Compounds from the flowers and fruits of Abelmoschus esculentus (L.) Moench
2021-11-05SihuiMiRuiGuoChuangXieXiaoxiaoHuang
Sihui Mi,Rui Guo,Chuang Xie,Xiaoxiao Huang
Key Laboratory of Computational Chemistry-Based Natural Antitumor Drug Research &Development,Liaoning Province,School of Traditional Chinese Materia Medica,Shenyang Pharmaceutical University,Shenyang 110016,China
Abstract The Abelmoschus esculentus (L.) Moench was previously reported to have various phytochemicals.Chemical fractionation yielded from the ethanolic extract of the A.esculentus ten compounds,including four alkaloids (4-6,8) and six phenolic acids (1-3,7,9-10).The structures of the compounds were elucidated through extensive spectra analyses and comparison of the experimental data with reported data.It is worth mentioning that compounds 1,2 and 5-10 were isolated from Abelmoschus esculentus (L.) for the first time.
Keywords:Abelmoschus esculentus (L.) Moench;phenolic acids;alkaloids
1 Introduction
Abelmoschus esculentuss
(L.) Moench belongs to Malvaceae family and is an annual herb of the Abelmoschus genus.The plant is native to Africa and has been planted in many countries around the world,mainly in tropical,sub-tropical,and warm temperate regions.In recent years,okra has also been widely cultivated in North and South China [1].It has become a part of the diet all over the world [2].Okra fruit has been paid increasing attention because of its health benefits [1].The edible part is rich in protein,unsaturated fatty acids,vitamins,and flavonoids [3,4].Previous investigations have revealed thatA.esculentuss
contains a wide variety of phytochemicals,including flavonoids,sterols,triterpenes,phenolic acids,amino acids,nucleotides and lipids [5,6].Besides,it also shows biological activity such as antioxidation,hypoglycemic,antiinflammatory and lipid-lowering effects [7,8].In order to search for the bioactive compounds inA.esculentuss
,we herein reported the isolation and identification of four alkaloids (4-6
,8
) and six phenolic acids (1-3
,7
,9-10
).It is important that compounds1
,2
and5-10
were isolated fromA.esculentus
for the first time.2 Materials and methods
2.1 Experimental instruments
NMR experiments were performed on Bruker ARX-400 and AV-600 spectrometers in CDCl(δ
7.26 andδ
77.2),with TMS as the internal standard.Chromatographic silica gel (200-300 mesh,Qingdao Marine Chemical Factory,Qingdao,China) and reversed phase C(RP-C) silica gel (50µ
m,Japan)were employed for column chromatography.RPHPLC separations were conducted using a Shimadzu LC-20AR series pumping system equipped with an SPD-20A UV detector,a YMC Pack ODS-A column(250 mm × 10 mm,5µ
m,YMC Company,Kyoto,Japan),TLC silica gel GF254 (Qingdao Marine Chemical Co.,China),Sephadex LH-20 (25-100µ
m,Green Herbs Science and Technology Development Co.,Ltd.China),and MCI resin (CHP20P,75-150 m,Mitsubish Chemical Corporation).2.2 Plant material
The plants ofA.esculentus
were collected in 2017 in Bozhou,Anhui and identified asA.esculentuss
dried flowers and fruits by professor Jincai Lu from the school of traditional Chinese medicine of Shenyang Pharmaceutical University.2.3 Extraction and isolation
The dried flowers and fruits ofA.esculentuss
(30 kg) were extracted with 70% EtOH three times(2 h for each time).The resulting solutions were filtered,combined,and concentrated under reduced pressure to obtain methanol residue (1200 g).The residue was suspended in water and partitioned successively with petroleum ether,ethyl acetate andn
-butanol to give the corresponding extracts:petroleum ether (40 g),ethyl acetate (360 g)andn
-butyl alcohol layer (220 g).The extract ethyl acetate (360 g) was separated by silica gel CC,using the gradient elution of CHCl-MeOH (50:1-1:1) to obtain three fractions A1-A3.Fraction A1 was further separated by MCI,using gradient elution of MeOH-HO (90:10-100:0) to obtain three fractions A1A-A1C.Fractions A2 and A3 were further separated by ODS,using gradient elution of MeOH-HO (30:70-100:0) to obtain five fractions A2A-A2B and A3A-A3C.The above subfractions were further separated by silica gel CC,preparative HPLC and semipreparative HPLC.Compounds1
(3.9 mg)and
9
(16.0 mg) wereobtained from fraction A2A.Compound
2
(50.0 mg) was obtained from fraction A3C.Subfraction A2B was separated to give compounds3
(24.0 mg) and6
(40.0 mg).Compounds4
(38.0 mg),5
(69.0 mg) and7
(42.0 mg) were purified from fraction A1C.Compound8
(9.1 mg) was obtained from fraction A3B.Compound10
(3.5 mg) was obtained from fraction A1B.
