EPR spectroscopy of whole blood and blood components: can we diagnose abnormalities?
2021-10-19DimitriSvistunenko
Dimitri A. Svistunenko
School of Life Sciences, University of Essex, Colchester, Essex CO4 3SQ, UK.
Abstract This mini-review gives a brief account of the emergence of the electron paramagnetic resonance (EPR)spectroscopy in the second half of the 20th century and reports the continuous wave EPR spectroscopy studies on human and animal blood. The question posed by this review is whether the EPR spectroscopy in the form it appeared 70 years ago is still able to provide useful information about different pathological conditions in humans, particularly in the area of diagnosis.
Keywords: EPR spectroscopy, biological tissues, paramagnetic metals, protein free radicals, human blood, animal blood
Introduction
Soviet physicist Yevgeny Zavoisky, while working on microwave radar systems in evacuation in the city of Kazan at the height of the 2ndWorld War, came across a phenomenon of 'paramagnetic losses' in some crystal substances. His observations were published in 1945[1–2]and promptly confirmed by several independent groups[3–5]. The name for the phenomenon was coined as electron paramagnetic resonance (EPR) and a full theory published in 1948[6]earned the author,Van Vleck, a Nobel prize in 1977. Electron spin resonance (ESR), a synonym of EPR, is also in use.
Indeed, it took more than 30 years to realise fully the true calibre of the discovery although from the very beginning it became clear that important applications in physics, chemistry and biology should follow. Paramagnetic states of molecules, speculated to be transient, were highly anticipated in many chemical and biochemical reactions.
Most molecules around us have an even number of electrons which come in pairs so that magnetic moment of each electron, associated with its spin, is cancelled out by the opposite magnetic moment of its counterpart. But redox chemistry in general and redox biochemistry in particular are all about juggling of electrons between molecules. Therefore, long before the discovery of EPR it has been realised that there must be paramagnetic states of molecules when the number of electrons is odd and the molecule therefore can be considered as a magnet. A typical example of paramagnetic species is free radicals formed transiently in redox reactions. Also, several metals when forming inorganic complexes might have one or more electrons unpaired thus forming an overall nonzero electron spin of the complex.
New method took off rapidly
Thus, the discovery of the EPR phenomenon has triggered very rapid development of the new research method, the EPR spectroscopy—for detection of the paramagnetic states of molecules. It became clear very soon that the method is very sensitive—different salts of paramagnetic transition metals gives clearly different signatures (EPR spectra) for the metals in different coordination[7]. This high sensitivity of the method to the very nature of the paramagnetic species has driven both the researchers' interests in many biological systems and the technological developments of the instruments.
Over a period of only 15 years since the first report of free radicals in biological materials[8], the EPR/ESR spectroscopy has emerged as an important research method in a score of laboratories around the world,including those in the USA[9–14], the UK[15–16],Australia[17–18], Soviet Union[19–22], Sweden[23–25], and Japan[26–28].
From the beginning of use of the new method, two groups of paramagnetic species have been brought into focus of study: metal active sites in proteins (and enzymes) and the transient free radical intermediates of some biochemical reactions. The studies of the metal sites have been prompted by the initial works on paramagnetic salts[3–4]. And the first reports of paramagnetic free radical intermediates[16–17], first found in the reactions of haem proteins with peroxides[29–30], have been followed by many publications on free radicals in organic and inorganic chemistry as well as biochemistry and biology in general. In addition to the obvious aim of understanding the mechanistic aspects of how biological molecules work, looking into individual paramagnetic metals and radicals has a common practical reason – to provide a tool for identifying the EPR signatures of these molecules in the EPR spectra of complex systems,such as cell cultures and tissues. It was thought that the ability to see and, possibly, quantify intermediates of individual biochemical processes in whole tissues could open up possibilities of recognising abnormalities in humans at early stages, when other tests are not able to detect them. Understandably, the tissue easily available without invasive sampling procedures has come into focus—blood. Logically, the EPR signatures of blood components were to be studied first.
