Research progress on the main chemical constituents and pharmacological effects of Piper betle L.
2021-11-05ZhiyongXuYufeiXiXiaoxiaoHuangShaojiangSong
Zhiyong Xu,Yufei Xi,Xiaoxiao Huang,Shaojiang Song
Key Laboratory of Computational Chemistry-Based Natural Antitumor Drug Research &Development,Liaoning Province,School of Traditional Chinese Materia Medica,Shenyang Pharmaceutical University,Shenyang 110016,China
Abstract The Piper betle,belonging to Piper genus of the Piperaceae family,is an evergreen and perennial creeper used in several traditional medicines.This plant has been known to possess antioxidant,antitumor,antibacterial,anti-inflammatory and antimalarial activities.A wide range of chemical compounds including alkaloids,simple phenylpropanoids,lignans,flavonoids,steroids,triterpenoids,sesquiterpenes,monoterpenes have been isolated from this plant.The present review summarized the information concerning phytochemistry and biological activity of P.betle,providing references for the future research of this plant.
Keywords:Piper betle L.;chemical constituents;biological activity;alkaloids;lignans
1 Introduction
Piper betle
L.,belonging to Piperaceae family,is native to Malaysia,but widely distributed in South and Southwest China.It is also cultivated in India,Sri Lanka,Thailand and other Southeast Asian countries [1].The roots ofP.betle
have been used as Chinese folk medicine for treating wind-cold cough,bronchial asthma,rheumatism and edema of pregnancy [2].The leaves ofP.betle
