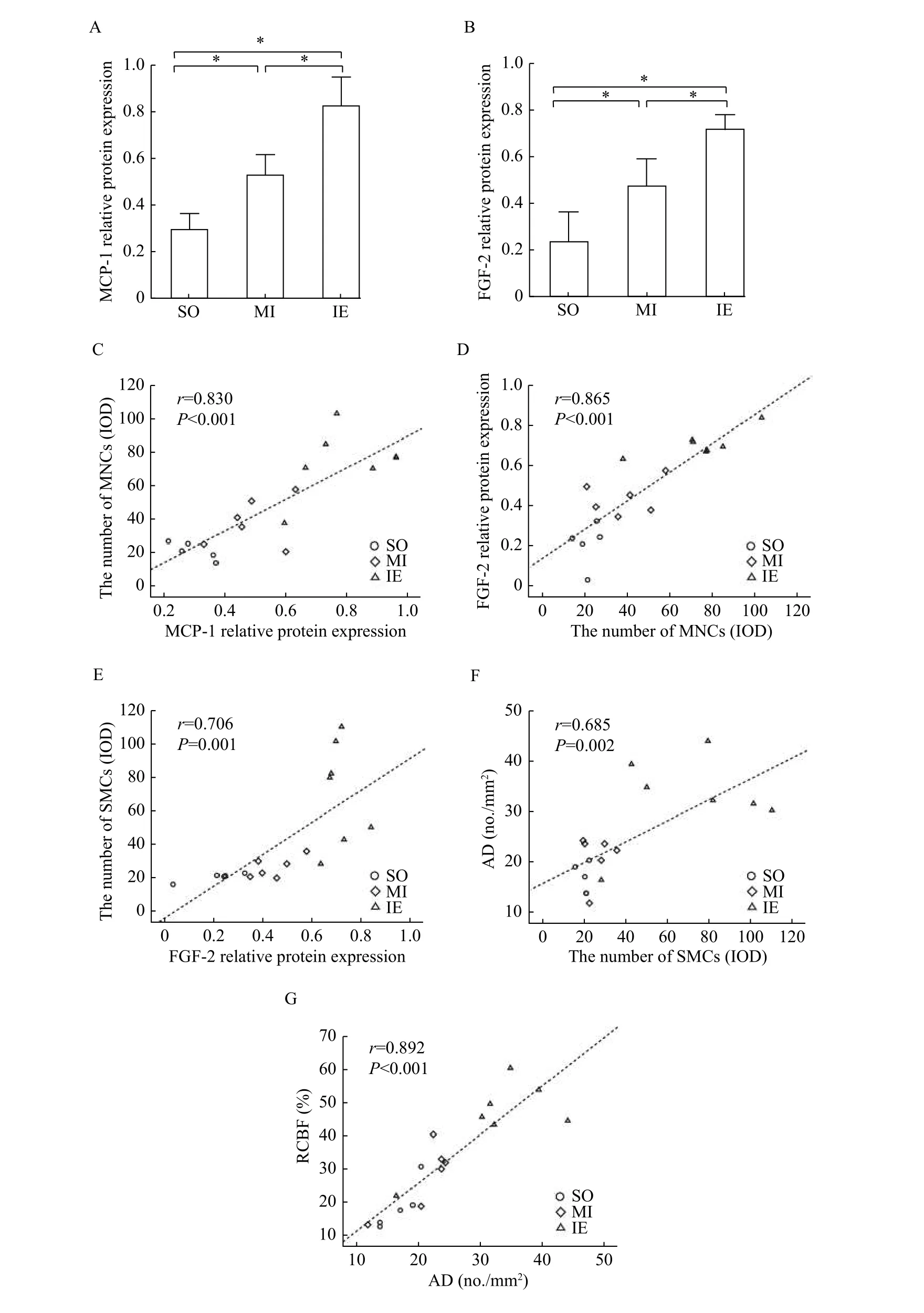
Fig. 3 The expression of MCP-1 and FGF-2 in ischemic myocardium and positive correlation analysis of molecular, cellular and histological parameters across groups. A and B: Total proteins were extracted from myocardial tissues and analyzed by Western blotting assay to determine the levels of MCP-1 (A) and FGF-2 (B) expression. C: Correlation between MCP-1 protein expression and MNCs number. D: Correlation between MNCs number and FGF-2 protein expression. E: Correlation between SMCs number and FGF-2 protein. F:Correlation between SMCs number and AD. G: Correlation between AD and RCBF. r: correlation coefficient. The number of MNCs and SMCs was represented by integrated optical density (IOD). Data are presented as mean±SD, *P<0.05. SO: sham-operation group; MI:myocardial infarction group; IE: isometric exercise group; MCP-1: chemoattractant protein-1; FGF-2: fibroblast growth factor-2; MNCs:monocytes; SMCs: smooth muscle cells; AD: arterial density; RCBF: relative collateral blood flow.
