Exosomal miR-29b found in aqueous humour mediates calcium signaling in diabetic patients with cataract
2021-11-08ChaoGaoXinLiuFanFanJiaNingYangXiYueZhouHengJunMeiXiaoLeiLinYiLuo
Chao Gao, Xin Liu, Fan Fan, Jia-Ning Yang, Xi-Yue Zhou, Heng-Jun Mei,Xiao-Lei Lin, Yi Luo
1First Affiliated Hospital, School of Medicine, Shihezi University, Shihezi 832000, Xinjiang Uygur Autonomous Region, China
2Eye Institute, Eye and ENT Hospital, College of Medicine,Fudan University, Shanghai 200031, China
3State Key Laboratory of Medical Neurobiology, Institutes of Brain Science and Collaborative Innovation Center for Brain Science, Shanghai Medical College, Fudan University,Shanghai 200031, China
4Shanghai Key Laboratory of Visual Impairment and Restoration, Science and Technology Commission of Shanghai Municipality, Shanghai 200031, China
5Key Laboratory of Myopia (Fudan University), Chinese Academy of Medical Sciences, National Health Commission,Shanghai 200031, China
Abstract
● KEYWORDS: exosomes; miR-29b; diabetes and cataracts; Ca2+; CACNA1C
INTRODUCTION
Cataract is the opacification of the crystalline lenses and a disease that can be caused by many factors. Diabetes is a complex metabolic disorder that also involves small blood vessels, often causing widespread damage to tissues, including the eyes. Cataracts are a common ocular complication of diabetes. Bilateral cataracts occasionally occur with rapid onset in severe juvenile diabetes, which is called true diabetic cataract. However, true diabetic cataracts are rare. Senile cataract in people with diabetes, which is called diabetes with cataracts (DMC) in our study, is more common. The potential mechanisms for the pathogenesis of diabetic cataracts are complicated and include the p38-MAPK signalling pathway[1-2],polyol pathway[3], and changes in inflammatory cytokines[4-10].However, the precise mechanism of DMC remains unclear.Ca2+exists in the endocytoplasmic reticulum and plays an important role in the processing of polypeptide chains in protein translation and post-translational processing.It was reported that abnormal distribution of Ca2+could lead to dysfunction of the endocytoplasmic reticulum and mitochondria, thus causing some metabolic diseases such as diabetes[11]. Previous studies have reported that Ca2+-CaM abnormalities exist in cataracts, and it was found that L-type calcium channels were extensively distributed in lens epithelial cells, and the inhibition of L-type calcium channels could lead to the formation of cortical cataract[12]. There are a variety of different types of channel playing a fundamental role in Ca2+homeostasis and cell signalling which, when activated, allow Ca2+entry into cells, including L-type calcium channels. The expression of L-type voltage-gated Ca2+channels in both lens epithelial and fiber cells is potentially an important route of Ca2+influx which may contribute to pathological Ca2+overload[13]. L-type calcium channels were partially translated from the gene CACNA1C, which was also found to be mutated in DMC[14]. Given the critical role of Ca2+in lens epithelial cells, further investigation on the role of Ca2+in DMC is required.
Exosomes are micro extracellular vesicles (30-150 nm) that can be secreted by almost all types of cells and contain nucleus acids, proteins and lipids[15]. Exosomes have been widely studied in diabetes, neural diseases, tumours, cardiovascular diseases,etc[16-26]. A previous study reported that there were abundant exosomes in the human aqueous humour (AH)[27].Exosomal microRNAs (miRNAs) play important roles in the mechanisms of diabetes[28-30]and diabetic retinopathy[31-33].However, the function and characteristics of exosomes and miRNAs in DMC remains unknown.
In this study, we found that decreased expression of miR-29b could upregulate the expression of CACNA1C, increase the concentration of Ca2+, and affect the apoptosis of human lens epithelial cells (HLECs) in patients with DMC. The role of miR-29b and calcium signalling in diabetic cataracts has not been reported, which might suggest a potential role of exosomal miRNAs in the pathogenesis of diabetic cataracts.
The purpose of the study was to investigate the potential function of exosomal miRNAs found in AH on calcium signaling in diabetic patients with cataract.
SUBJECTS AND METHODS
Ethical Approval The use of human AH samples from cataract eyes during surgery was approved by the Institutional Review Board of Eye and ENT hospital of Fudan University.This study was performed in accordance with the tenets of the Declaration of Helsinki for research involving human subjects.Written informed consent was obtained from every enrolled participant.