N
-trans
-coumaroyl tyramine (4
):white powder(MeOH);H-NMR (400 MHz,DMSO-d
):δ
7.38(2H,d,H-2/6),δ
6.79 (2H,d,H-3/5),δ
7.32 (1H,d,H-7),δ
6.40 (1H,H-8),δ
7.01 (2H,d,H-2′/6′),δ
6.67(2H,d,H-3′/5′),δ
2.64 (2H,t,H-7′),δ
3.33 (2H,dd,H-8′),δ
8.03 (1H,t,N-H),δ
9.53 (2H,brs,OH-4/4′).N
-trans
-Feruloyltyramine (5
):pale yellow solid(MeOH);H-NMR (400 MHz,DMSO-d
):δ
7.12(1H,s,H-2),δ
6.80 (1H,d,J
=8.0 Hz,H-5),δ
6.98(1H,s,H-6),δ
7.34 (1H,d,J
=15.7 Hz,H-7),δ
6.46(1H,d,J
=15.7 Hz,H-8),δ
7.02 (2H,d,J
=8.3 Hz,H-2′/6′),δ
6.70 (2H,d,J
=8.3 Hz,H-3′/5′),δ
2.66(2H,t,J
=7.9 Hz,H-7′),δ
3.35 (2H,dd,J
=12.7,6.5 Hz,H-8′),δ
8.02 (1H,s,N-H),δ
9.30 (1H,brs,OH),δ
3.80 (3H,s,OCH);C-NMR (100 MHz,DMSO-d
):δ
129.6 (C-1),δ
110.8 (C-2),δ
147.9(C-3),δ
148.4 (C-4),δ
115.7 (C-5),δ
121.6 (C-6),δ
139.0 (C-7),δ
119.0 (C-8),δ
165.5 (C-9),δ
126.4(C-1′),δ
129.5 (C-2′/6′),δ
115.2 (C-3′/5′),δ
155.7(C-4′),δ
34.5 (C-7′),δ
40.8 (C-8′),δ
55.6 (OCH).Perlolyrine (6
):yellow acicular crystal(MeOH);H-NMR (400 MHz,DMSO-d
):δ
8.37(1H,d,J
=5.1 Hz,H-3),δ
8.06 (1H,d,J
=5.1 Hz,H-4),δ
8.26 (1H,d,J
=7.8 Hz,H-5),δ
7.28 (1H,t,J
=7.8 Hz,H-6),δ
7.59 (1H,t,J
=7.6 Hz,H-7),δ
7.78 (1H,d,J
=7.6 Hz,H-8),δ
3.69 (2H,m,H-9),δ
7.21 (1H,d,J
=3.2 Hz,H-3′),δ
6.58 (1H,d,J
=3.2 Hz,H-4′),δ
4.67 (2H,s,CH),δ
5.48 (1H,t,OH),δ
11.26 (1H,brs,NH);C-NMR (100 MHz,DMSO-d
):δ
133.2 (C-1),δ
138.2 (C-3),δ
113.6 (C-4),δ
129.4 (C-4a),δ
120.6 (C-4b),δ
121.6 (C-5),δ
119.7 (C-6),δ
128.3 (C-7),δ
112.5 (C-8),δ
140.9(C-9),δ
130.5 (C-9a),δ
129.9 (C-1′),δ
156.8 (C-2′),δ
109.6 (C-3′),δ
109.0 (C-4′),δ
152.2 (C-5′),δ
55.9(CH).