are mainly used as mouth freshener,packaged and chewed by local people with the areca nut [3].The flowers of this plant are also the major ingredient that gives flavor and other properties to special chewing food [4].Studies on the chemical constituents ofP.betle
have shown that the essential oil is one of the major chemical constituents.Monoterpenes,sesquiterpenes,phenylpropanoids,alkaloids,lignans and steroids are also predominant chemical constituents [5].Previous studies onP.betle
showed that it had various pharmacological activities,such as antioxidant,antibacterial,anti-inflammatory and anticancer activities.This paper summarized the chemical constituents and pharmacological activities ofP.betle
,aiming to lay the foundation for further research.2 Studies of chemical constitutions
2.1 Amide alkaloids
There are about nineteen amide alkaloids isolated and identified fromP.betle
(Fig.1),including four piperidine-type amide alkaloids(1-4
),one pyrrolidine-type amide alkaloid (5
),isobutylamine-type amide alkaloids (6-10
),five lactam-type amide alkaloids (11-15
) and four ceramides (16-19
).They are piperdadine (1
) [6],piperine (2
) [6],dehydropipernonaline (3
) [8],piperolein B (4
) [8],piperyline (5
) [7],N
-isobutyl-2E
,4E
-octadienamide (6
) [7],N
-isobutyl-2E
,4E
dodecadienamide (7
) [8],pellitorine (8
) [6],(2E
,4E
)-N
-isobutyl-7-(3′,4′-methyl-enedioxyphenyl)-2,4-heptadienamide (9
) [8],guineensine (10
) [8],aristololactam B II (11