Collection of AH and Human Lens Epithelium We collected AH and human lens epithelium samples from 36 patients with DMC (age from 45-76 years old, fasting glucose 5.2-8.2 mmol/L, free of other ocular diseases, and lenticular opacity ranging C3-4, NO2-3, NC2-3, and P1-3 by LOCSIII)and 43 patients with age-related cataracts (ARC; age 62-85 years old, fasting glucose 5.0-6.2 mmol/L, free of other ocular diseases, C3-4, NO2-3, NC2-3, and P1-2) before cataract surgery at Eye and ENT Hospital of Fudan University. AH samples were obtained before the collection of lens epithelium samples. The lens epithelium samples were acquired by intact continuous curvilinear capsulorhexis during cataract surgery for ARC patients by the same experienced surgeon (Luo Y).All AH and human lens epithelium samples were stored in a freezer at -80℃ until the next step.
Isolation of Exosomes The AH samples from 36 patients with DMC were pooled together as the DMC group, and the AH samples from 43 patients with ARC were pooled together as the ARC group. Exosomes were isolated using ultracentrifugation. Procedures were as followed: take AH samples out and thaw in 27℃ water bath; 4℃, 2000 g, 10min,and remove supernatant; 4℃, 10 000 g, 30min, and take supernatant; 4℃, 110 000 g, 75min, and discard supernatant;Resuspend pellet and filter with 0.22 μm membrane; 4℃,110 000 g, 75min, and abandon supernatant.
TEM and NTA of AH Exosomes Purified exosomes were diluted in PBS. Samples 5 μL were absorbed onto copper grids and dried for 5min at room temperature. After that, a drop of 2% uranyl acetate solution was added for 20min, and the sample was air-dried and examined by transmission electron microscopy (TEM; Tecnai G2 Spirit BioTwin, FEI, USA).Particle size, concentration, and distribution of exosomes were determined by nanoparticle tracking analysis (NTA; ZetaView,Particle Matrix, Germany).
Exosomal RNA Extraction and miRNA Sequencing Analysis Exosomal RNA was extracted from the DMC group and the ARC group using the miRNeasy Micro Kit(217084, QIAGEN, Germany) according to the manufacturer’s guidelines. RNA libraries were prepared and sequenced on an Illumina HiSeq 2500 platform. Read counts were obtained by FeatureCounts software. FastQC software was used for quality control. Additionally, we used Cutadapt software to remove low-quality reads and high-quality reads were used to analyse miRNAs by mapping to the human reference genome using Bowtie software. A fold change >1.2 or <0.83 was considered to indicate differentially expressed miRNAs by DESeq2.
RNA Extraction of Epithelium Samples and qRT-PCR Lens epithelium samples from 43 patients with ARC were classified as ARC group. Epithelium samples from 36 patients with DMC were classified as DMC group. In each group, 4 to 5 epithelium samples were pooled together to obtain enough RNA. Total RNA from all epithelium samples was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and reverse transcribed with the RT reagent Kit (Takara Bio, Inc,Japan) according to the manufacturer’s protocol. Expression of mRNAs was detected using the SYBR Green detection kit (Takara, Japan) on the LightCycler 480II Real-Time PCR System (Roche, Switzerland). GAPDH was detected as the internal control. RNA expression was determined by the 2-ΔΔCTmethod.
HLEC Culture and Transfection HLECs were cultured in 35-mm culture Petri dishes with growth medium containing DMEM (Gibco, USA) with 10% foetal bovine serum (FBS;Gibco, USA). MiR-29b mimics (50 nmol/L) and inhibitors(100 nmol/L) were transfected into HLECs when the cells covered 70%-80% of the entire dish.
Examination of Ca2+in AH Samples and the Culture Supernatant of Cells Concentration of Ca2+in AH samples and the culture supernatant of cells was detected by using Calcium Assay Kit (Colorimetric) from Abcam (Cambridge,MA, USA) according to the manufacturer’s protocol. We added 50 μL of AH samples and the culture supernatant of cells to each well of the 96-well plates. Then 90 μL of the chromogenic reagent and 60 μL of calcium assay buffer were added into each well for 10min at room temperature protected from light. Absorbance was measured at a wavelength of 575 nm using an automatic microplate reader (Tecan,Switzerland). Concentration of Ca2+was equivalent to Abs/Vol(μg/μL). Abs referred to absorbance of AH samples and cell culture supernatant; Vol referred to the volume of AH samples and cell culture supernatant added to each well.
CCK-8 Cell Proliferation and Cytotoxicity Assay Cell viability was determined by using cell counting kit-8 (CCK-8)kit (Dojindo, Japan) according to the manufacturer’s protocol.Transfected cells were plated onto 96-well plates and cultured for 24h. CCK-8 (10 μL) was then added for 1h at 37℃. We used Tert-butyl hydroperoxide solution (TBHP) as an oxidative stimulus. Absorbance was measured at a wavelength of 450 nm using an automatic microplate reader (Tecan, Switzerland).The cell viability was equivalent to (At-Ab)/(Ac-Ab). At referred to absorbance of transfected cell groups; Ac referred to absorbance of controlled groups; Ab referred to absorbance of blank groups.
