Bacterial nanocellulose production and biomedical applications
2021-10-19FranoisBarja
François Barja
Microbiology Unit, Department of Botany and Plant Biology, University of Geneva, 1211 Genève-4, Switzerland.
Abstract Bacterial nanocellulose (BNC) is a homopolymer of β-1,4 linked glycose, which is synthesized by Acetobacter using simple culturing methods to allow inexpensive and environmentally friendly small- and large-scale production. Depending on the growth media and types of fermentation methods, ultra-pure cellulose can be obtained with different physio-chemical characteristics. Upon biosynthesis, bacterial cellulose is assembled in the medium into a nanostructured network of glucan polymers that are semitransparent, mechanically highly resistant,but soft and elastic, and with a high capacity to store water and exchange gasses. BNC, generally recognized as safe as well as one of the most biocompatible materials, has been found numerous medical applications in wound dressing, drug delivery systems, and implants of heart valves, blood vessels, tympanic membranes, bones, teeth,cartilages, cornea, and urinary tracts.
Keywords: bacterial nanocellulose, bacterial cellulose, biomedical applications
Introduction
The research involving biopolymers obtainable in large quantities from different natural sources is widely increasing. Remarkable discoveries in this field indicated the great potential for the development and use of innovative biomaterials in medical applications.One of the oldest and still very promising biopolymers is cellulose, the most abundant molecule on the earth[1]. It is a linear and extracellular homopolymer of β-1,4 linked glycose (Fig. 1), the essential component of plant cell walls. Cotton and wood are the main sources of cellulose for all products manufactured or derived from it. Moreover, cellulose is also synthesized by plankton and unicellular algae in oceans, and by different species of bacteria which can also be grown in culture[2−4].
In recent decades bacterial cellulose (BC), also named bacterial nanocellulose (BNC), is gaining more attention due to the unique self-assembling of secreted fibrils into nanostructured biomaterial possessing exceptional biophysical characteristics that are suitable for a variety of biomedical applications[5]. Moreover,BNC belongs to the category of materials that have been generally recognized as safe. BNC is produced by the fermentation of certain bacterial species including Gram-negative bacteria species such asAcetobacter(reclassified asKomagataeibacter),Rhizobium,Agrobacterium,Pseudomonas,Salmonella,Alcaligenes, as well as Gram-positive species such asSarcina ventriculi[1,4]. The most efficient production of bacterial cellulose comes fromAcetobacters,e.g.,A. xylinum,A. hansenii,A. pasteurianus,andKomagataeibacter europaeus.
Many reviews have been published recently focusing on the intrinsic properties, methods of production and impact of different chemical and physical factors (carbon and nitrogen sources,microelements, pH, temperature, oxygen,etc.), and potential applications of BNC[6−8]. Here, the overview of the current state of culture methods production,biosynthesis, unique structural properties, and biomedical applications will be presented in order to explain the paramount importance of BNC as a promising and highly biocompatible material obtainable from sustainable natural resources.