) [9],piperolactam A (12
) [9],aristololactam A II (13
) [9],cepharadione A (14
) [9],desmethylenesqualenyl deoxycepharadione-A (15
) [10],(2S
,3S
,4R
)-2-N
-[(2′R
)-2′-hydroxyl-pentacosanoylamino]-nonacosane-1,3,4-triol (16
) [11],(2S
,3S
,4R
,8E
)-2-N
-[(2′R
)-2′-hydroxytetracosanoylamino]-8-eicosylene-1,3,4-triol (17
) [11],pipercerebroside A (18
) [12],and pipercerebroside B (19
) [12].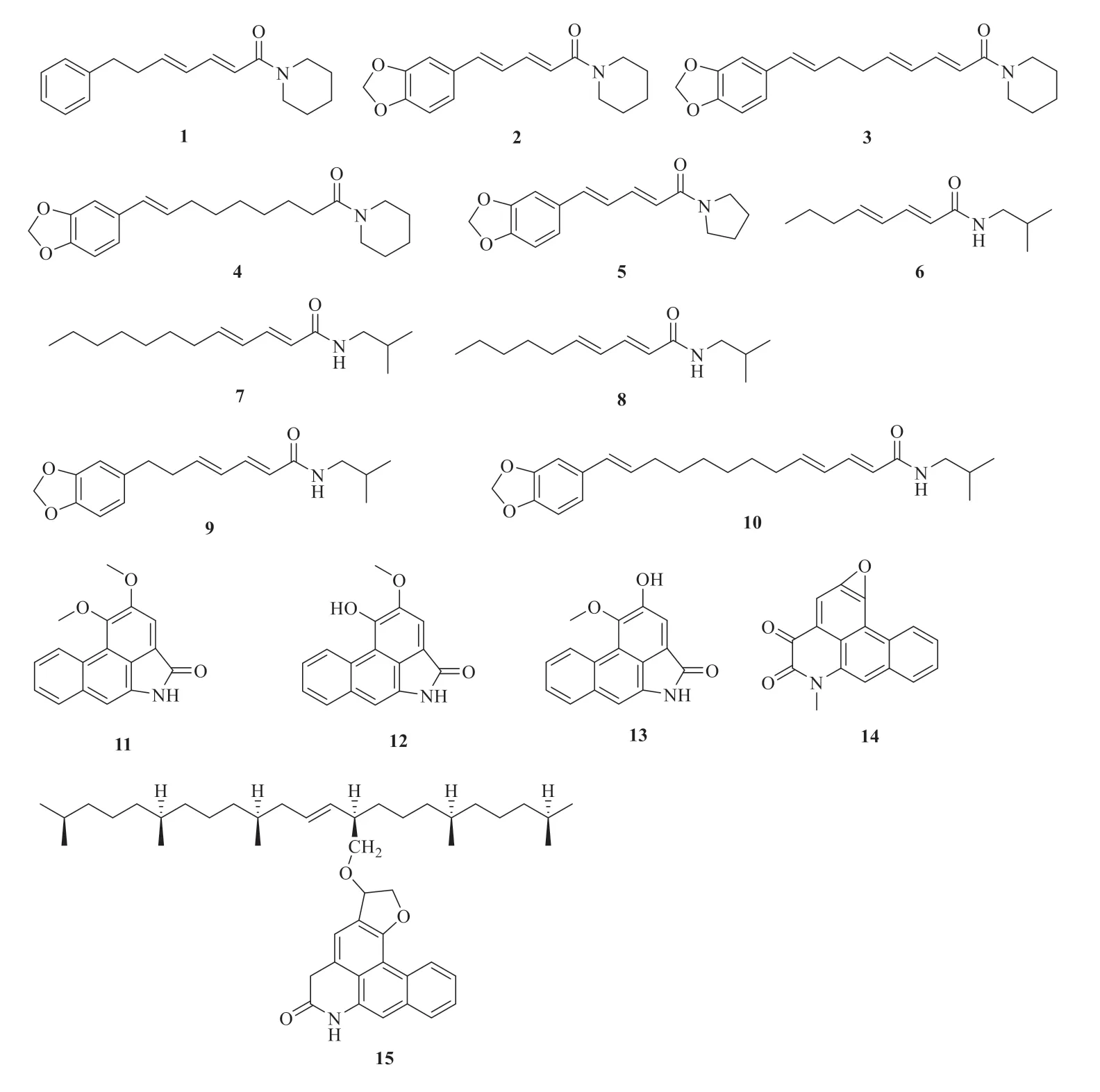
Fig.1 Chemical structures of amide alkaloids in P.betle
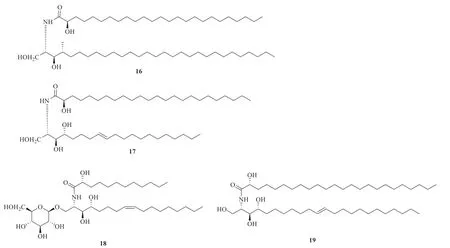
Continued fig.1
2.2 Phenylpropanoids
2.2.1 Simple phenylpropanoids
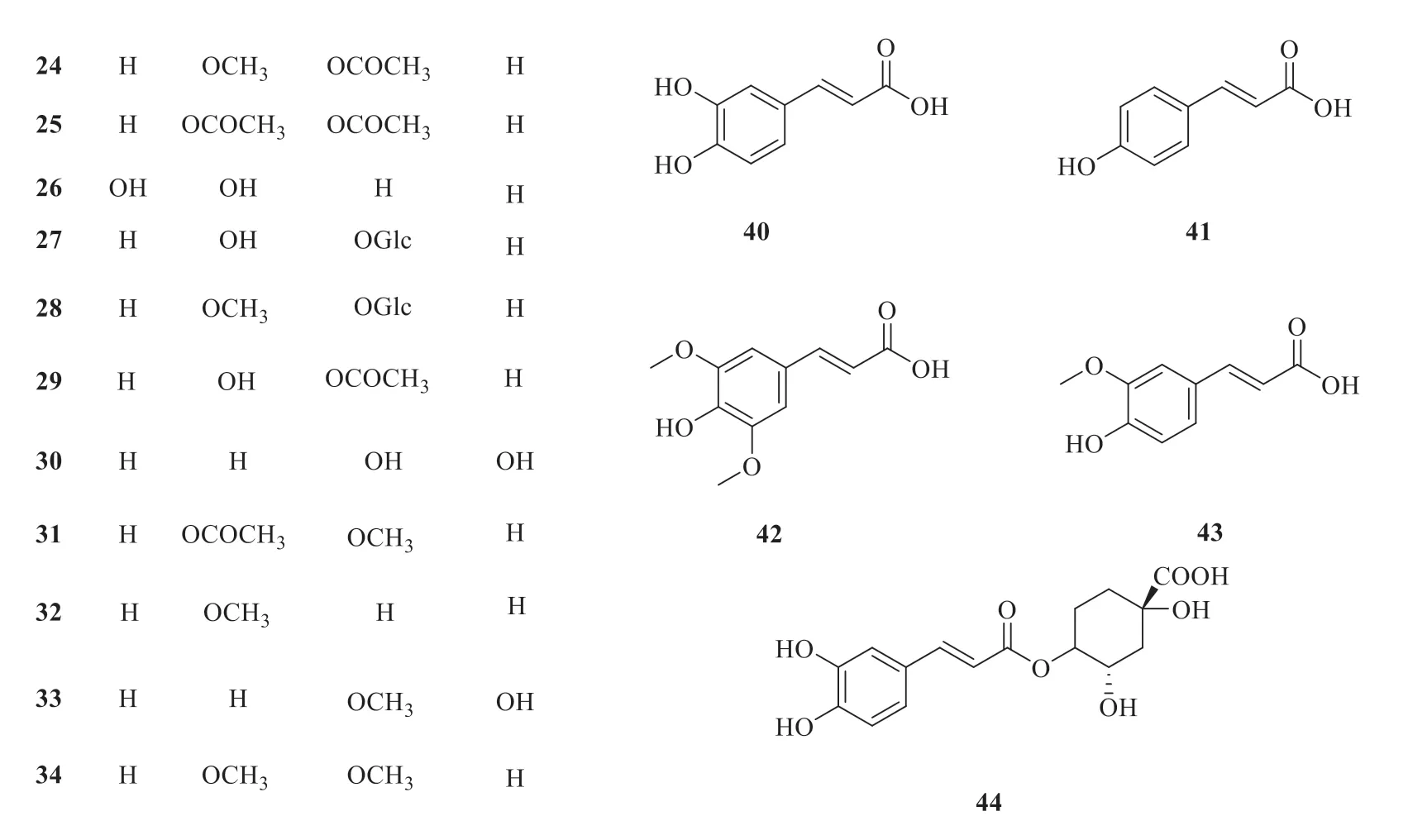
At present,twenty-five simple phenylpropanoids have been isolated fromP.betle
(Fig.2).They are 4-allylbenzene-1,2-diol (
20
) [13],eugenol (21
) [14],1-methoxy-2-hydroxy-4-allylbenzene (22
) [15],chavicol (23
) [15],1-methoxy-2-acetoxy-4-(2-propyly1) benzene (24
) [15],1-Acetoxy-2-acetoxy-4-(2-propenyl) benzene (25
) [15],4-allyl resorcinol(26
) [16],2-hydroxy-5-(2-propen-1-yl)phenyl-β
-D-glucopyranoside (27
) [17],3-hydroxyestragoleβ
-D-glucopyranoside (28
) [17],allylpyrocatechol monoacetate (29
) [18],allylpyrocatechol (30
) [19],isoeugenol acetate (31
) [20],estragole (32
) [21],o
-eugenol (33
) [22],methyl eugenol (34
) [23],hidroxychavicol acetate (35
) [19],safrole (36
) [24],anethole (37
) [21],isoeugenol (38
) [20],isoeugenol acetate (39
) [20],caffeic acid (40
) [25],p
-coumaric acid (41
) [25],sinapic acid (42