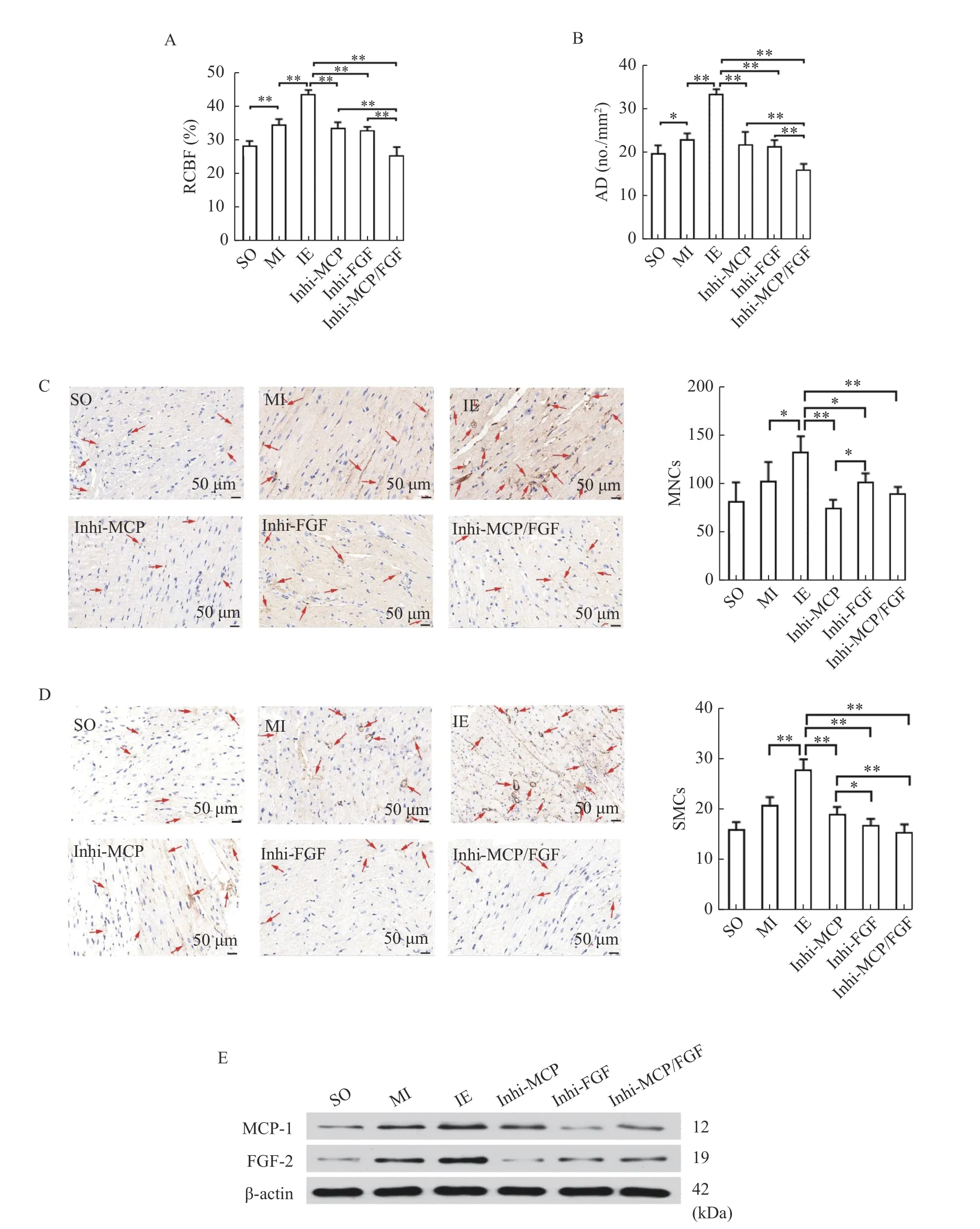
Fig. 4 MCP-1 or/and FGF-2 inhibitor decreased RCBF, AD, the number of MNCs and SMCs in AMI rats after IE training. Rats were treated with MI surgery, with or without IE training and intragastric administration of leflunomide or/and formononetin. n=6 for each group. A: Total relative collateral blood flows were determined by the microspheres methods. B: Total artery densities in the ischemic myocardium were determined by immunohistochemical staining. C: Immunohistochemical staining was performed with the indicated antibodies (CD68) to determine the number of MNCs in the ischemic myocardium. Scale bar: 50 μm. D: Immunohistochemical staining was performed with the indicated antibody (α-SMA) to determine the number of SMCs in the ischemic myocardium. Scale bar: 50 μm. The number of positive cells (red arrows) of MNCs, and SMCs were represented by integrated optical density. E: Total proteins were extracted from myocardial tissues and analyzed by Western blotting assay to determine the expression levels of MCP-1 and FGF-2. Data are presented as mean±SD. One-way ANOVA was used to analyze the significance between groups, *P<0.05; **P<0.001. SO: sham-operation group; MI:myocardial infarction group; IE: isometric exercise group; Inhi-MCP: MCP-1 inhibitor group; Inhi-FGF: FGF-2 inhibitor group; Inhi-MCP/FGF: MCP-1 and FGF-2 inhibitor group; MCP-1: monocyte chemoattractant protein-1; FGF-2: fibroblast growth factor-2; RCBF:relative collateral blood flow; AD: arterial density; MNCs: monocytes; SMCs: smooth muscle cells.
The numbers of MNCs and SMCs in myocardium were significantly increased in the IE training group compared to the MI, Inhi-MCP, Inhi-FGF, and Inhi-MCP/FGF groups. Furthermore, fewer MNCs were found in the Inhi-MCP group than in the Inhi-FGF group (P<0.05), and SMCs were fewer in the Inhi-FGF group compared with the Inhi-MCP group(P<0.05,Fig. 