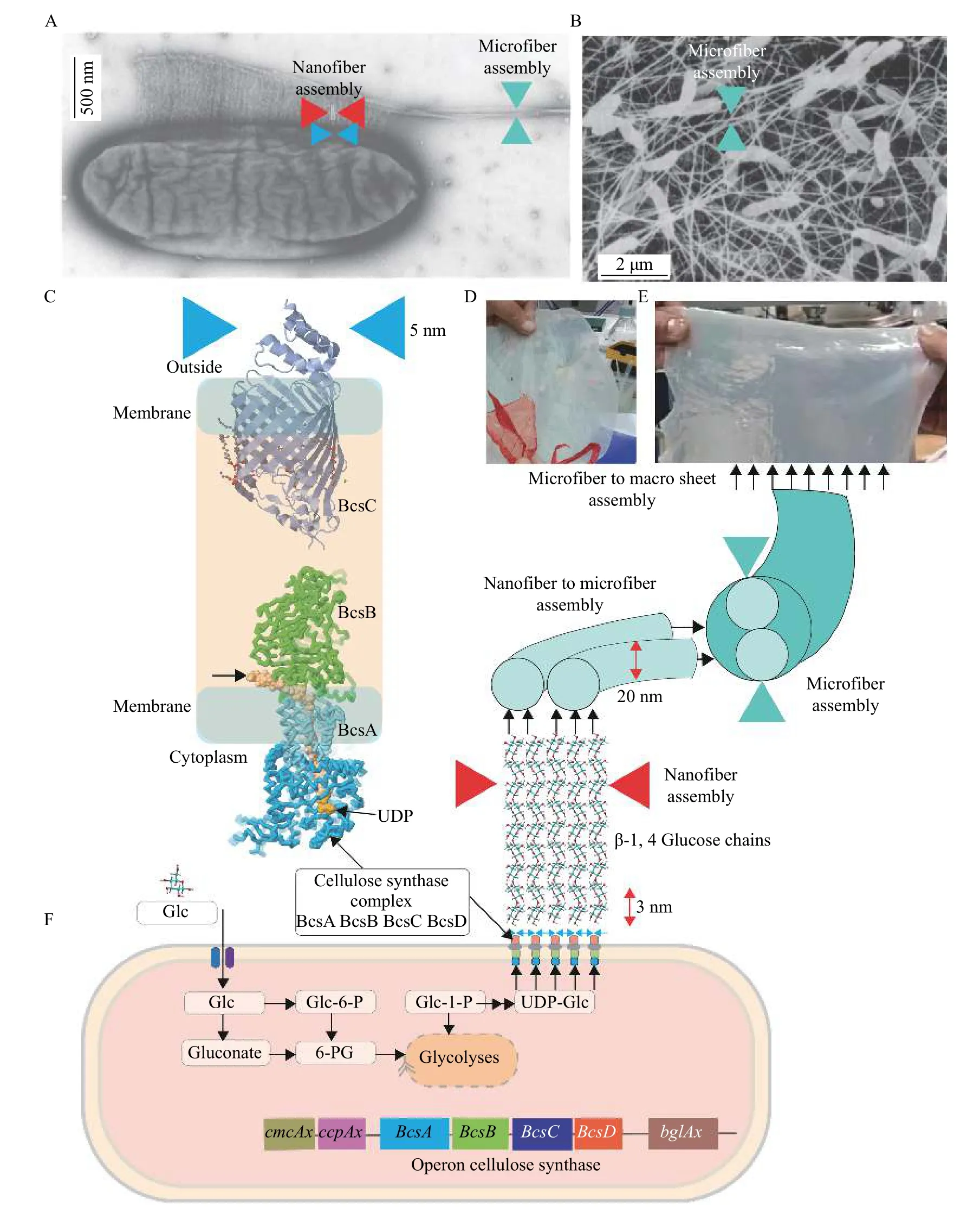
Fig. 1 Bacterial nanocellulose biosynthesis. A and B: Electron microscopy image of bacteria synthesizing cellulose and their assembly into nanofibers and microfibers in the medium. C: Cellulose synthase complex[9−13]. D: Dry bacterial nanocellulose sheet (red is a dyed piece). E: Wet bacterial nanocellulose sheet. F: Schematic representation of bacterial cell synthesizing cellulose assembling into nanofiber and microfiber structures. Metabolic pathway of cellulose synthesis from the glucose and cellulose synthase complex adapted from Lee et al[7]. Representation of the genetic structure found in Komagataeibacter xylinus and Komagataeibacter europaeus of the bcs operon and some other upstream or downstream genes also involved in cellulose production, adapted from Tonouchi[14]. Glc: glucose; Glc-6-P: glucose-6-phosphate; UDP-Glc: UDP-glucose.
