番茄SlN-like的克隆、表达与抗病毒功能
2021-10-29刘昌云李欣羽田绍锐王靖裴悦宏马小舟樊光进汪代斌孙现超
刘昌云,李欣羽,田绍锐,王靖,裴悦宏,马小舟,2,樊光进,汪代斌,孙现超
番茄的克隆、表达与抗病毒功能
刘昌云1,李欣羽1,田绍锐1,王靖1,裴悦宏1,马小舟1,2,樊光进1,汪代斌3*,孙现超1*
1西南大学植物保护学院,重庆 400715;2西南大学园艺园林学院南方山地园艺学教育部重点实验室,重庆 400715;3重庆烟草科学研究所,重庆400715
【目的】番茄()作为重要的蔬菜作物,其生长受到包括害虫、真菌、细菌和病毒等各种生物因素的危害。明确番茄抗性基因的抗病毒功能与机制,为番茄的抗病毒育种与抗病毒药剂的靶向开发提供理论依据。【方法】从茄科植物基因组数据库Solanaceae Genomics Network中获得的全长,并将其分为4段,利用融合聚合酶链式反应(fusion PCR)扩增获得的序列全长;通过生物信息学分析SlN-like的进化关系、蛋白特征、保守结构域、亚细胞定位以及互作关系;通过实时荧光定量PCR分析在番茄根、茎、叶、花和果实中的表达情况及其在烟草花叶病毒(tobacco mosaic virus,TMV)侵染后的叶片表达量;借助烟草脆裂病毒(tobacco rattle virus,TRV)介导的基因沉默技术(virus induced gene silencing,VIGS)沉默番茄内源,摩擦接种TMV-GFP于沉默植株,明确对病毒侵染的影响。实时荧光定量PCR分析沉默植株中脱落酸(abscisic acid)、茉莉酸(jasmonic acid)和乙烯(ethylene)激素相关基因的表达量及在外施乙烯利(ethephon,ETH)3、6、12、24 h后的表达情况,最终明确SlN-like调控激素途径响应病毒侵染的机制。【结果】通过分子克隆与融合PCR技术,从番茄品种Micro-Tom中克隆获得全长3 444 bp的,上传至NCBI获得序列号MW792493。通过生物信息学分析发现SlN-like含有TIR、NB-ARC和NACHT结构域,并与马铃薯()N-like(AAP44394.1)亲缘关系最近。在番茄各组织中均有表达,在茎中的表达量最高,其次是根、花,叶和果实中的表达量最低。TMV-GFP侵染番茄后第5、7天的表达显著高于PBS处理,分别是PBS处理的1.6和2.2倍,并且TMV-GFP侵染会使的表达持续升高。TRV载体介导沉默番茄的,发现沉默78.3%的不会影响番茄生长表型,但可促进TMV-GFP侵染;实时荧光定量PCR分析发现沉默植株中的表达显著降低,仅为对照组的12.5%;外施乙烯利处理番茄3 h后表达量升高,并在12 h达到最高峰,是对照组的2.71倍,24 h后恢复正常。【结论】番茄SlN-like属NBS-LRR类抗病蛋白,其表达受TMV侵染诱导,沉默促进TMV-GFP侵染,降低乙烯相关基因的表达,而外施乙烯利导致的差异表达,揭示了SlN-like作为正调控因子可能影响乙烯途径介导的番茄抗病毒防御。
番茄;SlN-like;烟草花叶病毒;基因表达;乙烯
0 引言
【研究意义】番茄()是茄科番茄属的一种一年生或多年生草本植物,在农业种植方面有着重要的地位。生产过程中,番茄会遭受高温、干旱等非生物胁迫或病毒、真菌等生物胁迫,造成生物组织的损伤,进而引起减产。其中由烟草花叶病毒(tobacco mosaic virus,TMV)引起的番茄病毒病是番茄生产过程的重要病害之一。挖掘并研究番茄抗病毒基因及其抗病毒机制,对番茄抗病毒育种及利用药剂诱导调控抗病毒基因防控病毒病具有重要意义。【前人研究进展】是最早发现的抗TMV基因,烟草可对包括TMV、番茄花叶病毒(tomato mosaic virus,ToMV)在内的绝大多数烟草花叶病毒组成员产生抗性,属TIR-NBS-LRR类植物抗性基因家族中的一员[1-2]。早期研究发现,TMV复制酶126 kD能够引起介导的超敏反应(hypersensitivity,HR)[3],而位于复制酶126 kD羧基端末端的约50 kD的解旋酶片段(p50)能够导致转录产物的积累,并有效地引起介导的HR反应[4-5],因此编码p50的核苷酸序列又被称为对应的无毒基因(avirulence,)。TMV侵染引起心叶烟()、三生烟(var.Samsun NN)等抗病品种坏死斑的形成是植物体自身防御病原微生物侵入所形成的细胞程序性死亡(programmed cell death,PCD)[6]。目前介导的TMV识别过程及其对下游抗病基因的诱导已经研究得非常透彻[7]。NRIP1(N receptor- interacting protein 1)是一种同时与N蛋白的TIR结构域和p50产生相互作用的硫氰酸酶(硫转移酶),NRIP1定位于叶绿体中。SPL6(squamosa promoter binding protein-like 6)是一种与N蛋白在核小体内互作的转录因子。在TMV未侵染的细胞中,N蛋白处于活性被抑制的状态,其表达极低,并且分布在细胞核和细胞质中[8]。此时核内的N蛋白不与SPL6结合。当TMV通过机械损伤进入植物体内后,病毒在细胞质中脱壳、复制、转录和翻译,p50激发子引起了NRIP1的重新定位。NRIP1与p50结合后,被细胞质中的N蛋白识别,三者形成NRIP1-p50-N复合体[9]。随着三者的结合,p50引起了N蛋白构象上的变化,而该变化可能需要ATP结合或水解。