Western Blot Protein was extracted by RIPA lysis buffer(Biotech Well, Shanghai, China). Equal amounts of proteins were resolved by SDS-PAGE using 5% acrylamide-containing gels, followed by electrophoretic transfer to PVDF membranes.The membranes were blocked with transfer buffer (Biotech Well, Shanghai, China) and incubated overnight with the monoclonal primary antibodies at a 1:200 dilution, followed by secondary antibodies at a 1:2000 dilution. The signaling of western blotting was then observed using ECL prime reagents(Biotech Well, Shanghai, China) and scanned using a Peiqing automatic gel imaging analysis system (Shanghai, China). The L-VOCC polyclonal antibody (21774-1-AP) was purchased from Proteintech Group (USA). The anti-GAPDH antibody and the goat anti-rabbit IgG (H+L) secondary antibody were purchased from Biotech Well (Shanghai, China).
MicroRNA Target Prediction Potential targets of miR-29b were predicted by the Targetscan database (http://www.targetscan.org)[34].
Statistical Analysis All data are shown as the mean±SD, and experiments were repeated three times. Statistical significance was determined by two-tailed Student’st-test, One-way ANOVA or Chi-square test using IBM SPSS 21.0 (USA).P<0.05 was considered statistically significant.
RESULTS
Baseline Characteristics in DMC and ARC Groups Totally 79 eyes were enrolled in the two groups, among which there were 36 eyes in DMC group and 43 eyes in ARC group. In DMC group, there were 22 males and 14 females, 17 right eyes and 19 left eyes. Mean age and mean fasting glucose of DMC group were 68.11±1.34y and 6.31±1.34 mmol/L. In ARC group, there were 21 males and 22 females, 21 right eyes and 22 left eyes. Mean age and mean fasting glucose of ARC group were 69.07±0.82y and 5.55±0.06 mmol/L. There were no statistical significance in the gender, right or left eye, and age between the two groups (Table 1). The fasting glucose between the two groups was statistically significant (Table 1).
Cup-shaped Phenotype Observed Using TEM and Particle Size Distribution of Exosomes Using NTA To identify the characteristics of exosomes, we used TEM to observe the morphology of exosomes isolated from AH, and observed a typical cup-shaped phenotype in both groups (Figure 1A)[35].The particle diameter was 129.8 nm in the ARC group and 116.3 nm in the DMC group. Particle sizes were mainly distributed at 80-120 nm, and the concentration reached more than E+10 particles/mL in both groups (Figure 1B)[35].
Differential Expression of miRNAs in AH Samples of the DMC and ARC Groups miRNA sequencing was used to examine the expression of miRNAs in AH samples of the DMC and ARC groups. A total of 552 miRNAs were obtained from all samples. Of them, 119 miRNAs were unchanged;295 miRNAs were upregulated and 138 miRNAs were downregulated in AH samples of the DMC group compared with the ARC group (fold change >1.2 in upregulated miRNAs, and fold change <0.833 in downregulated miRNAs).Among the 138 downregulated miRNAs, miR-29b was significantly downregulated in the DMC group compared with the ARC group (with fold change=0.286; Figure 2).In addition, CACNA1C was a potential target of miR-29b through the TargetScan website. We thus investigated the role of miR-29b in the regulation of HLECs function. Part of differentially expressed miRNAs were shown in Figure 2.
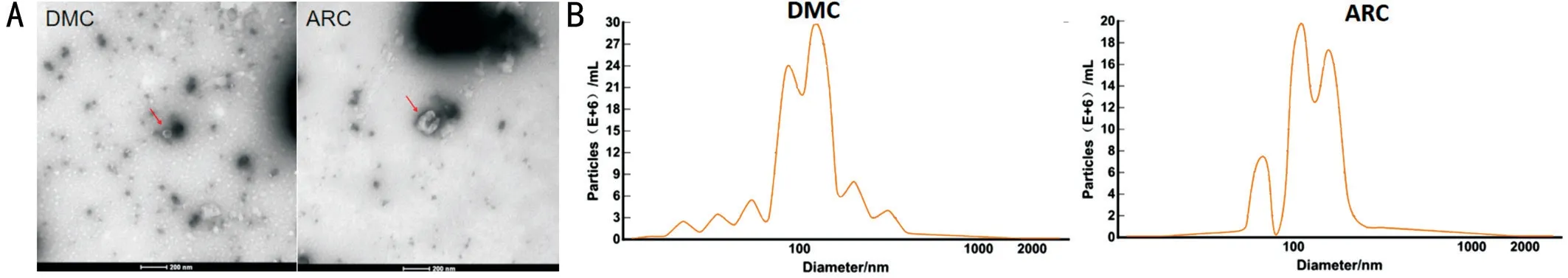
Figure 1 Identification of exosomes using TEM and NTA A: Typical cup-shaped phenotype under TEM in the DMC and the ARC group; B:Particle size distribution in DMC and ARC group by NTA.