Properties of bacterial nanocellulose
In 1886, Adrian Brown was the first to speak about BNC. Indeed, while studying the chemical actions ofBacterium aceti, he leaned closer to another acetic ferment known for its ability to form the "mother of vinegar". This did not have the same aspect asBacterium acetiand the film that formed on the surface of the medium was highly resistant and resembled, to the touch, an animal membrane. In addition, the results obtained for this film after treatment with different chemical solutions allowed Brown to affirm that this "vinegar plant" had all the characteristics of cellulose[15]. Following this discovery,this acetic ferment was renamed "Bacterium xylinum".Today, this bacterium is known asKomagataeibacter xylinus(or sometimesGluconacetobacter xylinus) and is the benchmark acetic bacterium for cellulose production.
BNC is chemically identical to plant cellulose (PC),but significant differences have been highlighted between them in terms of purity, macromolecular properties, and physical characteristics. Unlike PC,BNC has a high purity since it is devoid of lignin,hemicellulose, and pectin, allowing for a higher degree of polymerization and crystallization[16]. Thus,by replacing PC with BNC, long and restrictive purification processes are avoided, thereby decreasing pollution. BNC is composed of ultrafine fibers of 20 to 100 nm in diameter, which is about 100 times thinner than plant cellulose fibers, assembled in an ultrafine network (Fig. 1A−E)[17]. This unique nanomorphology allows it to retain up to 200 times its dry weight in water, which makes it highly resistant to wet conditions and offers excellent elasticity and conformability. These exceptional properties have been used, among other purposes, in the medical field to create bandages[8]. Indeed, BNC is a porous material, allowing the passage of antibiotics and other drugs while acting as a physical barrier against external infections. Moreover, thanks to its excellent ability to retain water, it accelerates the healing of wounds, as re-epithelialization improves while the wound remains wet[18−19].
BNC has an exceptional ability to maintain its shape (a high Young's modulus) as well as a high speed of sound transmission over wide frequency ranges, which has also enabled its use as a diaphragm for high frequencies in speakers and headphones[20].Furthermore, BNC has also been used in the paper industry, since the addition of a certain amount of BNC in the paper pulp makes it possible to produce high-quality paper with better tensile strength and four to five times better folding resistance[20]. Finally, the unique intrinsic characteristics of BNC have also been commercialized in areas such as the food industry,textiles, agriculture, and cosmetics[4,21]. Taking into account all of these properties, together with the fact that the use of PC leads to a decrease in forest resources and, consequently, many environmental problems, BNC offers an excellent alternative to cellulose produced by plants.
Synthesis of bacterial cellulose
The synthesis of bacterial cellulose is a complex,precise, and highly regulated process that takes place across several stages. It involves a large number of genes coding individual enzymes, present in catalytic complexes, and regulatory proteins (Fig. 1F). When glucose is used as a carbon source, BNC synthesis occurs in four key enzymatic steps: 1) phosphorylation of glucose to glucose-6-phosphate (Glc-6-P)viaglucokinase; 2) isomerization of Glc-6-P to Glc-1-Pviaphosphoglucomutase; 3) synthesis of uridine diphosphate glucose (UDP-glucose or UDPGlc), a direct precursor of cellulose,viaUDPG-pyrophosphorylase; and 4) polymerization of UDP-glucose into β-1,4 glucan chainsviathe cellulose synthase complex.
InK.europaeus, as inK.xylinus, cellulose synthase consists of 4 protein subunits: BcsA, BcsB, BcsC, and BcsD (Fig. 1C). The genes coding for these different proteins are under the control of a single promoter and thus form an operon called "bacterial cellulose synthase" (bcs) (Fig. 1C)[22]. The BcsA membrane protein is the catalytic subunit that synthesizes BNC and forms the transmembrane pore through the inner membrane (Fig. 1CandF). It consists of eight transmembrane segments and two cytoplasmic domains: a catalytic β-1,4-glycosyltransferase domain conserved between the four and five transmembrane helices and a C-terminal fragment containing a PilZ domain binding the cyclic secondary messenger diguanosine monophosphate (c-di-GMP). The activity of BcsA is stimulated by the secondary messenger cdi-GMP[22]. The binding of c-di-GMP to the PilZ domain of BcsA induces a conformational change that allows UDP-glucose to access the catalytic site. As a result, the cellulose synthase remains inactive or shows very low enzymatic activity in the absence of cdi-GMP[17]. Once activated, the catalytic domain polymerizes the UDP-glucose monomers into β-1,4-glucan chains.