4CandD). The IE training group showed the highest MCP-1 and FGF-2 levels in the ischemic myocardium compared to the MI, Inhi-MCP,Inhi-FGF, and Inhi-MCP/FGF groups. However, there were no differences in protein expression of MCP-1 or FGF-2 among the Inhi-MCP/FGF Inhi-MCP and the Inhi-FGF groups (Fig. 4E). These results suggested IE training might improve arteriogenesis following AMI through the pathway related to MCP-1 and FGF-2.
Optimized protocol of IE training
LVEF, ECs, and MNCs were significantly increased in the high-intensity IE training group(HIIE, received 100% load IE training 20 times/day for eight weeks,n=6) as compared to the MI group(allP<0.05). In addition, significantly reduced MIS was found in the low-intensity IE training group(LIIE, received 50% load IE training 20 times/day for eight weeks,n=6), the moderate-intensity IE training group (MIIE, received 75% load IE training 20 times/day for eight weeks,n=6), and the high-intesnsity group(HIIE, received 100% load IE training 20 times/day for eight weeks,n=6), compared with the MI group(allP<0.05). AD was elevated in the LIIE, MIIE, and HIIE groups (allP<0.05,vs.the MI group). The number of SMCs was also elevated in the MIIE and HIIE groups (bothP<0.05,vs.the MI group) (Fig. 5A).
In terms of training frequency, LVEF, and the number of ECs and MNCs were elevated in lowfrequency the IE training group (LFIE, received 100%load IE training 10 times/day for eight weeks,n=6),the moderate-frequency IE training group (MFIE,received 100% load IE training 20 times/day for eight weeks,n=6), and the high-frequency IE training group(HFIE, received 100% load IE training 30 times/day for eight weeks,n=6) in comparison with the MI group (P<0.05). Reduced MIS was found in the LFIE,MFIE, and HFIE groups compared with the MI group(allP<0.05). AD and SMCs increased significantly in the MFIE group as compared to the other groups (allP<0.05) (Fig. 5B).