结合ATP的N蛋白进入细胞核,与SPL6互作进而引起SPL6的转录激活功能,激活了下游抗性基因的表达[10]。此外,NRIP1-p50-N形成复合体的同时,N蛋白利用其LRR结构域与p50形成次级结合,释放出TIR-NBS区段以提高核酸结合能力,促成N蛋白的聚合。聚合后的N蛋白进入核内,与SPL6结合,激活并引起下游抗性基因的表达[11]。【本研究切入点】是的同源基因,同样含有TIR-NBS-LRR结构域,其TIR-NBS保守结构域区段同样可与TMV p50产生HR反应,而LRR并不能和p50产生HR反应[12],因此推测这可能与N-like与TMV的识别有关[13],并且可能行驶的第一种调控模式。但番茄中如何响应病毒侵染,如何调控寄主免疫防御知之甚少[14-15]。【拟解决的关键问题】通过克隆番茄抗性基因的cDNA全长,并对核酸序列和蛋白质序列进行生物信息学分析,利用实时荧光定量PCR技术明确的表达模式,通过病毒介导的基因沉默(virus-induced gene silencing,VIGS)分析对TMV-GFP侵染的影响,确定SlN-like的抗病功能,并明确SlN-like可能影响的激素通路,为解析SlN-like在番茄抗病应答中的调控机制提供科学依据,同时为番茄抗病品种的选育和抗病毒药剂的靶向开发提供理论依据。
1 材料与方法
试验于2020—2021年在西南大学植物保护学院植物免疫与植物病害生态防控实验室完成。
1.1 试验材料
1.1.1 供试菌株与植物 供试大肠杆菌()DH5和农杆菌()GV3101购自上海唯地生物技术有限公司;VIGS沉默载体和烟草花叶病毒荧光标记载体TMV-GFP(pSDK661)由清华大学刘玉乐教授课题组馈赠;供试番茄Micro-Tom品种在恒温培养室中播种,培养至4—6叶期左右备用。
1.1.2 试剂和引物 DNA纯化回收试剂盒和质粒提取试剂盒购自擎科兴业生物技术有限公司;总RNA提取剂、反转录试剂盒、高保真酶PrimeSTAR® GXL DNA Polymerase DNA聚合酶、T4 DNA连接酶购自大连TaKaRa公司;实时荧光定量试剂盒购自莫纳生物。引物和测序由上海生工生物工程公司完成。
1.2 SlN-like的克隆与分析
番茄Micro-Tom总RNA提取按照TaKaRa RNAisoPlus(Total RNA 提取试剂)试剂盒手册进行。根据茄科植物基因组数据库Solanaceae Genomics Network[16](https://solgenomics.net/)中预测到的番茄序列,将其分为4段分段扩增,引物详见表1。以Micro-Tom番茄的cDNA为模板,扩增获得4段PCR片段,并利用融合PCR将4段片段融合。将序列上传至NCBI,获得序列号。运用ExPASy ProtParam(https://web.expasy.org/protparam/)分析SlN-like的蛋白理化性质,TMHMM 2.0(http://www.cbs.dtu.dk/ services/)分析SlN-like的跨膜区域,Cell-PLoc 2.0(http://www.csbio.sjtu.edu.cn/bioinf/Cell-PLoc-2/)预测SlN-like的亚细胞定位[17],STRING(https://string- db.org/)分析SlN-like的互作网络,SMART(http:// smart.embl-heidelberg.de/)分析SlN-like的保守结构域,NCBI Blastp比对出与番茄SlN-like同源的N-like蛋白,利用MEGA X分析并制茄科植物N-like系统进化树。

表1 本研究所用到的引物
下划线的碱基为所加酶切位点 The underlined bases were the restriction enzyme sites
1.3 TMV接种
TMV接种方法详见文献[18]。将0.1 g带有TMV-GFP的鲜样叶片放置研钵中,加入石英砂,并使用pH 7.2—7.4的磷酸缓冲液研磨至匀浆状。获得的匀浆在5 000×离心机中离心3 min,取上清进行摩擦接种。每片叶片接种100 μL,每株植株接种两片叶。
1.4 SlN-like沉默载体的构建及浸润
沉默载体的构建基于烟草脆裂病毒(tobacco rattle virus,TRV)改造的沉默体系。首先根据的全长,利用Solanaceae Genomics Network的VIGS Tool(https://vigs.solgenomics.net/)分析获得的最佳沉默片段,设计含有H I和I的沉默片段引物,将其连接在同样被H I和I双酶切的pTRV2载体上,构建pTRV2:SlN-like载体。以空载体pTRV2为对照,将pTRV1和pTRV2:SlN-like转化至农杆菌GV3101中,过夜培养至OD600=0.6,等比混合后接种六叶期番茄。14 d后利用实时荧光定量PCR检测沉默效率。
1.5 实时荧光定量PCR
实时荧光定量PCR利用qTOWER2.0 real-time PCR(Analytikjena,Germany)和MonAmpTMChemoHS qPCR Mix(Monad,China)分析靶基因的相对表达水平。使用Primer3web(https://bioinfo.ut.ee/primer3/)软件根据每个基因的编码序列设计基因特异性引物。选择番茄作为内参,使用2-ΔΔCt法定量计算基因转录水平的相对变化[19-20]。