Haemoglobin pioneering studies
The pioneering method of optical spectroscopy in the early 20thcentury was used to study haemoglobin(Hb) reacting with H2O2[31]—and (amazingly!) the optical spectra changed, which was interpreted as H2O2forming an adduct to methaemoglobin (metHb).This conclusion was later challenged with a suggestion that the optical change was caused not by mechanistic addition of H2O2to the protein but by a redox reaction, and a free radical was suggested to be formed in the process[29]. A radical demonstrated by the newly available EPR spectroscopy[15]was shown to be on the polypeptide part of the protein[16]. A further series of papers investigating Mb and Hb reacting with peroxide demonstrated that the reported optical changes seen on H2O2addition were caused by an electron transfer and the chemistry of haem oxidation was revealed[17–18].
In a parallel line of the new method applied in biology, haem containing proteins and enzymes were in the spotlight. A series of papers were published in the late sixties by Blumberg, Peisach and the Whittenbergs, the giants of haem proteins studies. The paramagnetic state of the haem (when the iron is in the oxidised Fe3+form) was shown to give different EPR signatures, not only for the high spin (S=5/2) and low spin (S=1/2) Fe3+forms, but also for the same forms in different enzymes and proteins, or even for α- and βsubunits of the same Hb molecule, thus revealing a sensitivity to structural differences in the haem microenvironment[11,13,32–33].
EPR spectroscopy of whole tissues: the lyophilisation approach
Assessment of whole biological tissues by the newly arrived EPR spectroscopy had an important methodological issue. The dominant component of most tissues by mass is water. Water is not paramagnetic but its molecules are polar and a great deal of the microwave power sent to the sample is absorbed in a non-resonant way—in re-orienting the polar water molecules by the electromagnetic radiation oscillating with the microwave frequency (this is exactly how the microwave oven works). This makes it extremely difficult to detect the EPR absorption caused by the paramagnetic molecules. There are three ways to overcome this difficulty. The tissue can be lyophilised (vacuum dried), it could be frozen thus making the re-orientation of water molecules not possible, or the tissue could still be wet, and at a temperature above freezing point, but in a special geometry cell (so-called flat cell) that holds the sample in the spectrometer—to minimise non-resonance losses.
Historically, it was the lyophilisation approach that was used first in tissue studies by EPR spectroscopy.In 1954, Commoner, Townsend and Pake published a pioneering paper reporting EPR spectra of a range of plant and animal tissues (lyophilised)[8]. Free radical EPR spectra were analysed and quantified—to show a great diversity in free radical content in different biological tissues. Interestingly, the greatest diversity in free radical concentrations was found in plants—spreading over a 30–fold difference range[8].
The authors of this seminal paper also compared EPR spectra of normal mouse liver and those of a hepatoma showing a significantly different yield of the radicals (lower in the tumour). This certainly triggered a series of studies investigating into the correlation between the malignancy score and the free radical level in tissues (see, for example, review[34]).
It has been shown later that the free radical EPR signal in lyophilised tissues (of approximately 7 to 8 Gauss width) has very little to do with the biochemical and physiological status of the tissue but is rather bluntly caused by the ascorbic acid and the availability of oxygen during lyophilisation (the label 'artefact'EPR signal has been attached to the lyophilised tissues EPR spectra since)[20,35–36]. The line shape of the EPR signal of ascorbic acid radical in frozen solutions and of the signal of lyophilised tissues have been confirmed to be the same[37]. As the ascorbic acid concentration in plants varies in a much larger range than in the animal tissue, the observation by Commoner,Townsend and Pake[8]reporting that the free radical EPR signals in the lyophilised plants show a much larger range of intensities than in animal tissues becomes understandable.
It turned out that EPR of frozen tissues can also be informative. Free radical EPR spectra of frozen (not lyophilised) samples, being free from the ascorbic acid radical signal, are characterised by approximately 10 times lower integral intensity and a wider line width(14 to 15 Gauss[21]).
Continuous wave EPR spectra of frozen blood and components
Over the last 70 years, the EPR spectroscopy method has evolved from the early 9 GHz (X-band)continuous wave instruments to a spectacular complexity and diversity of instruments and methods including pulsed spectrometers, spectrometers operating on different microwave frequencies (covering a range of 1 to 350 GHz), new methodologies allowing structural and kinetics studies, spin labels and spin traps techniques for a range of applications, including the studies of causative relationship between nanoparticles and reactive oxygen species (ROS)generation, andex vivoandin vivoimaging. A detailed and relatively recent account of the evolution of EPR spectroscopy in biomedical research is given in the review[38].