BcsB is a periplasmic protein anchored to the inner membraneviaa single transmembrane helix (TM)interacting with BcsA (Fig. 1CandF). The presumed function of BcsB would be to guide the polymer through the periplasm towards the outer membrane using two carbohydrate binding domains[13]. In all cases, BcsB is crucial for the catalytic activity of BcsA because their interaction makes it possible to stabilize the BcsA TM region, which makes catalytically active synthase. In some species, BcsA and BcsB are fused into a single polypeptide.
The structure of the BcsC protein consists of a beta barrel inside the outer membrane preceded by a periplasmic domain containing a tetratricopeptide repetition, pointing to the involvement of this protein in the assembly of the complex (Fig. 1CandF). BcsC would also facilitate the passage of periplasmic cellulose out of the cell by forming a pore at the outer membrane. BcsC is required for cellulose synthesisin vivo, but notin vitro[23].
BcsD is a periplasmic protein whose presence is not essential for the activity of cellulose synthase.However, Wonget alhave shown in 1990 that mutations of theBcsDgene inK.xylinusproduced 40% less cellulose compared to wild types, suggesting that the BcsD protein is required for maximum synthesis inK. xylinus[24]. BcsD protein would then play a role in the extrusion or crystallization of cellulose sub-fibers. Nevertheless, the precise function and action mechanism of the BcsD protein remains uncertain.
Upstream from thebcsoperon, there are two genes that also play an essential role in the production of cellulose inA. xylinum:cmcAxandccpAx(Fig. 1F)[25−26].ThecmcAxgene encodes a carboxymethyl cellulase(CMCase) with endo-β-1,4-glucanase activity. Despite its cellulose hydrolysis activity, this enzyme increases the amount of cellulose produced when it is endogenously overexpressed or added to the culture medium exogenously. In fact, by degrading the cellulose, the CMCase enzyme makes it possible to reduce stress due to the tension that is created on the glucan chains during the formation of crystalline cellulose microfibrils. The hydrolysis activity of the cellulose of this protein, therefore, makes it possible to regulate the biosynthesis of cellulose.
The protein CcpAx ("cellulose complementing proteinA. xylinum"), encoded by theccpAxgene, is required for the biosynthesis of cellulosein vivo, in particular during the crystallization stage. In addition,the study of this protein by pull-down assay revealed that CcpAx interacts with the BcsD and is, therefore,an integral part of the terminal complex. Moreover,because of its low molecular weight and secondary structures rich in alpha helices, CcpAx facilitates protein-protein interactions during the assembly of the cellulose synthase complex. Indeed, Denget alhave demonstrated in 2013 that disruption of theccpAxgene results in a significant reduction in BcsB and BcsC levels, confirming the role of CcpAx as a cellulose biosynthesis regulator[27].
Downstream of thebcsoperon is thebglAxgene(Fig. 1CandF) coding for a β-glucosidase which belongs to the family 3 glycoside hydrolases. The role of this protein in the biosynthesis of cellulose is still unknown. Denget alhave, nevertheless, observed that a deletion of thebglAxgene causes a decrease in the biosynthesis of cellulose[27].
Some species of acetic bacteria, includingK.xylinusandK. europaeus, also have a secondbcsoperon that codes for a broad BcsAB fusion protein,as well as two additional genes:bcsXandbcsY.However, the products of these genes have not yet been characterized.