1.6 外施乙烯利
六叶期番茄喷施100 mmol·L-1的乙烯利(Aladdin),以喷施无菌水为对照,并在3、6、12、24 h取样,保存至-80℃冰箱用于下步试验。
1.7 数据处理与分析
所有试验和数据至少3个重复,数据表示为平均值±标准误(SE),统计分析采用SPSS软件student’s检验(*0.01<<0.05,**0.001<<0.01,***<0.001)和ANOVA单因素分析(LSD检验,<0.05)。
2 结果
2.1 SlN-like的克隆与分析
首先根据本氏烟TMV,在茄科植物基因组数据库Sol Network中比对出番茄的可能序列。根据序列结构将预测的番茄核苷酸分为4段,命名为P1—P4,长度分别为493、1 132、1 357和529 bp。以番茄Micro-Tom品种cDNA为模板,分别扩增4段片段,长度和预测相符,总长度3 444 bp,命名为。上传至NCBI,获得序列号MW792493。编码蛋白共1 147个氨基酸,ProtParam预测结果显示其理论分子量为130.48 kD,理论等电点为8.74,分子式为C5866H9357N1575O1671S56。TMHMM 2.0分析结果显示SlN-like不具有跨膜结构。Cell-PLoc 2.0亚细胞定位预测结果表明SlN-like可能定位于细胞质与细胞膜。STRING互作蛋白预测显示SlN-like可能与线粒体小核糖体亚基蛋白(mitochondrial small ribosomal subunit protein,Solyc01g081520.2.1)、半胱氨酸蛋白酶抑制剂(cysteine proteinase inhibitor,Solyc09g097850.1.1)、五肽重复PPR超家族蛋白(pentatricopeptide repeat superfamily protein,Solyc05g047540.2.1)和EDS1(enhanced disease susceptibility 1,Solyc06g071280.2.1)等蛋白存在互作。SMART预测SlN-like保守结构域表明SlN-like含有TIR、NB-ARC和NACHT保守结构域,为NBS-LRR类抗病蛋白。
根据SlN-like的氨基酸序列,在NCBI对比茄科植物中的SlN-like同源蛋白。下载茄科植物中10个物种的N-like氨基酸序列,利用MEGA X构建进化树。由图1可知,SlN-like与马铃薯()N-like(AAP44394.1)的氨基酸亲缘关系最近,与野生烟草()N-like(AKN63563.1)和中华辣椒()N-like(PHU14921.1)的亲缘关系较远。
2.2 SlN-like的组织表达及TMV侵染表达
利用实时荧光定量PCR检测在根、茎、叶、花和果实各组织中的表达量。结果显示,在茎中的表达量最高,其次是花和根,在叶和果实中的表达量最低(图2-A),表明表达具有组织差异性。为进一步明确在TMV侵染番茄过程中的表达,以PBS为对照,摩擦接种TMV-GFP,并于接种后第3、5、7天取样,利用实时荧光定量PCR检测TMV-GFP侵染番茄Micro-Tom后的表达量。结果显示,TMV-GFP侵染后3 d,处理组中的表达是对照组的1.4倍,但差异不显著。TMV-GFP侵染后5、7 d,处理组中的表达显著高于对照,分别为是对照组的1.6、2.2倍。TMV-GFP侵染后呈现表达逐渐上升趋势(图2-B),说明TMV-GFP侵染会诱导的表达,揭示SlN-like可能参与番茄抗TMV响应。
2.3 沉默SlN-like对TMV-GFP侵染的影响
为进一步明确对TMV-GFP侵染的影响,利用TRV介导的基因沉默技术沉默番茄内源。以TRV:00为对照,沉默14 d后,发现沉默植株与对照植株相比并无显著的表型差异(图3-A),说明沉默不会影响番茄生长;沉默效率检测表明,TRV:SlN-like植株中的表达量仅为对照的21.7%(图3-B),说明成功沉默。

图1 SlN-like及其同源基因系统发育分析

A:SlN-like的组织表达,统计分析采用ANOVA(LSD检测,P<0.05)Tissue expression of SlN-like, the statistical analyses were performed using One-way ANOVA (LSD’s test, P<0.05);B:TMV侵染后SlN-like的表达。统计分析采用Student’s t检验(**0.001<P<0.01,***P<0.001),每个处理进行3次生物学重复,每次生物学重复3株番茄,数值代表3次生物学重复的平均值±标准误SlN-like expression after TMV infection.The statistical analyses were performed using Student’s t-test (**0.001<P<0.01, ***P<0.001).The experiments were repeated three times with three plants each time.Values represent means±SE from three biological replications