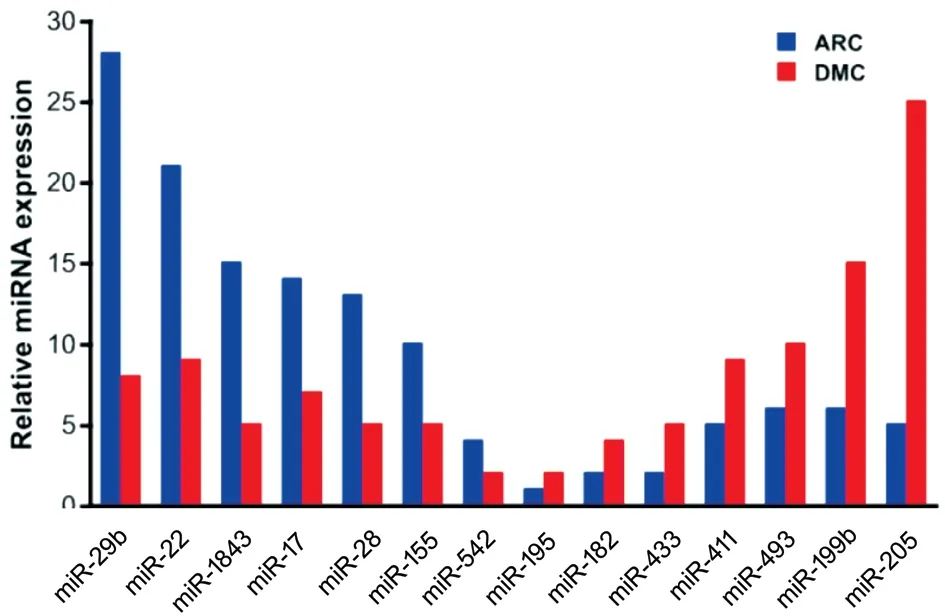
Figure 2 Part of downregulated and upregulated miRNA expression in the DMC group compared with the ARC group.
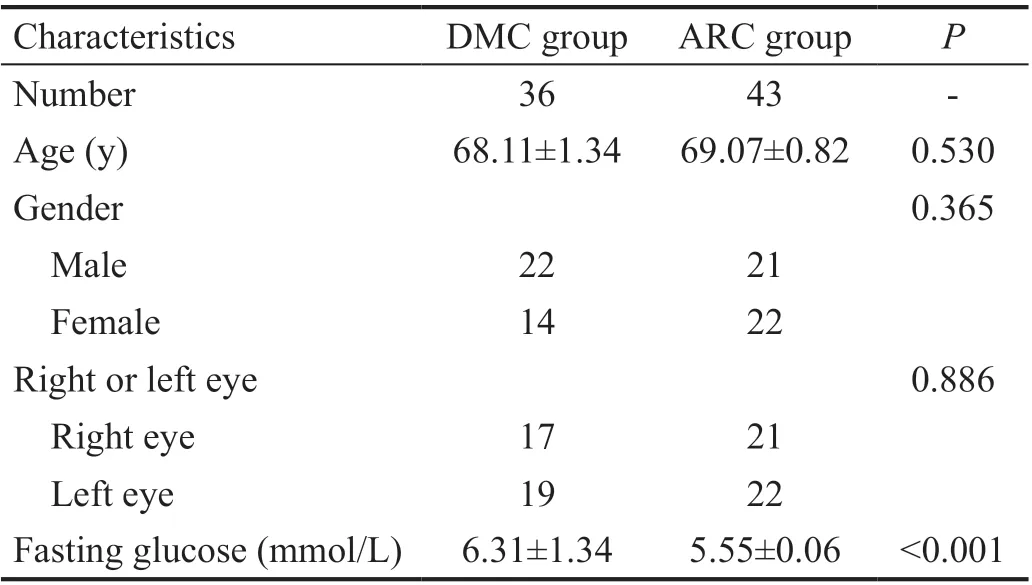
Table 1 Baseline characteristics in DMC and ARC groups
Differential CACNA1C Expression Between DMC and ARC Epithelium Samples CACNA1C mRNA expression was upregulated in DMC epithelium samples compared with that in ARC epithelium samples by quantitative real-time PCR(qRT-PCR), and the difference was statistically significant(Figure 3).
Different Concentration of Ca2+in AH Samples in the DMC and ARC Groups To determine the effect affected by the different expression of CACNA1C, we detected the concentration of Ca2+in AH samples in two groups. Mean concentration of Ca2+in AH samples was 0.075±0.005 μg/μL in the DMC group and 0.06±0.004 μg/μL in the ARC group.The concentration of Ca2+in AH samples was higher in the DMC group than that in the ARC group (25% more), and the difference was statistically significant (P=0.024; Figure 4).