For the comparison of training duration in six groups, LVEF, the number of ECs and MNCs were significantly increased in the moderate-duration IE training group (MDIE, received 100% load IE training 20 times/day for eight weeks,n=6) and the longduration IE training group (LDIE, received 100% load IE training 20 times/day for twelve weeks,n=6) as compared to the other groups (allP<0.05). Reduced MIS was observed in the MDIE and LDIE groups when compared to the other groups (bothP<0.05). AD was significantly increased in the short-duration IE training group (SDIE, received 100% load IE training 20 times/day for four weeks,n=6), MDIE, and LDIE groups as compared to the MI group (allP<0.05). The number of SMCs was only significantly increased in the MDIE group (Fig. 5C).
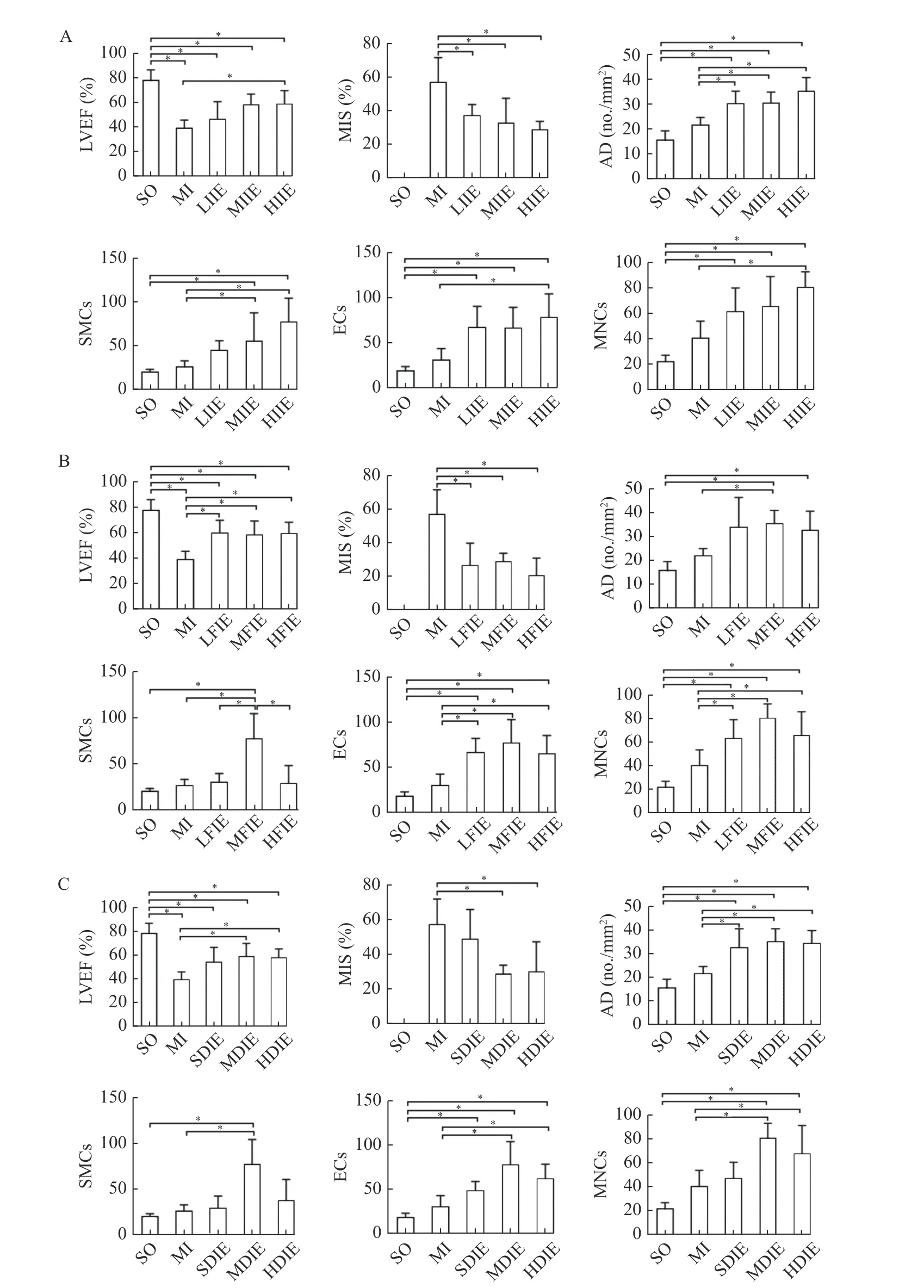
Fig. 5 Comparisons of LVEF, MIS, AD, ECs, MNCs and SMCs levels following IE training of different intensities, frequencies, and duration. Rats were treated with MI surgery, with or without IE training of different intensities, frequencies, and durations. A: Different intensities. B: Different frequencies. C: Different duration. *P<0.05. SO: sham-operation group; MI: myocardial infarction group; IE:isometric exercise group; LIIE: low-intensity isometric exercise group (IE training with a load of 50% for 20 times/day for 8 weeks); MIIE:moderate-intensity isometric exercise group (IE training with a load of 75% for 20 times/day for 8 weeks); HIIE/MFIE/MDIE: highintensity/moderate-frequency/moderate-duration isometric exercise group (IE training with a load of 100% for 20 times/day for 8 weeks);LFIE: low-frequency isometric exercise group (IE training with a load of 100% for 10 times/day for 8 weeks; HFIE: high-frequency isometric exercise group (IE training with a load of 100% for 30 times/day for 8 weeks); SDIE: short-duration isometric exercise group (IE training with a load of 100% for 20 times/day for 4 weeks); LDIE: long-duration isometric exercise group (IE training with a load of 100%for 20 times/day for 12 weeks); n=6 for each group. The number of positive cells of SMCs, ECs and MNCs were represented by integrated optical density. Data are presented as mean±SD. One-way ANOVA was used to analyze the significance between groups, *P<0.05. LVEF:left ventricular ejection fraction; MIS: myocardial infarction size; AD: arterial density; ECs: endothelial cells; MNCs: monocytes; SMCs:smooth muscle cells.
Discussion
The current study explored the effects, optimal protocol, and mechanisms of a long-term IE training on arteriogenesis in MI rat model. At the endpoint of the study, LVEF is increased after IE training, while the infarct area was decreased significantly. At the histological level, increased RCBF and AD suggested that IE could improve reperfusion and affect cardiac function. The results have also demonstrated increased proliferation of ECs, MNCs, and SMCs, strongly suggesting arteriogenesis following IE training. These findings corroborate the previous studies[9,21,25]. IE training of different intensity, duration, and frequency have been investigated and the results showed that 100% load IE training 20 times/day for eight weeks is the most efficient and effective protocol. Upregulated MCP-1 and FGF-2 are observed following IE training,which in turn promotes the recruitment of MNCs and SMCs, and then improves arteriogenesis in the remote ischemic myocardium.