A:SlN-like沉默后的番茄表型Tomato phenotype after SlN-like silenced;B:TRV:SlN-like的沉默效率检测。统计分析采用Student’s t检验 (***P<0.001),每个处理进行3次生物学重复,每次生物学重复10株番茄,数值代表3次生物学重复的平均值±标准误silencing efficiency detection in TRV:SlN-like.The statistical analyses were performed using Student’s t-test (***P<0.001).The experiments were repeated three times with ten plants each time.Values represent means±SE from three biological replications
对成功沉默的植株进行攻毒试验。接种TMV-GFP于沉默植株TRV:SlN-like和对照组TRV:00,于接种后第3、5、7天在手持紫外灯下观察TMV-GFP的荧光斑点数(图4-A),并取样利用实时荧光定量PCR对TMV进行定量分析,明确沉默后TMV-GFP的侵染情况(图4-B)。结果显示,沉默后接种TMV-GFP第3、5天时,沉默植株中接种叶的荧光斑点数明显多于对照植株,实时荧光定量PCR也显示同样结果。沉默后接种TMV-GFP第7天,实时荧光定量PCR表明沉默植株系统叶中TMV的含量显著高于对照组,为对照的4.58倍,说明沉默会促进TMV-GFP侵染,SlN-like可能作为正调控因子抑制TMV-GFP侵染。

A:TRV:SlN-like和TRV:00接种TMV-GFP后的症状图Symptoms after inoculation with TMV-GFP in TRV:SlN-like and TRV:00;B:TRV:SlN-like和TRV:00中TMV MP含量检测。统计分析采用Student’s t检验(**0.001<P<0.01,***P<0.001),每个处理进行3次生物学重复,每次生物学重复10株番茄,数值代表3次生物学重复的平均值±标准误 Detection of TMV MP content in TRV:SlN-like and TRV:00.The statistical analyses were performed using Student’s t-test (**0.001<P<0.01, ***P<0.001).The experiments were repeated three times with ten plants each time.Values represent means±SE from three biological replications
2.4 沉默SlN-like对激素相关基因表达的影响
为探讨SlN-like参与抗病毒防御的机制,以TRV:00为对照,对沉默植株中乙烯(ethylene,ET)相关基因、脱落酸(abscisic acid,ABA)相关基因以及茉莉酸(jasmonic acid,JA)相关基因的表达进行实时荧光定量PCR分析。结果显示,沉默后,乙烯途径相关基因的表达量呈现极显著下降,仅为对照的12.5%。而脱落酸相关基因与茉莉酸相关基因的表达量未出现明显变化(图5),说明沉默可能会降低乙烯含量,揭示SlN-like可能参与乙烯介导的抗病毒防御。

统计分析采用Student’s t检验(**0.001<P<0.01,***P<0.001),每个处理进行3次生物学重复,每次生物学重复3株番茄,数值代表3次生物学重复的平均值±标准误The statistical analyses were performed using Student’s t-test (**0.001<P<0.01, ***P<0.001).The experiments were repeated three times with three plants each time.Values represent means±SE from three biological replications。图6同The same as Fig.6
2.5 外施乙烯利对SlN-like表达的影响
沉默会降低乙烯相关基因的表达,揭示沉默可能影响乙烯合成。为明确乙烯对表达的影响,试验以无菌水为对照,通过外施乙烯利(ethephon,ETH),并分别在外施后3、6、12、24 h取样,提取总RNA,利用实时荧光定量PCR检测的表达量。结果显示,外施乙烯利后3 h,的表达与无菌水处理并无显著性差异,而后开始上升;外施乙烯利后6 h,的表达持续上升,并在12 h达到最高,是对照组的2.71倍。之后的表达开始降低,24 h时恢复到与对照组相等水平(图6),说明外施乙烯利会促进的差异表达,进一步验证了通过介导乙烯通路参与抗病毒防御。
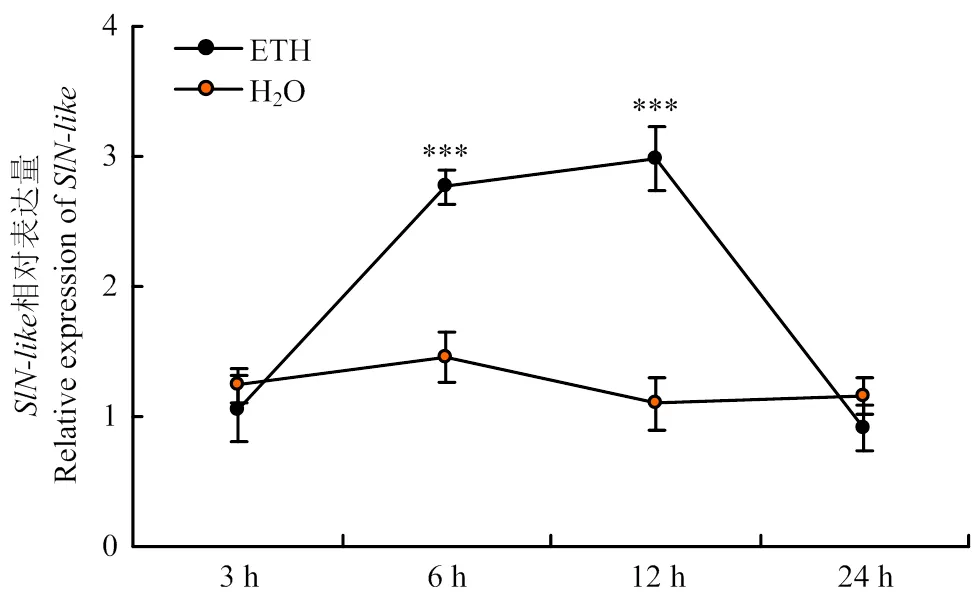
图6 外施乙烯利后SlN-like的表达
3 讨论