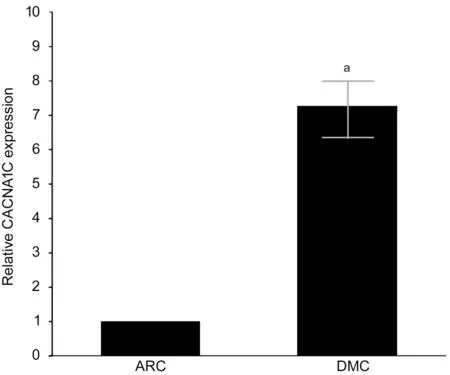
Figure 3 Expression of CACNA1C mRNA detected by qRTPCR aStatistically significant compared with ARC.
MiR-29b Mimics and Inhibitors Significantly Changed CACNA1C Expression in HELCs To determine whether exosomal miR-29b influenced the expression of CACNA1C in HELCs, we used miR-29b mimics and inhibitors to transfect HLECs and then detect the expression of CACNA1C mRNA of HLECs. miR-29b mimics significantly downregulated CACNA1C expression in HELCs, while miR-29b inhibitors significantly upregulated CACNA1C expression in HELCs(Figure 5A). Additionally, negative control (NC) mimics and NC inhibitors were used as comparators of miR-29b mimics and inhibitors in Western blot analysis. Western blot analysis showed that transfection of miR-29b mimics led to a reduction in CACNA1C expression, while NC mimics did not. By contrast, transfection of miR-29b inhibitors led to increased CACNA1C expression, while NC inhibitors did not (Figure 5B). The results were consistent with the results observed in epithelium samples.

Figure 4 Concentration of Ca2+ of AH samples in the DMC and ARC groups aStatistically significant compared with ARC.
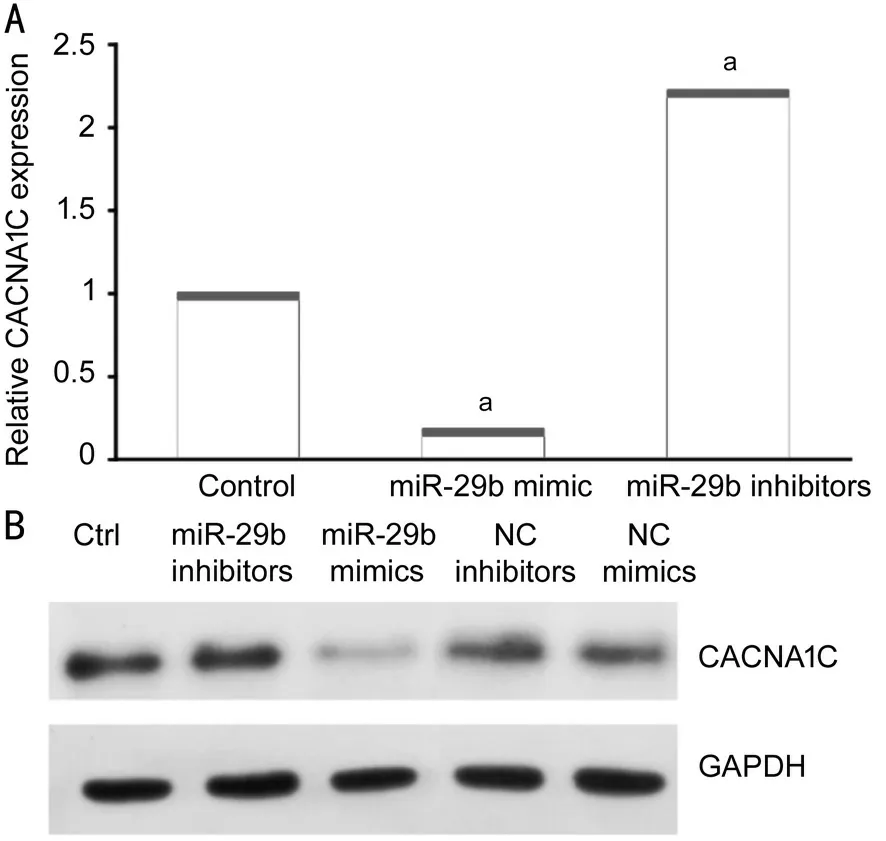
Figure 5 MiR-29b mimics and inhibitors changed CACNA1C expression in HELCs A: Expression of CACNA1C mRNA was affected by miR-29b mimics and inhibitors as demonstrated using qRT-PCR; B: Protein levels of CACNA1C were affected by miR-29b mimics and inhibitors as shown using Western blotting. aStatistically significant compared with ARC.
Different Concentration of Ca2+in the Culture Supernatant of Cells Transfected with miR-29b Mimics and Inhibitors To determine whether different expression of CACNA1C regulated by miR-29b affected the concentration of Ca2+, we then detect the concentration of Ca2+in the culture supernatant of cells transfected with miR-29b mimics and inhibitors. Mean concentration of Ca2+in the culture supernatant of cells was 0.09±0.005 μg/μL in the control group, and 0.078±0.01 μg/μL transfected with miR-29b mimics, and 0.106±0.002 μg/μL transfected with miR-29b inhibitors. The concentration of Ca2+was higher in the culture supernatant of cells transfected with miR-29b inhibitors than that in normal culture supernatant of cells. The concentration of Ca2+was 26.4% higher in the culture supernatant of cells transfected with miR-29b inhibitors than that in the culture supernatant of cells transfected with miR-29b mimics (Figure 6). The result was similar to that in AH samples.