Oxidative stress at the organism level is a frequent consequence of erythrocytes lysis and haemoglobin release to the blood flow (or myoglobin released from damaged muscle). These haem proteins, without the in-cell protection by catalase and superoxide dismutase, become active producers of ROS, and EPR spectroscopy allows detection and quantitation of these species.
Nano systems have been used to modulate the ROS production. It has been show that silver nanoparticles applied toArabidopsis thalianacould cause formation of ROS, shown by EPR to be mitigated by tissue ascorbic acid[39]. On the other hand, nontoxic hydrophilic carbon cluster nanoparticles were demonstrated to convert superoxide to O2and hydrogen peroxide thus providing protection against O2•−[40]—notably more efficient than by most singleactive-site enzymes. The nanoconstructs accommodating membrane proteins have been shown to be effective systems for studying conformational changes in proteins. Styrene-maleic acid lipid particles as nanocontainers for a spin labelled transmembrane proteins complex allowed EPR studies of light induced conformational changes; importantly, EPR allowed kinetic studies of such changes[41]. Bifunctional spin labels in combination with similar nanoparticles Lipodisq were used for structural studies of a voltage-gated potassium channels[42].
Continuous wave EPR spectra of frozen blood,plasma, and cells, as published by different researchers, are collated inFig. 1, which allows the comparison of different experimental spectra within a common frame[43–50].
Spectra inFig. 1AandBdemonstrate that human and rat venous blood are characterised by almost identical EPR spectra. In assigning different EPR signals in the spectra, an analysis of the blood components can help. It appears quite clear that the EPR signals withg-factors 4.28 and 2.05 are caused by the components of blood plasma (Fig. 1C),whereas theg≈5.9 andg≈2 EPR signals, as well as the three component signal withg-factors at 2.59, 2.18 and 1.83 (Fig. 1D), are associated with the cell fraction of blood. The 4.28 signal is caused by the high spin ferric iron in the plasma protein transferrin[51]. The Cu2+ions in the other plasma protein ceruloplasmin are responsible for the signal that gives the main (g⊥) component at 2.05 and a parallel componentg||that is split into four lines(difficult to see in the spectra but obvious in the model ceruloplasmin spectra[43–44].