PCI and coronary artery bypass grafting (CAGB)have been applied in AMI patients in clinical practice for decades. Although PCI and CAGB can effectively achieve vascular recanalization, a substantial number of patients need a well-tolerated and non-invasive intervention[26]. The current study focused on the use of IE training to improve cardiac function, which may avoid surgical contraindications. As a promising intervention to improve blood flow reperfusion, IE training has progressed rapidly[27]. However, several challenges are hampering its application in AMI patients, including the limited evidence regarding the effects of IE training in case of totally occluded lesion and the lack of suitable training programs. Therefore,evaluating the long-term beneficial effects of IE training initiated in the acute stage of MI and exploring the optimal training plans are essential for its clinical application.
There are two distinct types of vessel growth in ischemia: angiogenesis and arteriogenesis. Angiogenesis features the sprouting of new capillaries from pre-existent capillaries. Yet the newly generated capillary networks consisting of EC tubes lack stabilizing structures[28]. In contrast, arteriogenesis features the formation of mature and functional arteries by remodeling the pre-existent interconnecting arterioles after the arterial occlusion[29]. Angiogenesis only marginally improves the local blood supply while arteriogenesis improves blood flow more efficiently with complete reperfusion after arterial occlusion[30].
Increased MCP-1 and FGF-2 are the most critical molecular features of arteriogenesis[31]. It has been reported that plasma levels of MCP-1 are significantly increased in AMI patients with well-developed collateral circulation[32], suggesting that MCP-1 is closely related to the presence of coronary collaterals.Another study showed that the expression level of MCP-1 is increased in the left ventricle in the first 24 hours in myocardial infarction rats[33]. MCP-1 is a potent chemokine that regulates the recruitment of MNCs[34]. When the blood monocyte concentration is pharmacologically increased, arteriogenesis is accelerated after femoral artery ligation[35]. There is a reduction in the accumulation of MNCs followed by abnormal flow restoration in MCP-1 deficient mice[36].After recruitment, monocytes secrete fibroblast growth factor (FGF), the main driver of arteriogenesis[37].Yuet alfound that the promotion of arteriogenesis in the ischemic myocardium is associated with the increase of FGF[38]. FGF-2 upregulation, which is dependent on its receptor FGFR-1, improves cell proliferation of SMCs[11]. It has also been reported that de-differentiation, proliferation, and migration of vascular SMCs are important characteristics of arteriogenesis[39].