植物病毒严重威胁着作物的产量与品质。作物在对抗病毒过程中,演化出一系列抵御病毒侵染的机制,其中包括病原体相关分子模式触发的免疫反应(pathogen- associated molecular patterns-triggered immunity,PTI)和效应因子触发的免疫反应(effector-triggered immunity,ETI)。PTI和ETI是植物免疫防御中不可或缺的防御机制,但ETI防御速度更快、强度更强。TMV是危害最为严重、寄主范围最广的植物病毒[21],位列十大植物病毒之首,一旦发生极难防治[22]。在与植物病毒的长期协同进化中,某些作物品种进化出一些抵御病毒侵染的抗性基因,称为(resistance gene)基因[8]。大部分编码核苷酸结合位点-富含亮氨酸重复结构域(nucleotide binding site and leucine rich repeat domains,NBS-LRR)。NBS-LRR类抗病蛋白能够特异性地识别病原组分的无毒蛋白,从而激活效应因子触发的植物免疫防御反应[23]。植物NBS-LRR 蛋白具有针对细菌、病毒及真菌病原体“基因对基因(gene for gene concept)”的抗性,即植物和病原菌中的无毒基因存在一对一的关系,并共同构成了一个全面的病原体检测系统[24]。目前已从拟南芥()、水稻()、马铃薯等作物中鉴定出多个NBS-LRR类抗性基因,而分离自烟草野生种粘烟草()的便是NBS-LRR类抗病基因之一[25-28]。烟草编码蛋白与TMV解旋酶蛋白p50发生互作,并通过识别p50诱发HR反应,造成PCD以限制TMV的进一步扩张侵染。是植物中鉴定的第一个TIR-NBS-LRR(toll-interleukin-1 receptor/nucleotide-binding site/leucine-rich repeat)类抗病基因,其包含N末端结构域、NB-ARC结构域(APAF-1,disease resistance proteins,CED-4,亦称NBS结构域)及C端的LRR结构域。则是的同源基因,且烟草编码蛋白含有TIR-NBS-LRR结构域。通过克隆,并对其保守结构域进行分析,发现编码蛋白包含TIR-NBS-NACHT结构域,这与烟草N-like存在差异[12]。研究表明,烟草N-like的TIR-NBS参与TMV p50介导的HR反应,而LRR并不影响p50依赖的HR反应。SlN-like与烟草N-like仅在TIR-NBS存在相似性,表明SlN-like可能同样能与p50存在HR反应。同样,本研究表明TMV侵染会促进的表达(图2-B),而沉默则促进TMV-GFP的侵染(图4),进一步验证了SlN-like可能参与p50的识别并引起番茄抗病毒防御,但SlN-like与p50的直接识别证据仍需进一步探索。
乙烯是一种被人们熟知且已经被广泛应用于农业的小分子气体植物激素,是植物三大抗逆激素之一[29-30]。ERF(ethylene-responsive factor)转录因子家族基因是乙烯信号传递调控因子EIN3/EIL1的直接作用目标,其通过GCC-box直接与乙烯诱导基因启动子的顺式作用元件结合,进而调控植物的生物与非生物胁迫反应[31]。多项研究表明乙烯是植物免疫防御的正调节因子[32-34]。抗病蛋白RPW8.1(resistance to powdery mildew 8.1)结合并稳定ACC氧化酶4(acyl-CoA oxidase 4,ACO4),而ERF59能与启动子结合并抑制其表达,从而反馈抑制RPW8.1介导的细胞死亡及抗病机制[35];ERF转录因子能与病程相关蛋白(pathogenesis-related protein,PR)启动子结合,调控PR蛋白表达,引起抗病防御[36];外源施用乙烯会诱发拟南芥植株系统性积累防卫素,并提高植物防御素的转录和翻译[37]。乙烯对植物病毒侵染也存在调控,但研究相对较少。花椰菜花叶病毒(cauliflower mosaic virus,CaMV)的症状产生可能是由于P6与乙烯相关基因的互作引起[38];NbALD1通过介导水杨酸和乙烯途径响应芜菁花叶病毒(turnip mosaic virus,TuMV)侵染[39];乙烯介导植株对番茄丛矮病毒(tomato bushy stunt virus,TBSV)的极端抗性[40]。本研究表明,沉默后乙烯相关基因的表达量降低(图5),而外施乙烯利后的表达量升高(图6),揭示可能通过影响乙烯含量引起抗病防御。
笔者研究团队前期研究发现,番茄IP-L(interaction
protein L)、SlHIN1(harpin-induced gene 1)以及SlSYTA(Synaptotagmin A)均可在TMV侵染后高表达,但三者对病毒侵染的影响不尽相同[20,41-42]。通过对番茄抗性基因的挖掘与机制解析,对于理解以外壳蛋白互作蛋白IP-L为起点,SlHIN1、SlN-like为核心,运动蛋白互作蛋白SlSYTA为终点的影响病毒侵染的关系链至关重要,可为番茄的抗性遗传育种提供理论依据。
4 结论
在番茄中克隆并得到具有NBS-LRR结构域的抗病蛋白SlN-like,其表达受TMV侵染诱导,并且作为植物正调控因子抑制TMV-GFP侵染。沉默降低了的表达,外施乙烯利引起的差异表达,表明SlN-like通过介导乙烯通路参与抗病毒防御。
[1] Marathe R, Anandalakshmi R, Liu Y, DINESH-Kumar S P.The tobacco mosaic virus resistance gene,.Molecular Plant Pathology, 2002, 3(3): 167-172.