) [26],ferulic acid (43
) [26],and chlorogenic acid (44
) [27].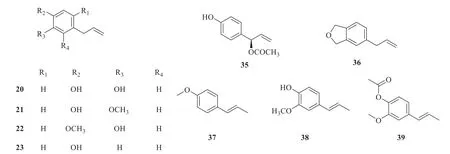
Fig.2 Chemical structures of simple phenylpropanoids in P.betle
Continued fig.2
2.2.2 Lignans and neolignans
A number of lignans and neolignans have been isolated and identified fromP.betle
(Fig.3).They are bis-chavicol dodecanoyl ester(45
) [14],γ
-(γ
′isohydroxychavicol)-chavicol octanyl ether (46
) [28],bis-hydroxychavicol dodecanoyl ester (47
) [14],2-(γ
′-hydroxychavicol)-hydroxychavicol (48
) [9],piperol A (49
) [29],piperbetol (50
) [29],methylpiperbetol (51
) [29],piperol B (52
) [29],pinoresinol (53
) [8],and syringaresinol (54
) [8].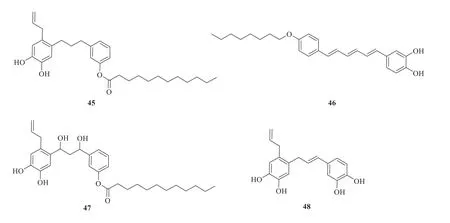
Fig.3 Chemical structures of lignans and neolignans in P.betle

Continued fig.3
2.3 Flavonoids and flavone glycosides
Fifteen flavonoids and flavone glycosides have been isolated and identified fromP.betle
(Fig.4).They are 8-C
-hexosyl-luteolin (55
) [30],8-C
-hexosyl-apigenin (56
) [30],2”-O
-hexosyl-8-C
-hexosyl-apigenin (57
) [30],2”-O
-rhamnosyl-8-C
-hexosyl-apigenin (58
) [30],2”-O
-hexosyl-6-C
-hexosyl-luteolin (59
) [30],2”-O
-rhamnosyl-6-C
-hexosyl-luteolin (60
) [30],rutin (61
) [25],myricetin (62
) [31],quercetin (63
) [31],apigenin(64
) [26],kaempferol(65
) [26],(2S
)-4′hydroxy-2,3-dihydroflavonone-7-O
-β
-D-glucoside(66
) [32],catechin (67
) [31],epicatechin (68
) [31],and naringenin (69
) [31].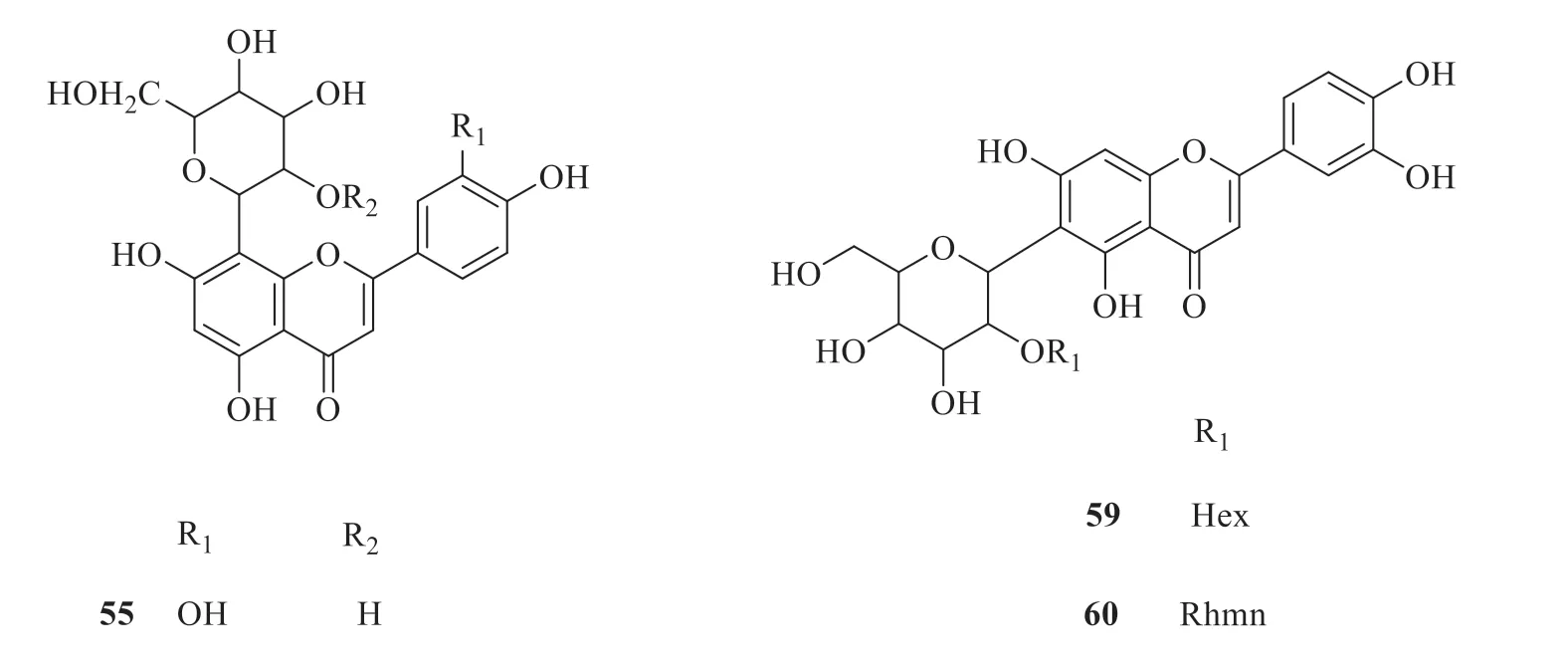