Role of miR-29b in the Regulation of Cell Viability of HLECs We further investigated the effect of miR-29b on the viability of HLECs. Both under normal conditions and under oxidative stress, miR-29b inhibitors significantly reduced the viability of HLECs compared with miR-29b mimics, and the difference was statistically significant (Figure 7A, 7B). Under oxidative stress, the viability of HLECs transfected with miR-29b mimics was slightly reduced, but the difference was not statistically significant (Figure 7C). However, the viability of HLECs transfected with miR-29b inhibitors was significantly reduced, and the difference was statistically significant (Figure 7D).
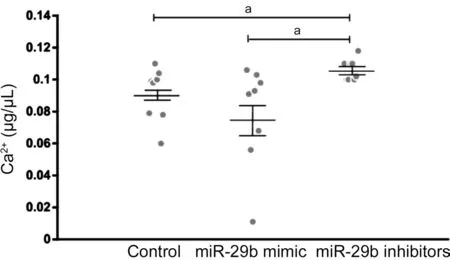
Figure 6 Concentration of Ca2+ in normal cell culture supernatant and in cell culture supernatant transfected by miR-29b mimics and inhibitors aStatistically significant compared with ARC.
DISCUSSION
Cataracts are more common in patients with diabetes than individuals without diabetes; their symptoms are similar to those of ARC, while their progression is more rapid. Recently,exosomes have been widely studied in many fields, especially diabetes. To reveal a possible mechanism linking DMC, we investigated the role of exosomal miR-29b and Ca2+in HLECs.Ca2+is an important factor in biological activities. More importantly, calcium might induce conformational damage to A-crytallin and accelerate the development of the cataracts[36].Ca2+influx channel, including L-type voltage-gated Ca2+channel, is a powerful way of rapidly increasing intracellular Ca2+during cell signalling and the possibility of different combinations of channels enables a high degree of functional diversity between cell types. The L-type Ca2+channel blocker was found to be effective in attenuating cataract formation[13].However, the precise mechanism by which Ca2+plays its role still remains unclear. In our study, we detected the concentration of Ca2+in AH samples from patients with DMC and patients with ARC, and found that the concentration of Ca2+in the AH samples was higher in DMC than that in ARC, showing a potential relationship among Ca2+and DMC.In addition, the mRNA expression of CACNA1C, which is partially translated into an L-type calcium channel protein that might lead to the different concentrations of Ca2+in the two groups, was upregulated in DMC epithelial samples compared with ARC epithelial samples.
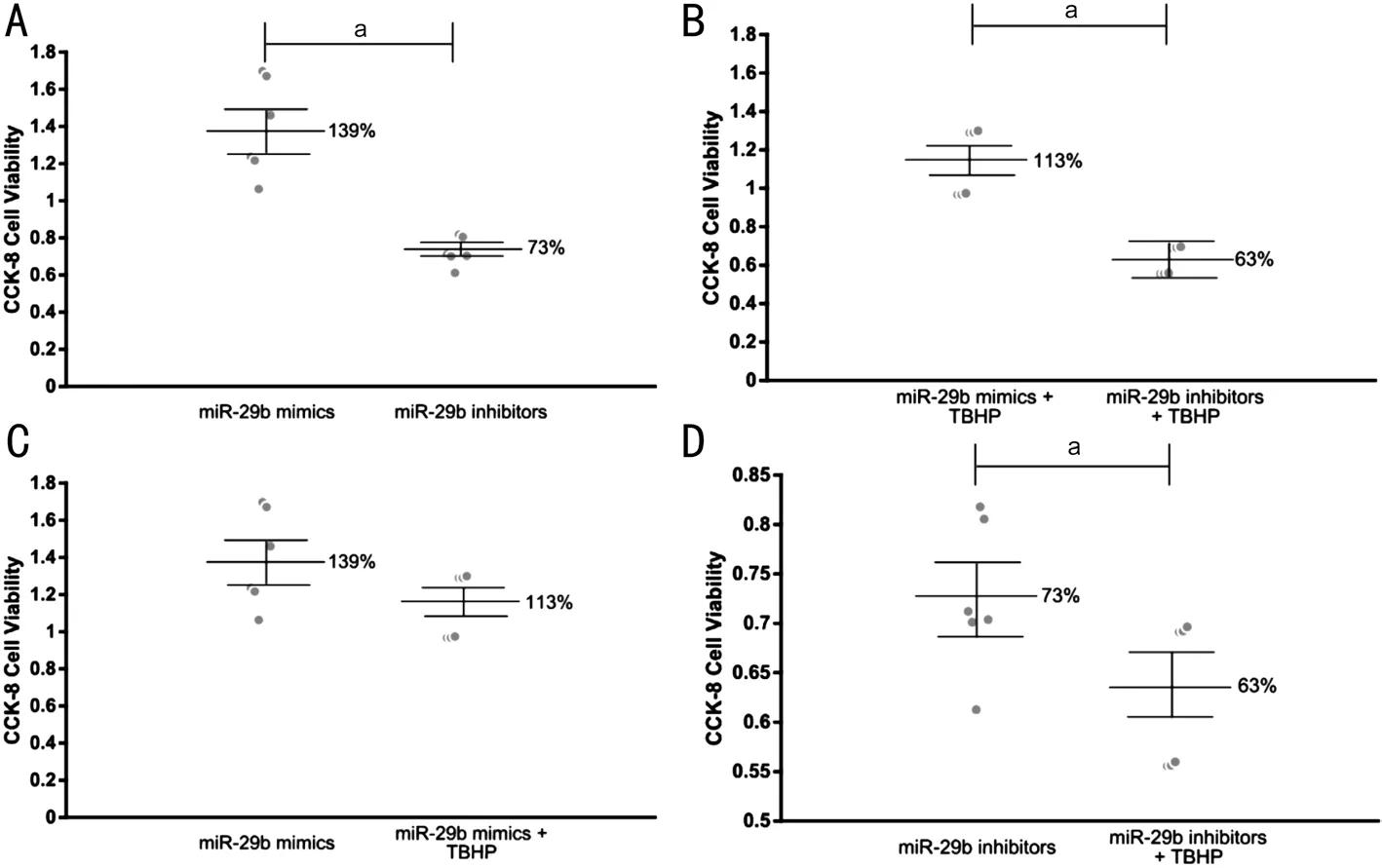
Figure 7 Cell viability measured by CCK-8 A: The viability of HLECs trancfected with miR-29b mimics and inhibitors in normal conditions;B: The viability of HLECs trancfected with miR-29b mimics and inhibitors under oxidative stress; C: The viability of HLECs trancfected with miR-29b mimics in normal conditions and under oxidative stress; D: The viability of HLECs trancfected with miR-29b inhibitors in normal conditions and under oxidative stress. aStatistically significant compared with ARC.