[2] Padgett H s, Beachy R n.Analysis of a tobacco mosaic virus strain capable of overcominggene-mediated resistance.The Plant Cell, 1993, 5(5): 577-586.
[3] 王倩, 刘贯山.烟草N基因及其介导的抗TMV信号转导分子机制.中国烟草科学, 2016, 37(3): 93-99.
WANG Q, LIU G S.The tobacco N gene and the signal transduction of-mediated TMV resistance.Chinese Tobacco Science, 2016, 37(3): 93-99.(in Chinese)
[4] Whitham S, McCormick S, Baker B.The N gene tobacco confers resistance to tobacco mosaic virus in transgenic tomato.Proceedings of the National Academy of Sciences of the United States of America, 1996, 93(16): 8776-8781.
[5] Erickson F L, Holzberg S, Calderón-Urrea A, Handley V, Axtell M, Corr C, Baker B.The helicase domain of the TMV replicase proteins induces the-mediated defence response in tobacco.The Plant Journal, 1999, 18(1): 67-75.
[6] Greenberg J t.Programmed cell death in plant-pathogen interactions.Annual Review of Plant Physiology and Plant Molecular Biology, 1997, 48: 525-545.
[7] DINESH-Kumar S P, Tham W H, Baker B j.Structure-function analysis of the tobacco mosaic virus resistance gene.Proceedings of the National Academy of Sciences of the United States of America, 2000, 97(26): 14789-14794.
[8] Whitham S, DINESH-Kumar S P, Choi D, Hehl R, Corr C, Baker B.The product of the tobacco mosaic virus resistance gene: similarity to Toll and the interleukin-1 receptor.Cell, 1994, 78(6): 1101-1115.
[9] DINESH-Kumar S P, Whitham S, Choi D, Hehl R, Corr C, Baker B.Transposon tagging of tobacco mosaic virus resistance gene: its possible role in the TMV--mediated signal transduction pathway.Proceedings of the National Academy of Sciences of the United States of America, 1995, 92(10): 4175-4180.
[10] 张恒木, 陈剑平, 程晔.烟草抗烟草花叶病毒基因的研究.浙江农业学报, 2001, 13(2): 55-60.
ZHANG H M, CHEN J P, CHENG Y.Study on the resistant geneof tobacco to tobacco mosaic virus (TMV).Acta Agriculturae Zhejiangensis, 2001, 13(2): 55-60.(in Chinese)
[11] Padmanabhan M S, Ma S, Burch-Smith T M, Czymmek K, Huijser P, Dinesh-Kumar S P.Novel positive regulatory role for the SPL6 transcription factor in theTIR-NB-LRR receptor- mediated plant innate immunity.PLoS Pathogens, 2013, 9(3): e1003235.
[12] Gao J S, Sasaki N, Kanegae H, Konagaya K i, Takizawa K, Hayashi N, Okano Y, Kasahara M, Matsushita Y, Nyunoya H.The TIR-NBS but not LRR domains of two novel N-like proteins are functionally competent to induce the elicitor p50-dependent hypersensitive response.Physiological and Molecular Plant Pathology, 2007, 71: 78-87.
[13] Zhang G Y, Chen M, Guo J M, Xu T W, Li L C, Xu Z S, Ma Y Z, Chen X P.Isolation and characteristics of the CNgene, a tobacco mosaic virus resistance N gene homolog, from tobacco.Biochemical Genetics, 2009, 47(3/4): 301-314.
[14] 谢鹏.TMV抗性基因介导的烟草过敏性反应及同源基因克隆[D].合肥: 安徽农业大学, 2013.
XIE P.Studies on hypersensitive response mediated by TMV resistance geneand cloning of homologous gene in[D].Hefei: Anhui Agricultural University, 2013.(in Chinese)
[15] 谢鹏, 胡丽, 蔡永萍, 林毅, 高俊山.TMV抗性基因介导的烟草过敏性反应研究.核农学报, 2013, 27(12): 1809-1816.
XIE P, HU L, CAI Y P, LIN Y, GAO J S.Studies on hypersensitive response mediated by TMV resistance genein.Journal of Nuclear Agricultural Sciences, 2013, 27(12): 1809-1816.(in Chinese)
[16] Fernandez-Pozo N, Menda N, Edwards J D, Saha S, Tecle I Y, Strickler S R, Bombarely A, fISHER-York T, Pujar A, Foerster H, Yan A, Mueller L A.The sol genomics network (SGN)—from genotype to phenotype to breeding.Nucleic Acids Research, 2015, 43(Database issue): D1036-D1041.
[17] Chou K C, Shen H B.Cell-PLoc 2.0: An improved package of web-servers for predicting subcellular localization of proteins in various organisms.Natural Science, 2010, 2(10): 1090-1103.
[18] Lv X, Xiang S Y, Wang X C, Wu L, Liu C Y, Yuan M T, Gong W W, Win H, Hao C, Xue Y, Ma L S, Cheng D Q, Sun X C.Synthetic chloroinconazide compound exhibits highly efficient antiviral activity against tobacco mosaic virus.Pest Management Science, 2020, 76(11): 3636-3648.
[19] Liu C Y, Pu Y D, Peng H R, Lv X, Tian S R, Wei X F, Zhang J, Zou A H, Fan G J, Sun X C.Transcriptome sequencing reveals that photoinduced geneaffects the expression ofto response to virus infection in.Physiological and Molecular Plant Pathology, 2021, 114: 101613.