The polymerization and crystallization of cellulose are two coupled processes occurring consecutively(Fig. 1EandF). The degree of crystallization limits the rate of polymerization. The first step comprises the polymerization of numerous β-1,4 glucan chains.These are then secreted outside the cell through a linear array of pores on the outer membrane (Fig. 1A,E, andF). The assembly of β-1,4 glucan chains outside the cell is a precise and hierarchical process.First, sub-fibrils form, consisting of 10 to 15 chains of nascent glucans. Then, these sub-fibrils assemble and crystallize to form fibrils, which then combine to form a cellulose nanofiber and microfiber comprised of about 1000 individual glucan chains[17](Fig. 1E).
The thick gelatinous membrane or "mother of vinegar" that is observed during the culture of acetic bacteria under static conditions is composed of an ultrafine network of these cellulose nanofibers (3 to 8 nm, as shown inFig. 1A,B,DandE).
Culture methods for bacterial nanocellulose production
An important feature of BNC is that it can be produced in a semi-continuous static culture with a simple and low-cost medium, thus representing an interesting alternative for developing industries. The nanocellulose synthesized by different bacteria under a variety of possible culturing conditions has shown to possess diverse structural properties that are defining their particular physicochemical characteristics and morphologies suitable for many biomedical and industrial applications.
There are currently three methods for cellulose production, static, agitated, and bioreactor-based bacterial culturing. As mentioned above, macro morphology, owning various physicochemical properties resulting from particular types of cellulose assemblies into nano- and micro-structures, is quite different between these methods. Therefore, the applications of BNC with specific characteristics will determine the choice of a production method. In the following subsections description of bacteria strains,physicochemical parameters of the medium, such as pH, nutrients composition, oxygenation, carbon and nitrogen sources, and temperature on the production and characteristics of BNC by three culturing methods will be briefly reviewed.
Static culture method
The static culture method is a conventional and commonly used approach for BNC production.Different shapes and sizes of containers are filled with a culture medium at a pH between 4.5 and 6.5, and incubated for 2 to 20 days at a suitable temperature between 25 to 30 °C. BNC produced by the static culture method comprises a hydrogel pellicle formed at the air-culture medium interface[21,28]. The subfibrils of cellulose are continuously crystallized into microfibrils and extruded from linear pores at the wall-membrane of the bacteria, formed by selfassembling, overlapping, and intertwined cellulose ribbons in parallel-disorganized planes (Fig. 1A,B,DandF). This results in an accumulation of gelatinous cellulose membrane at the top of the culture medium(Fig. 1DandE).
The thickness of the formed BNC depends on the culture time. Once the membrane is formed, it is purified by washing it in a solution containing sodium hydroxide, neutralized with acetic acid, and rinsed with deionized water until the BNC becomes translucid. After purification, membranes can be dried or stored moist (Fig. 1DandE). The produced pellicle shows a 3D network structure with high stretch resistance, flexibility, and porosity. This static culture method is a relatively simple technique and is widely used for BNC production.
This method is unsuitable in such a form for largescale industrial production because of the costly synthetic media and the low yield rates of BNC synthesis. One of the major problems is caused, in particular, by the insufficient oxygenation of the static cultures of strictly aerobic bacteria. In an attempt to solve some of these issues, studies have focused on the feasibility of using fruit juices and/or agriculturaland industrial-based waste as carbon and nutrient sources[29−30]. The resulting morphology, microstructure, and intrinsic properties of BNC are almost the same as those produced from standard media as a control. Meanwhile, these studies have demonstrated the potential of waste products for eco-friendly and low-cost BNC production.