Fig.4 Chemical structures of flavonoids and flavone glycosides in P.betle
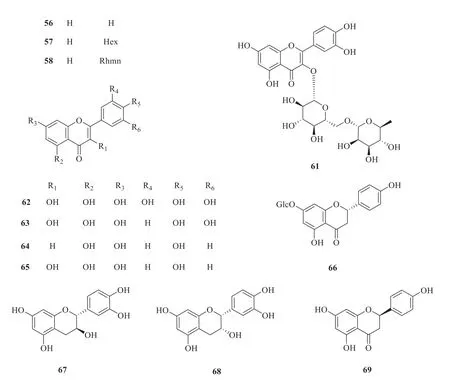
Continued fig.4
2.4 Steroids and their glycosides
About seven steroids and their glycosides have been isolated and identified fromP.betle
at present (Fig.5).They are 6-β
-hydroxystigmast-4-en-3-one (70
) [32],stigmasterol (71
) [32],stigmast-4-en-3-one (72
) [16],stigmast-4-en-3,6-dione(73
) [16],β
-sitosterol (74
) [16],β
-daucosterol(75
) [32],andβ
-sitosterol-3-O
-β
-D-glucoside-6′-O
palmitate (76
) [32].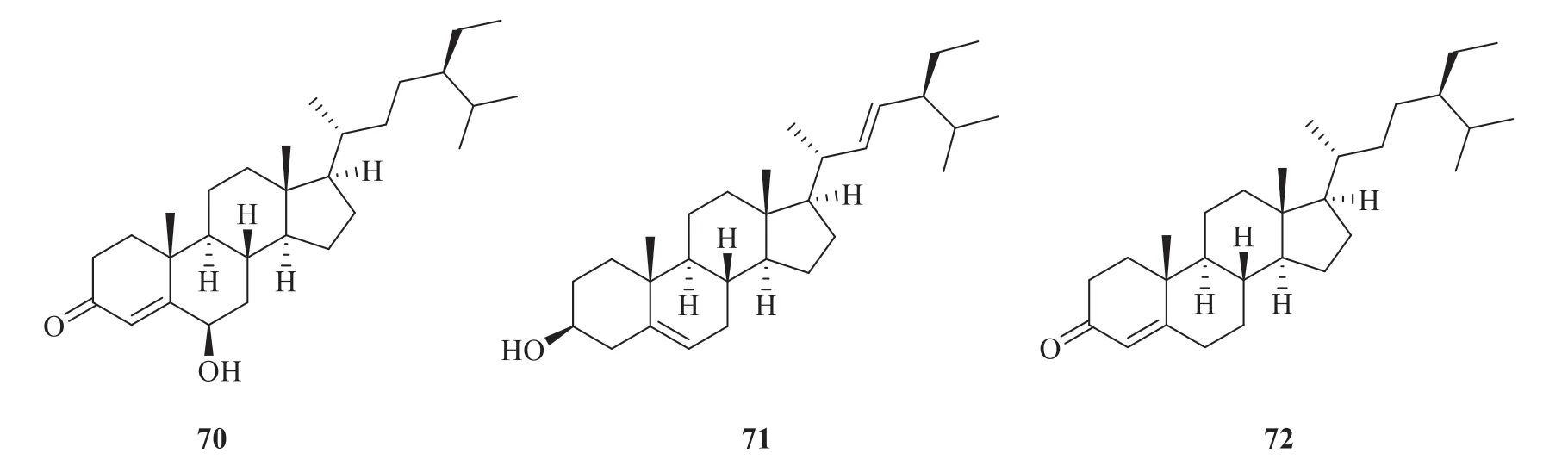
Fig.5 Chemical structures of steroids and their glycosides in P.betle

Continued fig.5
2.5 Terpenes
2.5.1 Triterpenoids and diterpenoids
Three triterpenoids and one diterpenoid,namely oleanolic acid (77
) [32],23-hydroxyurs-12-en-28-oic acid (78
) [32],β
-amyrin (79
) [7]and phytol (80
) [33],were isolated fromP.betle
(Fig.6).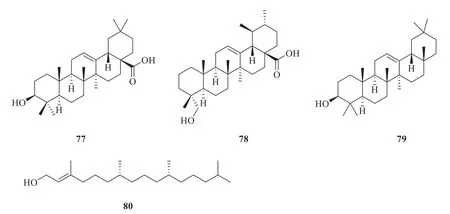
Fig.6 Chemical structures of triterpenoids and diterpenoids in P.betle
2.5.2 Sesquiterpenes
Till now,thirty-four sesquiterpenes have been reported fromP.betle
(Fig.7),including caryophyllene (81
) [24],caryophyllene oxide (82
)[24],viridiflorene (83
) [21],allo
-aromadendreneepoxide (8 4
) [2 1],globulol (8 5
) [3 3],allo
-aromadendrene (8 6
) [2 2],ledol(87
) [22],aciphyllene (88
) [21],bicycloelemene(89
) [23],α
-cubebene (90
) [23],β
-cubebene(91
) [23],β
-bourbonene (92
) [23],α
-humulene(9 3
) [2 3],2H
-cyclopentacy cloo cten e,4,5,6,7,8,9-hexahydro-1,2,2,3-tetramethyl (94