[20] 潘琪, 刘旭旭, 彭浩然, 蒲运丹, 张永至, 叶思涵, 吴根土, 青玲, 孙现超.番茄的克隆及表达分析.中国农业科学, 2017, 50(15): 2936-2945.
PAN Q, LIU X X, PENG H R, PU Y D, ZHANG Y Z, YE S H, WU G T, QING L, SUN X C.Cloning, expression analysis of.Scientia Agricultura Sinica, 2017, 50(15): 2936-2945.(in Chinese)
[21] Knapp E, Lewandowski D J.Tobacco mosaic virus, not just a single component virus anymore.Molecular Plant Pathology, 2001, 2(3): 117-123.
[22] SCHOLTHOF K B G, ADKINS S, CZOSNEK H, PALUKAITIS P, JACQUOT E, HOHN T, HOHN B, SAUNDERS K, CANDRESSE T, AHLQUIST P, HEMENWAY C, FOSTER G D.Top 10 plant viruses in molecular plant pathology.Molecular Plant Pathology, 2011, 12(9): 938-954.
[23] Dangl J L, Jones J D.Plant pathogens and integrated defence responses to infection.Nature, 2001, 411(6839): 826-833.
[24] Van Der Biezen E A, Jones J D.Plant disease-resistance proteins and the gene-for-gene concept.Trends in Biochemical Sciences, 1998, 23(12): 454-456.
[25] Jiang G, Liu D, Yin D, Zhou Z, Shi Y, Li C, Zhu L, Zhai W.A rice NBS-ARC gene conferring quantitative resistance to bacterial blight is regulated by a pathogen effector-inducible miRNA.Molecular Plant, 2020, 13(12): 1752-1767.
[26] Hulbert S H, Webb C A, Smith S M, Sun Q.Resistance gene complexes: evolution and utilization.Annual Review of Phytopathology, 2001, 39: 285-312.
[27] Guo Y L, Fitz J, Schneeberger K, Ossowski S, Cao J, Weigel D.Genome-wide comparison of nucleotide-binding site- leucine-rich repeat-encoding genes in.Plant Physiology, 2011, 157(2): 757-769.
[28] Luo S, Zhang Y, Hu Q, Chen J, Li K, Lu C, Liu H, Wang W, Kuang H.Dynamic nucleotide-binding site and leucine-rich repeat-encoding genes in the grass family.Plant Physiology, 2012, 159(1): 197-210.
[29] 黎家, 李传友.新中国成立70年来植物激素研究进展.中国科学: 生命科学, 2019, 49(10): 1227-1281.
LI J, LI C Y.Seventy-year major research progress in plant hormones by Chinese scholars.Scientia Sinica Vitae, 2019, 49(10): 1227-1281.(in Chinese)
[30] 左建儒, 漆小泉, 林荣呈, 钱前, 顾红雅, 陈凡, 杨淑华, 陈之端, 白永飞, 王雷, 王小菁, 姜里文, 萧浪涛, 种康, 王台.2019年中国植物科学若干领域重要研究进展.植物学报, 2020, 55(3): 257-269.
Zuo J R, Qi X Q, Lin R C, Qian Q, Gu H Y, Chen F, Yang S H, Chen Z D, Bai Y F, Wang L, Wang X J, Jiang L W, Xiao L T, Zhong K, Wang T.Achievements and advance in Chinese plant sciences in 2019.Chinese Bulletin of Botany, 2020, 55(3): 257-269.(in Chinese)
[31] Zhao H, Yin C, Ma B, Chen S Y, Zhang J S.Ethylene signaling in rice and: New regulators and mechanisms.Journal of Integrative Plant Biology, 2021, 63(1): 102-125.
[32] Ding Y, Murphy K M, Poretsky E, Mafu S, Yang B, Char S N, Christensen S A, Saldivar E, Wu M, Wang Q,.Multiple genes recruited from hormone pathways partition maize diterpenoid defences.Nature Plants, 2019, 5(10): 1043-1056.
[33] Yang D L, Yang Y, He Z.Roles of plant hormones and their interplay in rice immunity.Molecular Plant, 2013, 6(3): 675-685.
[34] Aerts N, Pereira Mendes M, van Wees S C.Multiple levels of crosstalk in hormone networks regulating plant defense.The Plant Journal, 2021, 105(2): 489-504.
[35] Zhao Z X, Feng Q, Liu P Q, He X R, Zhao J H, Xu Y J, Zhang L L, Huang Y Y, Zhao J Q, Fan J, Li Y, Xiao S Y, Wang W M.RPW8.1 enhances the ethylene-signaling pathway to feedback-attenuate its mediated cell death and disease resistance in.New Phytologist, 2021, 229(1): 516-531.
[36] Tang D, Christiansen K M, Innes R W.Regulation of plant disease resistance, stress responses, cell death, and ethylene signaling inby the EDR1 protein kinase.Plant Physiology, 2005, 138(2): 1018-1026.
[37] Alazem M, Lin N S.Roles of plant hormones in the regulation of host-virus interactions.Molecular Plant Pathology, 2015, 16(5): 529-540.
[38] Geri C, Love A J, Cecchini E, Barrett S J, Laird J, Covey S N, Milner J J.mutants that suppress the phenotype induced by transgene-mediated expression of cauliflower mosaic virus (CaMV) gene VI are less susceptible to CaMV-infection and show reduced ethylene sensitivity.Plant Molecular Biology, 2004, 56(1): 111-124.