Agitated culture method
The agitated culture method has been put forward as a way to improve production rates and increase dissolved oxygen in the medium culture. However, it has been reported that in spite of increasing dissolved oxygen in the medium for a similar duration as in the static culture method, either the same quantity of BNC is produced or, in certain instances, lower amounts are obtained[28]. Furthermore, the BNC shapes are spherelike, cocoon-like, pellet-like, or even irregular clump masses that are very different from those obtained by the static culture methods[21]. These changes in morphology, related to the lower crystallinity and intrinsic mechanical properties of BNC are the result of the lower degree of polymerization and assembly of BNC into nanofibers. The genetic instability of some bacteria under agitated conditions is one of the factors that may also contribute to reduced BNC productivity.Moreover, this technique is not suitable for all types of bacterial strains.
Bioreactor cultures
Bioreactor cultures with continuous cultivation using a rotating disc or airlift with a constant oxygen transfer have reported higher levels of BNC production. However, the BNC crystallinity, elasticity,and polymerization degree were reported to be lower than those of bacterial cellulose produced by agitated or static culture methods[4,21].
In the classical airlift fermentation bioreactor oxygen is continuously transferred from the bottom into the culture medium for providing a suitable oxygen supply. The process is energy efficient and involves less shear stress when compared to stirredtank reactors. An airlift bioreactor for BNC production was firstly reported by Chaoet al[31]. Different configurations of airlift bioreactors have been proposed, such as the modified airlift bioreactor proposed by Wu and Li[32]. The resulting BNC membranes have a higher water-holding capacity than that of BNC produced by static cultivation. Also, the elastic modulus can be changed by varying the number of net plates. Considering the advantages as well as disadvantages of the three methods above,further research has to be conducted to optimize industrial production with higher productivity at lower costs.
Applications of bacterial cellulose
The unique physico-chemical properties of BNC are suitable for various applications in the biomedicine field, as well as in agri-food, paper, textiles,cosmetics, and other biomaterial industries. For an effective future in regenerative medicine, biomaterials with specific characteristics need to be further developed to fulfill their increased demands.Nowadays, BNC, alone or combined with other components (e.g., biopolymers or nanoparticles), has gained great importance in the medical field. BNC is proved to reinforce epithelialization, necessary for rapid healing, through facilitating the appropriate cell adhesion, proliferation, migration, and differentiation[19,33−34]. BNC-based biomaterials can maintain a moist wound environment, blood and exudate absorption, gas exchanges, thermal insulation, and minimal tissue adhesion, while being nontoxic[19,33−34].
One of the first applications involving the use of bacterial cellulose in a form of membranes is for wound dressing, particularly for burn victims[35].Indeed, thanks to its advantage over classical materials, BNC with its high wet strength and permeability, and little irritation, is used as temporal artificial skin for wound covering. Several BNC membranes are currently marketed; one of the first was Biofill biomembrane with demonstrated efficacy in over 300 cases[36]. Various trademarks, like Bionext,Membranecell, and Xcell are also commercialized.The beneficial effects of these membranes over the conventional gauzes or synthetic materials such as Tegaderm, Cuproplan, Aquacell or Xeroform, include maintenance of a moist environment, removal of exudates, adaptation to the wound surface, as well as protection against infections and local pain reduction,that are collectively leading to accelerated healing through enhancing epithelization and tissue regeneration.
In addition, different biomolecules can be integrated into the BNC to extend its functionalities. In 2008 Maneerunget alhave described and utilized a silver-containing BNC (BNC-Ag) as a wound dressing for its bacteriostatic and bactericide effects[37]. Such silver-cellulose materials present a great benefit in avoiding several types of infections caused byE. coli,S. aureus,K. pneumoniae,B. subtilis,and P. aeruginosa.The problem in this case, however, is the cytotoxic effect of silver nanoparticles[37].
Furthermore, BNC has also been successfully loaded with many drugs like ibuprofen, tetracycline,lidocaine, diclofenac, vaccarin, benzalkonium chloride,and doxorubicin, and applied as a transdermal drug delivery system, as reviewed by Pichethet al[19].Moreover, an adapted profile of sustained drug release was introduced in a form of functionalized biomaterials, such as modification of the drug content and by changing the structure of BNC with x-rays irradiation[38]. The use of comfortable, transparent, and thin BNC sheets as drug reservoirs has great potential to further provide a more suitable environment for tissue repair and accelerate re-epithelialization processes by combining the sustained and sensitive release of healing agents.