Exosomes have been widely studied in diabetes, neural diseases, tumours, cardiovascular diseases,etc[16-26]. Abundant exosomal miRNAs, including miR-486, miR-204, miR-184,etc., have been found in human AH[27]. Exosomal miRNAs play important roles in the mechanisms of diabetes[29-30].In diabetes-associated ocular diseases (such as diabetic retinopathy), exosomal miRNAs play an important role by affecting the integrity of the vascular endothelium[32]. It has also been reported that pancreatic β cell derived exosomal miR-15a might damage the retina by targeting Akt3 and causing diabetic retinopathy[31]. In addition, plasma exosomes carrying IgG can damage retinal vessels[33], and retinal pigment epithelial (RPE) cell-derived exosomes can participate in immunoregulation by killing targeted monocytes[37-38], thus causing diabetic retinopathy. In addition, miRNAs play important roles in regulating the function of coding genes.Downregulation of miR-2113 inhibits high glucose-induced mesenchymal activation and fibrosis[39]. Interaction of miR-30a with circHIPK3 can regulate the expression of VEGFC,FZD4 and WNT2, thus changing the viability and apoptosis of retinal pigmented epithelial cells[40]. More importantly,our previous study showed that upregulation of miR-193a caused by downregulation of circHIPK3 can regulate cataract formation by targeting CRAYY[41]. However, there are few studies reporting the function and characterization of exosomes and miRNAs in DMC.
In our previous study, we used a bioinformatic approach to analyse the correlated functions of the differentially expressed miRNAs, and found that the coding genes of the upregulated miRNAs were mainly associated with proteoglycans in cancer and that the coding genes of the downregulated miRNAs were mainly associated with AGE-RAGE signalling pathway in diabetic complications. Among the upregulated exosomal miRNAs, miR-551b was highly expressed and could downregulated CRYAA expression, thus affecting the viability and apoptosis of HLECs[35].In this study, we assessed the AH as a microenvironment of lens epithelial cells, and we focused on its influence on the crystalline lens and sequenced exosomal miRNAs in the DMC and ARC groups. Therefore, we isolated exosomes from the AH by ultracentrifugation and identified them through NTA and TEM. We then sequenced exosomal miRNAs; we revealed that miR-29b was downregulated in patients with DMC and compared these data with those from patients with ARC.MiR-29b was previously reported to be significantly changed across the diabetes spectrum and associated with measures of pancreatic islet β cell function and glycaemic control[42].In cultured trancfected cells, the concentration of Ca2+was 26.4% higher in the culture supernatant of cells transfected with miR-29b inhibitors than in the culture supernatant of cells transfected with miR-29b mimics and was almost equivalent to that in AH samples (25%). The results of our study revealed that the downregulation of miR-29b caused upregulation of CACNA1C expression and an increase in the concentration of Ca2+, resulting in decreased viability of HLECs. In addition,HLECs transfected with miR-29b inhibitors exhibited increased sensitivity to oxidative stress. However, the mechanisms of calcium homeostasis and signaling in the lens were very complicated. Evidence showed that functional expression of L-type voltage-gated Ca2+channels in the intact lens might affect either oscillations of membrane potential or translens short-circuit current in the intact rabbit lens[13]. Since endoplasmic reticulum was the store of intracellular Ca2+, the increased Ca2+of extracellular fluid might be an interactive mechanism for increased Ca2+within the cell. Here, we identified a novel mechanism by which the function of HLECs in DMC might be regulated through exosomal miR-29b/CACNA1C/Ca2+, in which the interaction of extracellular and intracellular Ca2+need to be investigated in the future study(Figure 8).