H
-indole-3-carboxaldehyde (8
):light yellow powder (MeOH);H-NMR (400 MHz,CDCl):δ
8.96 (1H,brs,H-1),
δ
7.86 (1H,d,
J
=2.9 Hz,H-2),δ
8.33 (1H,m,H-4),
δ
7.31 (1H,d,
J
=5.5 Hz,H-5),δ
7.35 (1H,d,
J
=5.5 Hz,H-6),δ
7.45 (1H,m,H-7),
δ
10.07 (1H,s,CHO).2-hydroxyphenylacetic acid methyl ester (
9
):white powder (MeOH);H-NMR (400 MHz,CDCl):δ
7.39 (1H,d,J
=7.6 Hz,H-3),δ
7.29 (1H,t,J
=7.6 Hz,H-4),δ
7.07 (1H,t,J
=7.6 Hz,H-5),δ
6.88 (1H,t,J
=7.6 Hz,H-6),δ
7.79 (1H,brs,OH),δ
3.70 (3H,s,CH).P
-methoxy benzoic acid (10
):white needle crystal (MeOH);H-NMR (400 MHz,CDCl):δ
7.95(2H,d,J
=8.5 Hz,H-2/6),δ
6.88 (2H,d,J
=8.5 Hz,H-3/5),δ
3.89 (3H,s,OCH).3 Results and discussion
Structural elucidation of all isolated compounds fromA.esculentuss
(Fig.1) was performed by comparing their spectral data with literature values.Among these compounds,compounds1
,2
and5-10
were reported as the first time isolated from the plant.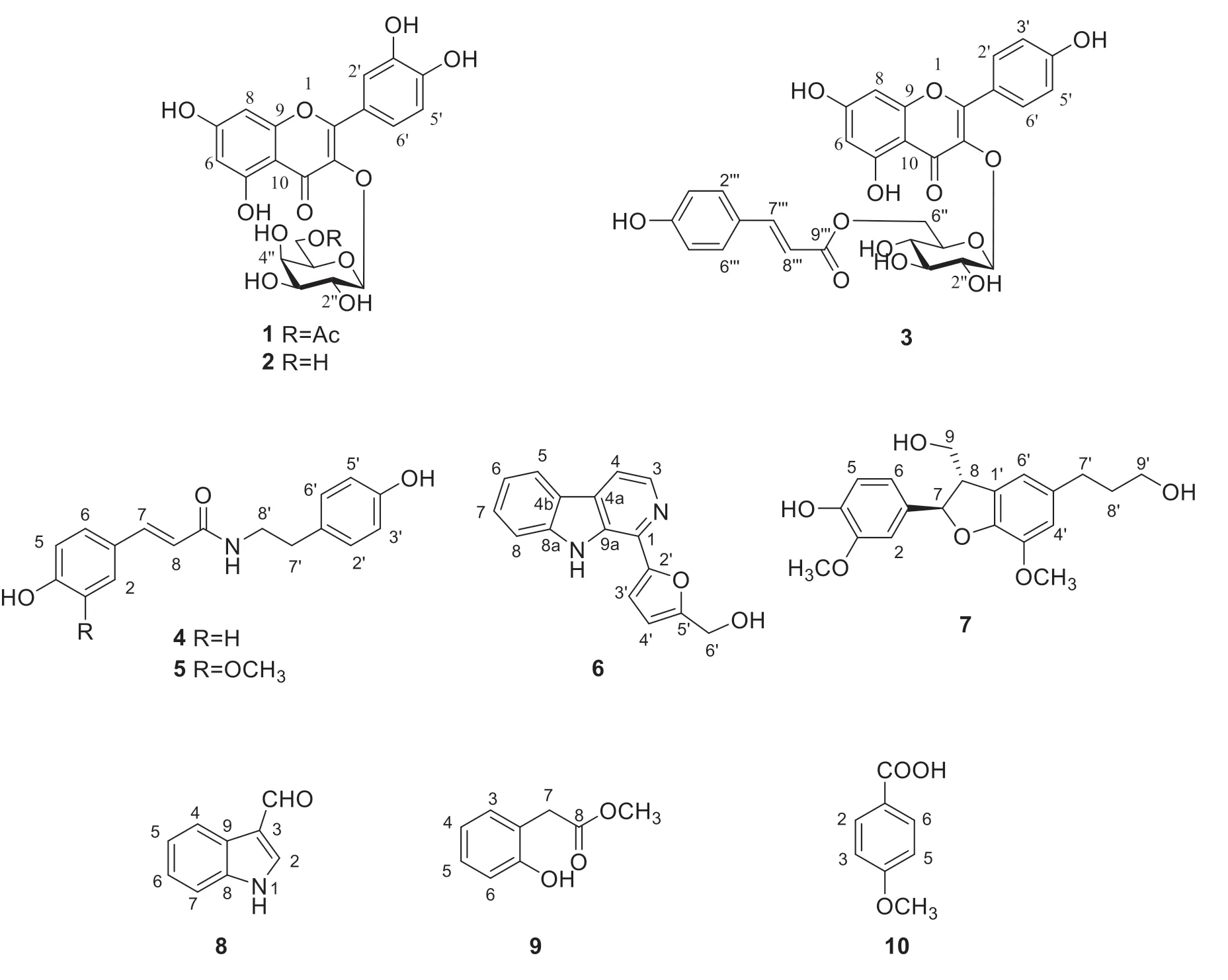
Fig.1 Chemical structures of compounds 1-10
Compound1
was isolated as a pale yellow acicular crystal.TheH-NMR spectrum showed the signals of three ABX systems protons atδ
7.63 (dd,J
=8.5,2.0 Hz,H-6′),6.81 (d,J
=8.5 Hz,H-5′) and 7.51 (d,J
=2.0 Hz,H-2′) on B-ring,two aromatic proton signals atδ
6.18 (s,H-6)and 6.39 (s,H-8) on A-ring protons,together with four phenolic hydroxyl protons atδ
12.61 (s),10.88 (s),9.76 (s) and 9.17 (s).From the above data,it can be easily deduced that there is a flavone skeleton in the molecule.In addition,an anomeric proton signal was found at
δ
5.31 (d,
J
=7.8 Hz,H-1′′) and four proton signals atδ
3.38-4.07.An anomeric carbon signal of
β
-D-glucose was observed atδ
101.8 (C-1′′).TheH-NMR also showed one methyl proton signal at
δ
1.73 (s).TheC-NMR spectrum showed 23 carbon signals.Six of the carbon signals were attributed to a glucose unit,while the remaining carbon signals included two benzene rings,two carbonyl carbon signals and one methyl carbon signal.The structure was confirmed based on analysis ofH andC-NMR data and the comparison of the experimental spectroscopic data with those in the literature [9].Therefore,the structure of compound