) [33],germacrene D (95
) [22],α
-copaene (96
)[21],α
-cadinene (97
) [22],α
-amorphene(98
) [23],α
-cadinol (99
) [22],β
-selinene(100
) [23],γ
-cadinene (101
) [20],γ
-muurolene(102
) [23],eudesm-7(11)-en-4-ol (103
) [33],cadina-1(2),4-diene (104
) [22],cadalene(105
) [22],epicubenol (106
) [22],cis
-calamenene(107
) [22],δ
-cadinol (108
) [22],1,1,4a-trimethyl-5,6-dimethylenedecahydronaphthalene(109
) [33],1-oxaspiro[2.5]octane,5,5-4-(3-methyl-1,3-butadienyl) (110
) [33],β
-elemene (111
) [22],γ
-elemene (112
) [22],δ
-elemene (113
) [22],and (E
)-nerolidol (114
) [22].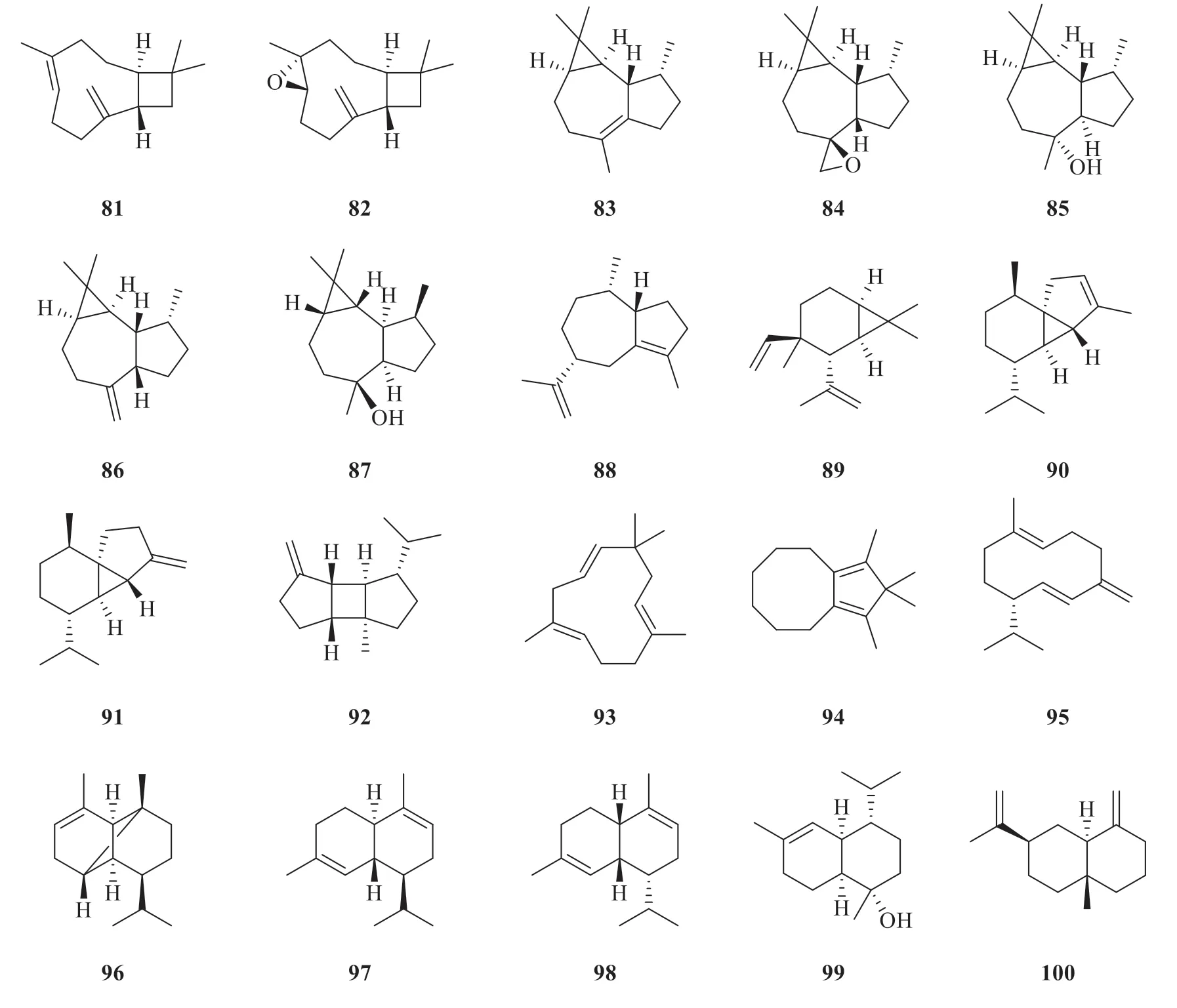
Fig.7 Chemical structures of sesquiterpenes in P.betle
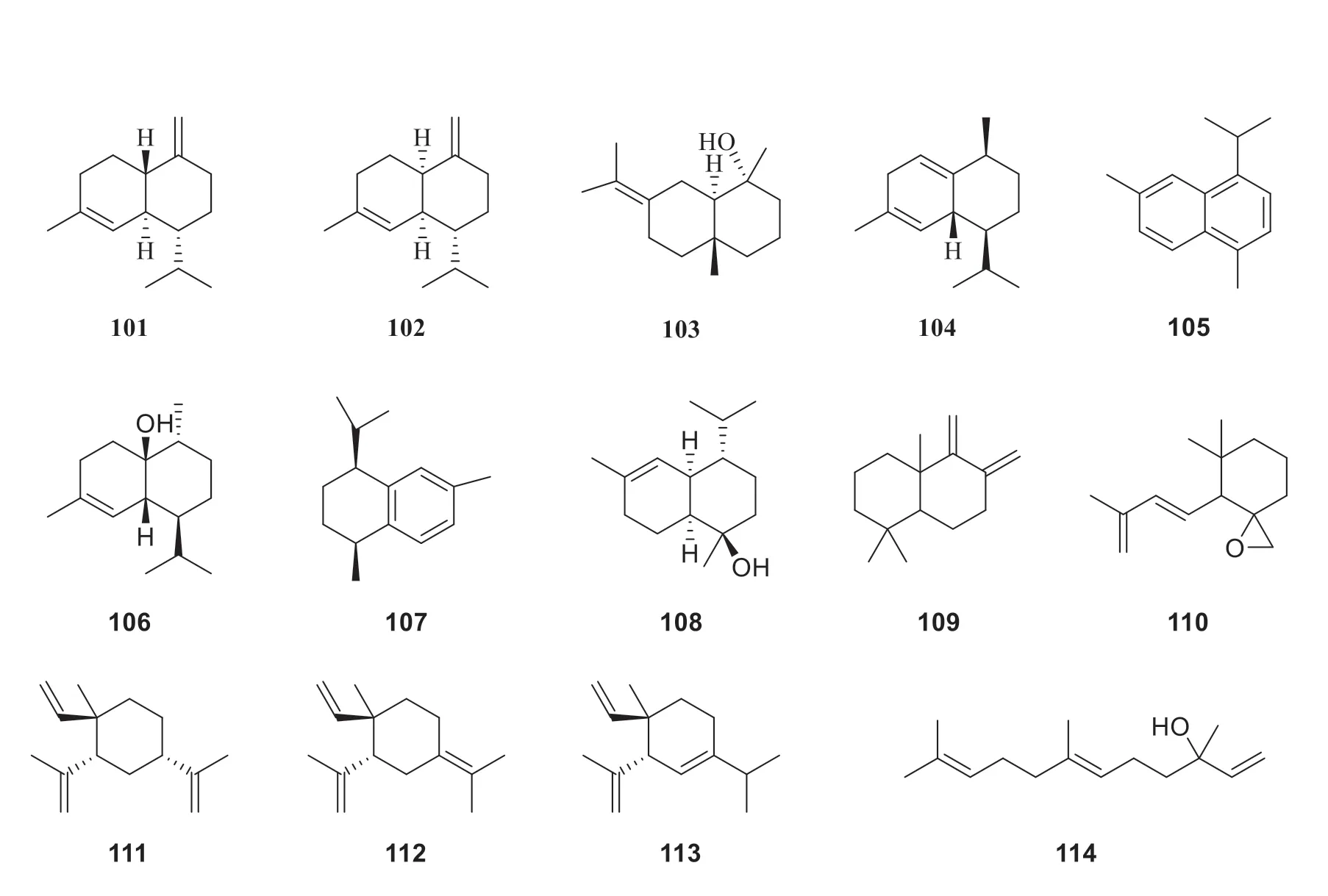
Continued fig.7
2.5.3 Monoterpenes
About twenty-seven monoterpenes have been isolated fromP.betle
(Fig.8).They areα
-pinene (115
) [21],β
-pinene (116
) [23],camphene(117
) [23],bornyl acetate (118
) [22],eucalyptol(119
) [21],5-caranol (120
) [33],borneol(121
) [22],α
-limonene (122
) [21],α
-phellandrene(123
) [20],α
-terpinene (124
) [22],β
-phellandrene(125
) [21],terpinolene (126
) [22],γ
-terpinene(127
) [23],p
-cymene (128
) [23],α
-terpineol(129
) [22],α
-thujene (130
) [23],sabinene(131
) [22],2-isopropenyl-5-methylcyclohexanol(132
) [33],terpinen-4-ol (133
) [22],terpinen-1-ol (134
) [23],nitrosothymol (135
) [33],cumene(136
) [21],linalool (137
) [21],cis
-geraniol(138
) [21],citral (139
) [21],β
-ocimene (140
) [23],andβ
-myrcene (141
) [23].