The current study detected high MCP-1 and FGF-2 protein levels, and increased number of MNCs and SMCs in the IE training group compared with SO and MI groups, suggesting that IE training is capable of upregulating MCP-1 and FGF-2, which in turn helps recruit MNCs and SMCs. The positive correlation between MCP-1 protein expression and MNC number indicates that MNC recruitment might be triggered by MCP-1[14]. Furthermore, a positive correlation between MNCs and FGF-2, and decreased number of SMCs in the Inhi-FGF group have also been observed,demonstrating that MNCs might release FGF-2 and enhance adhesion and migration of SMCs. In the current study, RCBF and AD are markedly elevated in the IE training group compared with SO, MI, Inhi-MCP, and Inhi-FGF groups. Lower levels of AD and RCBF are observed in Inhi-MCP/FGF group compared with Inhi-MCP and Inhi-FGF groups.Positive correlations are also found between SMCs and AD, as well as AD and RCBF. Taken together,these findings indicate the possible synergetic effects of IE-mediated upregulation of MCP-1 and FGF-2 in improving arteriogenesis.
Compared with passive IE training induced by electrical stimulation in previous research, active IE training (e.g., the rats gripping on the fence in this study) is more applicable in clinical practice[40].Meanwhile, the training protocol of active IE is promiscuous and rarely studied. In previous studies,isometric exercises were generally applied for a short time (e.g., 1 minute for one time or 2 minutes for four times)[9,41]. Yet, intensity, frequency, and duration are all important components of the training protocol. Our results showed that 100% load IE training 20 times/day for eight weeks may serve as an efficient mode. The rats' limb muscles achieve complete static contraction when in 100% load, compared with the low and moderate intensity. However, high frequency(30 times/day) or long-term duration (twelve weeks)show poor performance, which might be attributed to decreased exercise tolerance of MI rats with overburden or fatigue. Our observations are also consistent with the results of a recent review comparing the effects of exercise on cardiovascular diseases, recommending that six to eight weeks of exercise is the most effective in rats with MI-induced cardiac dysfunction[42].
Although associations among arteriogenesis and MCP-1/FGF-2 have been assessed in multiple levels,the mechanisms of that pathway might involve other mediators, which needs further exploration. In addition, these hypotheses should be confirmed in bothin vivoandin vitrosettings. Although the remote effects of IE training have been demonstrated in previous studies[21,43], tracing molecules and cells homing to the inflammation site should be performedviain vivofluorescent labeling.
Acknowledgments
This study was supported by the research grants from the National Natural Science Foundation of China (Grant No. 8177244, No. 81902288, and No.82072546) and Nanjing Municipal Science and Technology Bureau (Grant No. 2019060002). The authors gratefully acknowledge Peng Huang and Ruyi Zhang for their technical assistance and guidance.
RECEIVE IMMEDIATE NOTIFICATION FOR EARLY RELEASE ARTICLES PUBLISHED ONLINE
To be notified by e-mail whenJournalearly release articles are published online, sign up at jbr-pub.org.cn.
杂志排行
THE JOURNAL OF BIOMEDICAL RESEARCH的其它文章
- Paclitaxel-induced stress granules increase LINE-1 mRNA stability to promote drug resistance in breast cancer cells
- Construction of miRNA-mRNA network reveals crucial miRNAs and genes in acute myocardial infarction
- Ovalicin attenuates atopic dermatitis symptoms by inhibiting IL-31 signaling and intracellular calcium influx
- Target specificity of selective bioactive compounds in blocking α-dystroglycan receptor to suppress Lassa virus infection: an in silico approach
- Protective effects on acute hypoxic-ischemic brain damage in mfat-1 transgenic mice by alleviating neuroinflammation
- Application of a continuous respiratory sound monitoring system in thoracic surgery