1
was identified as quercetin-3-O
-(6’’-O
-acetyl)-β
-D-galactranoside.TheH andC-NMR spectrums of compound2
displayed similar signals to compound1
.According to the comparison of NMR data,they had the same structure skeleton.The sugar moiety of compound
2
showed one hydroxyl proton signal atδ
5.12-4.43(m),the absence of signal of acetyl and the presence of a rhamnose.Based on the above results and the comparison with the data in the literature [10],compound
2
was identified as hyperin.TheH-NMR spectrum of compound3
showed four AA′BB′ system protons atδ
7.99 (d,
J
=8.8 Hz,H-2′,H-6′),6.85 (d,
J
=8.8 Hz,H-2′,H-6′),7.36(d,
J
=8.5 Hz,H-2′′,6′′),and 6.79 (d,J
=8.5 Hz,H-3′′,5′′),and two olefinic protons atδ
7.35 (1H,d,
J
=13.9 Hz,H-7′′′) and 6.10 (1H,d,J
=13.9 Hz,H-8′′′).In addition,an anomeric proton atδ
5.45(d,
J
=7.3 Hz,H-1′′) and four signals atδ
3.17-3.37 (m) indicated the presence ofβ
-glucose.The key to elucidating the structure of this compound is the two proton signals on glucose 6′′ atδ
4.28 (d,J
=10.5 Hz,H-6′′) and 4.03 (m,H-6′′).TheH-NMR also showed two typical hydroxyl protons of flavonoid atδ
12.57 (s) and 10.35 (brs).TheC-NMR spectrum showed 30 carbon resonances consisting of two carbonyl carbons and three benzene rings.Based on the above data and the comparison with the data in the literature [11],compound3
was identified as kaempferol-3-O
-[6’’-O
-(trans
-p
-coumaroyl)]-β
-D-glucopyranoside.TheH-NMR spectrum of compound4
showed two olefinic protons atδ
7.32 (d,
J
=15.7 Hz,H-7)and 6.40 (d,J
=15.7 Hz,H-8),four AA’BB’ system protons atδ
7.38 (d,
J
=8.5 Hz,H-2,6),6.79 (d,J
=8.5 Hz,H-3,5) andδ
7.01 (d,
J
=8.3 Hz,H-2’,6’),6.69 (d,J
=8.3 Hz,H-3’,5’),one proton signal atδ
8.03 (t,
J
=5.3 Hz,N-H) for NH and an active hydrogen proton signal atδ
9.53 (brs,OH).At the high field,two proton signals appeared at
δ
2.64(t,
J
=7.3 Hz,H-7’) and 3.33 (t,J
=7.3 Hz,H-8’).Based on the above data and the comparison with the data in the literature [12],compound4
was identified asN
-trans
-coumaroyl tyramine.Compound5
was obtained as a yellowish solid.Analysis of its NMR data showed that it was highly similar to compound4