Fig.8 Chemical structures of monoterpenes in P.betle
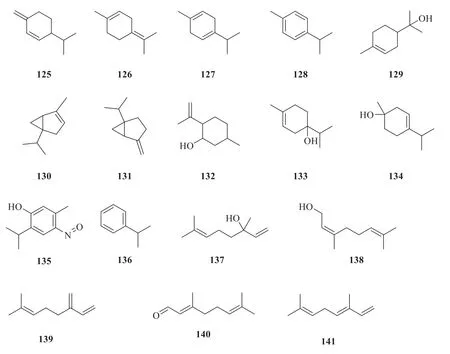
Continued fig.8
2.6 Other compounds
Except for the above-mentioned compounds derived fromP.betle
(Fig.9),there are also some other compounds.They areα
-ethylglucoside(142
) [32],3-cyclohexene-1-methanol (143
) [21],4-butylbenzyl alcohol (144
) [21],1-n
-dodecanyloxy resorcinol (145
) [10],gallic acid (146
) [25],syringic acid (147
) [25],1-naphthalenol(148
) [33],L
-ascorbic acid(149
) [25],decanal(150
) [23],1-decanol (151
) [23],dodecanal(152
) [23],14-methylpentadecanoic acid methyl ester (153
) [33],6,9-heptacosadiene (154
) [34],1-hexadecanol,2-methyl (155
) [34],methyl hexacos-7-enoate (156
) [34],6-hydroxytridecanoate(157
) [33],and pentatriacontanol (158
) [34].
Fig.9 Chemical structures of other compounds in P.betle
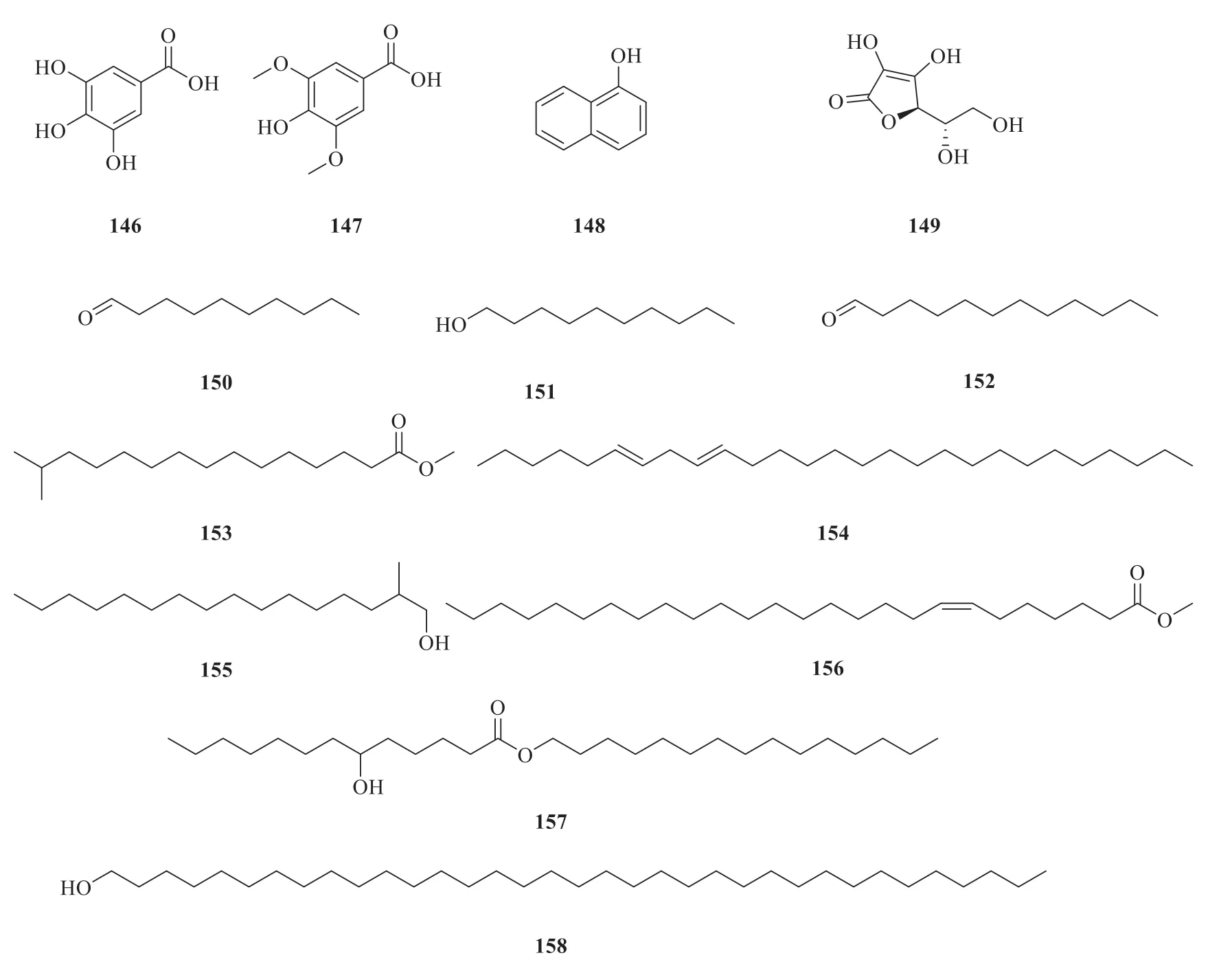
Continued fig.9
3 Research progress of pharmacological effects
3.1 Antioxidant activity
There is strong evidence that the extracts and compounds fromP.betle
possess antioxidant activity.Singh et al.evaluated the radical scavenging activity using 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay and the research showed that significant difference(P
<0.05) in the DPPH scavenging activity existed among the extracts of different solvent (methanol,ethanol,hydrous ethanol and water) at the concentration of 976.56µ
g/mL,of which the highest free radical scavenging was exhibited by methanol fraction (74.20 ± 0.92%) followed by positive control(72.68 ± 0.67%) [35].Atiya et al.found compounds20
and46
had antioxidant DPPH free radical scavenging activity with ICvalues of 4.61 and 4.12 mg/mL,while ascorbic acid as a standard antioxi dantdrug had the ICvalue of 3.42 mg/mL [28].Sarkar et al.found that allylpyrocatechol (30
) exhibited antioxidant properties by reducing phorbol-myristate-acetateinduced generation of reactive oxygen species and superoxide in murine peritoneal macrophages [36].3.2 Antitumor activities
Abrahim et al.found that the ethyl acetate extracts ofP.betle
leaves showed high inhibitory effect against the proliferation of MCF-7 cells(IC=65μ
g/mL) by increasing the activities of catalase and superoxide dismutase [37].Ng et al.demonstrated that 4-allylpyrocatechol (20
) andP.betle
crude extract significantly mitigated the 5-FU dosage and enhanced the cytotoxicity of the drug in killing colon cancer cells (HT 29 cells) [38].Two new phenylpropanoid analogues,bis-chavicol dodecanoyl ester (45
) and bishydroxychavicol dodecanoyl ester (47
),isolated from the leaves ofP.betle
by Atiya et al.[14],were tested for the cytotoxicity against two human oral cancer cell lines (AW13516 and AW8507).Results showed that they exhibited significant effect with the ICvalues of 19.61 and 23.01μ
g/mL for compound45
,and 10.25 and 13.12μ
g/mL for compound47
.Desmethylenesqualenyl deoxy-cepharadione-A(15
) was also isolated from the leaves ofP.betle
by Atiya et al.[10] and evaluation of cytotoxic activity against human hepatoma cell line (PLC-PRF-5)showed moderate effect with the ICvalues of 31.01μ
g/mL.3.3 Antibacterial activity
Garg et al.found that the essential oil from the leaves ofP.betle
showed high zone of inhibition against four keratinophilic fungi,namelyArthrodemuz benhamiae
(65 mm),Microspomm gypseum
(62 mm),Trichophyton mntagrophytes
(60 mm) andCtenomyces serratus
(69 mm) [39].Ramji et al.found that the decrease in volatile sulfur compounds (VSC) produced by the oral anaerobic bacteriaFusobacterium nucleatum
was primarily due to the anti-microbial activity of theP.betle
ether extracts and allylphenols [18].Nordin et al.investigated the effects ofP.betle
extract on the growth profile and the ultrastructure of commonly isolated oral candidal cells [40].The result showed that theμ