[39] WANG S, HAN K, PENG J, Zhao J, Jiang L, lu Y, Zheng H, LIN L, Chen J, Yan F.mediates resistance to turnip mosaic virus by regulating the accumulation of salicylic acid and the ethylene pathway in.Molecular Plant Pathology, 2019, 20(7): 990-1004.
[40] Sansregret R, Dufour V, Langlois M, Daayf F, Dunoyer P, Voinnet O, Bouarab K.Extreme resistance as a host counter-counter defense against viral suppression of RNA silencing.PLoS Pathogens, 2013, 9(6): e1003435.
[41] 彭浩然, 潘琪, 魏周玲, 蒲运丹, 张永至, 吴根土, 青玲, 孙现超.番茄抗性相关基因的克隆、表达与抗病毒功能.中国农业科学, 2017, 50(7): 1242-1251.
PENG H R, PAN Q, WEI Z L, PU Y D, ZHANG Y Z, WU G T, QING L, SUN X C.Cloning, expression and anti-virus function analysis of tomato resistance-related gene.Scientia Agricultura Sinica, 2017, 50(7): 1242-1251.(in Chinese)
[42] 彭浩然, 蒲运丹, 张永至, 薛杨, 武改霞, 青玲, 孙现超.ToMV外壳蛋白互作IP-L蛋白的亚细胞定位及表达分析.中国农业科学, 2017, 50(17): 3344-3351.
PENG H R, PU Y D, ZHANG Y Z, XUE Y, WU G T, QING L, SUN X C.Subcellular localization and expression analyses of IP-L protein interacting with ToMV coat protein.Scientia Agricultura Sinica, 2017, 50(17): 3344-3351.(in Chinese)
Cloning, Expression and Anti-Virus Function Analysis of
LIU ChangYun1, LI XinYu1, TIAN ShaoRui1, WANG Jing1, PEI YueHong1, MA XiaoZhou1,2, FAN GuangJin1, WANG DaiBin3*, SUN XianChao1*
1College of Plant Protection, Southwest University, Chongqing 400715;2Key Laboratory of Horticulture Science for Southern Mountainous Regions of Ministry of Education, College of Horticulture and Landscape Architecture, Southwest University, Chongqing 400715;3Chongqing Tobacco Science Research Institute, Chongqing 400715
【Objective】As an important vegetable crop, tomato () is endangered by various biological factors including pests, fungi, bacteria and viruses.The objective of this study is to clarify the antiviral function and mechanism ofresistance gene, and to provide a theoretical basis for the genetic breeding of antiviraland the targeted development of the antiviral agents.【Method】The full length ofwas obtained from the Solanaceae Genomics Network database and was divided into four segments, fusion PCR was used to amplify the full length of sequence.Bioinformatics was used to analyze the evolutionary relationship, protein characteristics, conserved domains, subcellular location and interaction relationship of SlN-like.real-time fluorescent quantitative PCR was used to analyze theexpression inroots, stems, leaves, flowers and fruits and its response after tobacco mosaic virus (TMV) infection.endogenouswas silenced using tobacco rattle virus (TRV)-mediated gene silencing technology, and the silent plants were inoculated with TMV-GFP to clarify the influence ofon virus infection.The expressions of abscisic acid (ABA), jasmonic acid (JA) and ethylene (ET) hormone-related genes in silenced plants, and the expression ofafter application of ethephon (ETH) for 3, 6, 12 and 24 h were analyzed by real-time fluorescence quantitative PCR to investigate the mechanism of SlN-like regulatory hormone pathway in response to virus infection.【Result】Through molecular cloning and fusion PCR technology, a 3 444 bpwas cloned fromvariety Micro-Tom, and uploaded to NCBI to obtain the sequence number MW792493.Through bioinformatics analysis, it was found that SlN-like contains TIR, NB-ARC and NACHT domains, and is closely related toN-like (AAP44394.1).expressed in all tissues of, with the highest expression in stems, followed by roots, flowers, leaves and fruits.After TMV-GFP infectionat 5th and 7th day, theexpression level was higher than that of PBS treatment, and TMV-GFP infection would cause the expression ofto increase continuously.TRV vector induced silencing ofin, and it was found that silencing 78.3% ofdid not affect tomato growth phenotype, but silencingpromoted the infection of TMV-GFP.Real-time fluorescent quantitative PCR analysis found that the expression of-silent plants was significantly reduced, only 12.5% of that in the control group.The expression ofincreased after 3 h of external application of ethephon, and reached the highest peak at 12 h, which was 2.71 times that of the control group, and returned to normal at 24 h.【Conclusion】SlN-like belongs to the NBS-LRR disease-resistant protein family, its expression is induced by TMV infection.Silencingcan promote TMV-GFP infection and reduce the expression of ethylene-related gene, while external application of ethephon resulted in the differential expression of, revealing that SlN-like participates inantiviral defense through the ethylene pathway.
; SlN-like; tobacco mosaic virus (TMV); gene expression; ethylene
2021-04-02;
2021-04-24
国家自然科学基金(31870147,31670148)、西南大学大学生创新创业训练计划(X202010635495)、中国烟草总公司重庆公司科技项目(A20201NY02-1306,B20211-NY1315,B20202NY1338)
刘昌云,E-mail:15228920380@163.com。通信作者孙现超,E-mail:sunxianchao@163.com。通信作者汪代斌,E-mail:467572562@qq.com
(责任编辑 岳梅)