except for one more methoxyl group.TheH-NMR data of5
revealed three ABX system protons atδ
6.98 (s,H-6),6.80 (d,
J
=8.0 Hz,H-5) and 7.12 (s,H-2) in contrast with four AA’BB’ system protons of compound4
.TheC-NMR data showed 18 carbon signals,including one carbon of the amide group,two olefinic carbons and 12 carbons of aromatic rings.Based on the above results and the comparison with data in the literature [12],compound5
was identified asN
-trans
-Feruloyltyramine.TheH-NMR spectrum of compound6
showed an aryl hydroxyl methyl proton atδ
4.67 (s,H-6),four aromatic protons at
δ
8.26 (d,
J
=7.8 Hz,H-5),7.28 (t,
J
=7.8 Hz,H-6),7.59 (d,
J
=7.6 Hz,H-7) and7.78 (d,
J
=7.6 Hz,H-8),two olefinic protons at 8.37 (d,J
=5.1 Hz,H-3),and8.06 (d,
J
=5.1 Hz,H-4),and one signal for NH atδ
11.26(brs,N-H).The signals at
δ
7.21 (d,
J
=3.2 Hz,H-3′) and 6.58 (d,J
=3.2 Hz,H-4′) belonged to H-3′and H-4′ of the furan ring.TheC-NMR spectrum showed 16 carbon signals assigned to 5 quaternary carbons,8 methines,and 3 methylenes.Among the 16 carbon signals,there were 11 aromatic or olefinic carbons (δ
140.9,138.2,133.2,130.5,129.4,128.3,121.6,120.6,119.7,113.6,112.5),two oxygenated methines (δ
152.2,156.8) and an oxygenated methylene (
δ
55.9).Based on the detailed comparison of the NMR data of compound
6
with those in the literature [13],compound6
was identified as perlolyrine.TheH-NMR spectrum of compound7
showed the existence of three aromatic proton signals atδ
6.91 (s,H-2),6.76 (s,H-5) and 6.69 (d,J
=4.4 Hz,H-4′),two methylene protons atδ
2.54 (t,
J
=3.5 Hz,H-7′) and 1.68 (m,H-8′),an oxygenated methylene at 3.42 (t,J
=6.4 Hz,H-9′),and two methoxy protons atδ
3.77 (s) and 3.74 (s).There were also typical proton signals atδ
5.41 (d,
J
=10.6 Hz,H-7),3.61 (dd,J
=10.6,6.8 Hz,H-8)and 3.69 (m,H-9),accounting for the CH(O)-CH-CH(O) unit.All the assignments suggested compound7
might be a dihydrobenzofuran-type neolignan.Based on the above results and the comparison with the data in the literature [14],compound7
was identified as (−)-7R
,8S
dihydrodehydro-coniferyl alcohol.TheH-NMR spectrum of compound8