-values of the treated cells were significantly different from those of the untreated cells (P
<0.05) and the candida population was also significantly reduced from an average of 13.44 × 10to 1.78 × 10viable cell counts (CFU)/mL.3.4 Anti-inflammatory activity
Sarkar et al.found that allylpyrocatechol(20
) reduced dextran-mediated acute inflammatory response in rats [41].It reduced the edema by 74.19%,while the established anti-inflammatory drug caused 83.87% reduction in edema volume.Lin et al.tested thein vitro
anti-inflammatory effects of 2-(γ’
-hydroxychavicol)-hydroxychavicol(48
) [9].The result showed that compound48
exhibited inhibitory effects on the generation of superoxide anion and the release of elastase in human neutrophils (ICof 8.59 and 13.14μ
M/mL,respectively).3.5 Antimalarial activity
The methanolic leaf extract ofP.betle
was investigated for its antimalarial activity againstPlasmodium berghei
(NK65) during early and established infections by Aladhroey et al.[42].The result showed that the leaf extract demonstrated significant (P
<0.05) schizonticidal activity in all three antimalarial evaluation models and the chemosuppression exhibited by the highest dose of the extract (400 mg/kg) was comparable to that of the standard drug pyrimethamine,with chemosuppression of 73.04% in prophylactic antimalarial activity.3.6 Antidiabetic activity
Diabetes mellitus is a chronic metabolic disorder caused by an improper balance of glucose homeostasis,which has a significant impact on health,life quality and life expectancy of patients,as well as on the health care system [43].Oliveira et al.investigated the inhibitory effect of the extract on the activity ofα
-glucosidase enzyme by performing the antidiabetic activityin vitro
[44].Ethanol and aqueous extracts fromP.betle
leaves showed strong capacity of inhibitingα
-glucosidase in a concentration-dependent way,with the ICvalues of 0.069 and 0.257 mg/mL,respectively,the first being more effective.3.7 Other activities
Faizal et al.concluded that the ethanol extracts ofP.betle
leaves could reduce uric acid levels in hyperuricemic male white rats [45].The results showed that average uric acid level ofP.betle
leaves extract group decreased from 8.09 mg/dL (pretest) to 2.02 mg/dL (post-test).Zeng et al.found that piperol A (49
),piperbetol (50
),methylpiperbetol (51
) and piperol B (52
) could selectively inhibit the washed rabbit platelet aggregation induced by platelet activating factor (PAF) in a concentration-dependent manner [29].4 Conclusion
The scientific research onP.betle
shows that this plant has great biological potential.This study summarized the chemical constituents isolated fromP.betle
,including amide alkaloids,phenylpropanoids,steroids,terpenes and other compounds.Besides,this study also reviewed the previous reports on the pharmacological properties ofP.betle
,such as antioxidant,anti-tumor,antibacterial,anti-inflammatory and antimalarial activities.These attempts are aimed at providing crucial materials for further development and research.It is necessary to find new compounds with activity potential from this species using modern tools like chromatography,Nuclear Magnetic Resonance (NMR) and other functional genomics techniques.杂志排行
Asian Journal of Traditional Medicines的其它文章
- Network pharmacology-based analysis on three amicoumacintype isocoumarin compounds from an endophytic bacterium in Houttuynia cordata
- Compounds from the flowers and fruits of Abelmoschus esculentus (L.) Moench
- A network pharmacology-based study on the anti-hepatoma effect of Phellodendri Chinensis Cortex
- Research progress on chemical constituents in Sophora alopecuroides L.and their pharmacological activities
- Contribution Regulations for Asian Journal of Traditional Medicines