It has been reported that by increasing BNC's hydrophilic properties with surface modification using chitosan and carboxymethyl cellulose, the proliferation of the retinal pigment epithelium and keratinocytes has been improved[39]. By incorporating polyvinyl alcohol on BNC, Wanget alhave demonstrated in 2010 the increase in light transmittance and UV absorption[40]. These BNC-based materials have great potential to be used as eye scaffolds and replace the less biocompatible poly(methyl)methacrylate or hydroxyapatite materials generally used. Hence, BNC composites offer a high bioengineering potential for eye diseases. In this context, several methodologies have been developed to produce stable contact lenses for the correction of presbyopia, astigmatism, and myopia[41]. Therefore, contact lenses based on BNC have a high potential for use as wound dressings after eye surgery, improving the recovery of ocular problems.
BNC is also used for the production of cardiovascular implants. Klemmet alhave demonstrated that BNC is an excellent biomaterial for artificial vessels and developed a specific material BActerial SYnthesized Cellulose (BASYC) to be successfully implanted in the carotid arteries of rats and pigs with a long stability period and without any complications[42−43]. The same group developed a prototype BNC implant tubes, whose diameter and length could vary. Such cellulose-based tubes show mechanical properties similar to small diameter blood vessels and have been successfully implemented in the carotid artery of pigs and rats[42−43]. Indeed, BNC has many mechanical properties superior to those of other synthetic materials (polypropylene, polyethylene,terephthalate, cellophane) usually used for this purpose. For example, BNC offers better shape retention and tear resistance. In addition, a study by Fink 2009 showed that BNC has a lower risk of blood clots compared to other materials[44].
Another approach involves BNC as a natural graft for tympanic membranes in patients suffering local perforation. BNC occludes the wound and reduces pain, bleeding and hematomas compared to the current material in the use of Gelfoam[45]. Bacterial cellulose can also be used as a framework for the creation,inter alia, of bone and cartilage tissue[46−47].
Indeed, BNC has many inherent advantages such as biocompatibility, high resistance, biodegradability,and non-toxicity, as reviewed above. It can also be synthesized in a pure form by different acetic bacteria.Moreover, its architecture and porosity can be controlled during the culturing process. For all these reasons, BNC represents an ideal biomaterial for the design of 3D models mimicking the architecture of the extracellular matrixes of native tissues[48].
Conclusions
BNC, a chemically pure natural biopolymer produced by microorganisms, is being progressively recognized and used in different biomedical applications as a highly biocompatible material for wound dressing, and drug delivery systems, as well as for implants of heart valves, blood vessels, tympanic membranes, bones, teeth, cartilages (meniscus, ear,etc.), cornea, and urinary tracts. In addition, numerous industries such as food packaging, desert foods, facial masks, scrubs, transparent optical films, sensors,battery separators, water treatment, electric and magnetic conductors, high fidelity speakers, and highquality paper have taken advantages of applications of BNC based on its unique physicochemical properties and simple eco-friendly production.
Acknowledgments
The authors are grateful for financial assistance from the INNOGAP (Unitec), Sciences Innovation HUB, and Botany and Plant Biology Department of University of Geneva.
杂志排行
THE JOURNAL OF BIOMEDICAL RESEARCH的其它文章
- Editorial commentary on the special issue of Advances in Nanomedicine
- Superior in vitro anticancer effect of biomimetic paclitaxel and triptolide co-delivery system in gastric cancer
- Nanobody-based immunosensing methods for safeguarding public health
- Hybrid lipopolymer vesicle drug delivery and release systems
- EPR spectroscopy of whole blood and blood components: can we diagnose abnormalities?
- Mechanotransduction, nanotechnology, and nanomedicine