showed one aldehyde proton atδ
10.07 (s),a signal for NH at
δ
8.96 (brs,N-H),and four aromatic protons at
δ
8.33 (m,H-4),7.31 (m,H-5),7.35 (m,H-6),and 7.45 (m,H-7).Based on the above results and the comparison with the data in the literature [15],compound
8
was identified as 1H
-indole-3-carboxaldehyde.TheH-NMR spectrum of compound9
indicated the presence of one phenolic hydroxyl proton signal atδ
7.79 (brs),four aromatic protons at
δ
7.39 (d,
J
=7.6 Hz,H-3),7.29 (t,
J
=7.6 Hz,H-4),7.07 (t,J
=7.6 Hz,H-5) and 6.88 (d,J
=7.6 Hz,H-6),one methoxy proton atδ
3.70 (s,OCH),and one methylene proton signal at
δ
2.95 (s,H-7).Based on these data and the comparison with the data in the literature [16],compound9
was determined to be 2-hydroxyphenylacetic acid methyl ester.A detailed comparison of the NMR data of compound10
with the data in the literature revealed that compound10
wasp
-methoxy benzoic acid [17].TheH-NMR spectrum of compound10
showed a set of AA’BB’ system protons atδ
7.95 (d,
J
=8.5 Hz,H-2,6) and 6.88 (d,J
=8.5 Hz,H-3,5),and a methoxyl group atδ
3.89 (s,6’-OCH).Therefore,the structure of compound
10
was determined.4 Conclusion
Ten compounds(1-10)
were isolated and elucidated from theAbelmoschus esculentuss
.It is noteworthy that compounds1
,2
and5-10
were reported for the first time inthis plant and even in its family.Our findings might provide a valuable information source for further study on the biological activity of this plant.The present study also indicated that
A.esculentuss
might have broad prospects in the study of natural products.杂志排行
Asian Journal of Traditional Medicines的其它文章
- Network pharmacology-based analysis on three amicoumacintype isocoumarin compounds from an endophytic bacterium in Houttuynia cordata
- A network pharmacology-based study on the anti-hepatoma effect of Phellodendri Chinensis Cortex
- Research progress on the main chemical constituents and pharmacological effects of Piper betle L.
- Research progress on chemical constituents in Sophora alopecuroides L.and their pharmacological activities
- Contribution Regulations for Asian Journal of Traditional Medicines