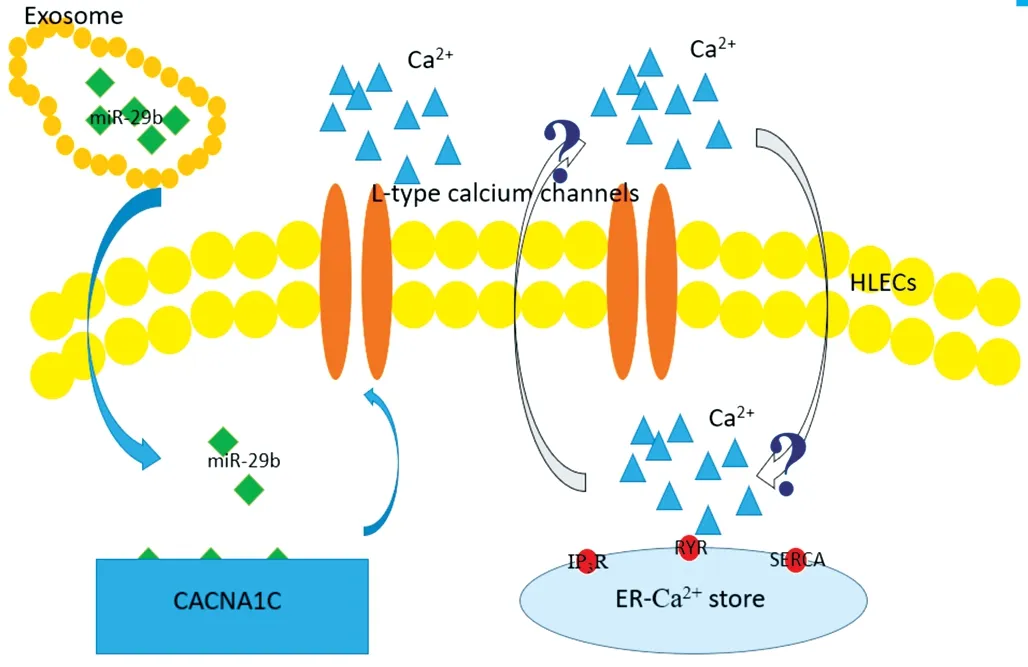
Figure 8 Possible regulation of function HLECs through exosomal miR-29b/CACNA1C/Ca2+ Down-regulation of exsomal miR-29b upregulated the expression of gene CACNA1C, increasing expression of L-type voltage-gated Ca2+ channels and Ca2+ of extracellular fluid.The increased Ca2+ of extracellular fluid might be an interactive mechanism for increased Ca2+ within the cell, of which the precise mechanism remained uncertain in the study.
In conclusion, exosomes isolated from human AH contained abundant miRNAs. A significantly expressed miRNA,miR-29b, could affect the concentration of Ca2+and regulate HLEC processes by upregulating CACNA1C.
ACKNOWLEDGEMENTS
Foundation:Supported by the National Natural Science Foundation of China (No.81870645).
Conflicts of Interest:Gao C, None; Liu X, None; Fan F,None; Yang JN, None; Zhou XY, None; Mei HJ, None; Lin XL, None; Luo Y, None.
杂志排行
International Journal of Ophthalmology的其它文章
- Intraluminal stenting versus external ligation of Ahmed glaucoma valve in prevention of postoperative hypotony
- Visual acuity after intravitreal ranibizumab with and without laser therapy in the treatment of macular edema due to branch retinal vein occlusion: a 12-month retrospective analysis
- Dexamethasone intravitreal implant (Ozurdex) in diabetic macular edema: real-world data versus clinical trials outcomes
- Comparative analysis of the clinical outcomes between wavefront-guided and conventional femtosecond LASlK in myopia and myopia astigmatism
- Reliability of the ocular trauma score for the predictability of traumatic and post-traumatic retinal detachment after open globe injury
- Vitreous function and intervention of it with vitrectomy and other modalities