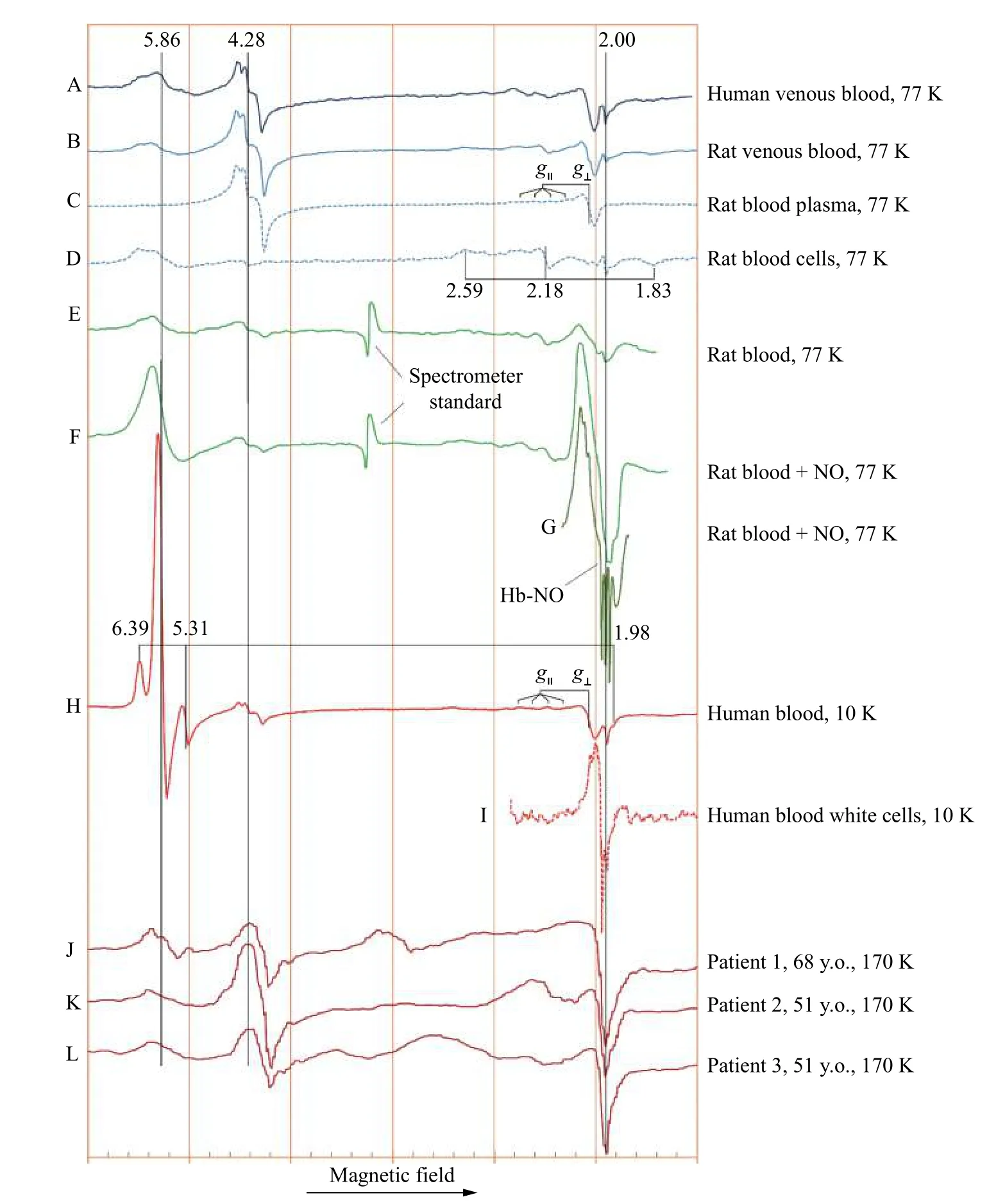
Fig. 1 Low temperature X-band (9 GHz microwave source) EPR spectra of human and rat blood, and blood components. Spectra published in different papers have been scanned and digitised using UN-SCAN-IT 6.0 (Silk Scientific, USA). As slightly different microwave frequencies were used in different studies, the same EPR signals appear at slightly different values of the magnetic field (the horizontal axis). Because of that, the values of the field, increasing from left to right, are not shown in the figure, but rather the orange gridlines, drawn at a 500 Gauss interval, indicate the overall scale of the scan. All spectra have been stretched/compressed to a common horizontal scale by making the same distance between two reference EPR signals, typically the g=4.3 from transferrin and g=2.00 from free radical or metHb. The g-values of the key EPR signals are indicated. The g|| and g⊥ values in the blood (H) and plasma (C) spectra (the latter at the value of about 2.05) are from the Cu2+ ion in the protein ceruloplasmin[43–44]. The temperature values at which EPR measurements have been taken, are indicated. The references to the original papers are as follows: A[45], B–D[46], E–G[47], H[48], I[49], J–L[50]. See detailed description of the spectra in the text.
The cell fraction of blood is characterised by three signals. One is from the free radicals atg=2.00. This signal is not seen at a high intensity in the spectra presented inFig. 1but was characterised in detail in ref.[45]and was shown to be caused by the free radicals formed on the Hb molecule as a result of ferric haem interacting with H2O2. The other two EPR signals are from ferric haem in Hb. The signal atg≈6 is caused by the ferric haem in the high spin (S=5/2) state[17,33,52].Another component of it is atg≈2, which is close to the g-factor of free radicals and therefore not easy to be distinguished. The ferric haem of Hb also can attain a low spin state (S=1/2), in which case it gives a rather different EPR signature—the three-component signal at theg-values of 2.59, 2.18 and 1.83 (Fig. 1A, B, D,andE)[33,52].
The two spectra of rat blood at 77 K inFig. 1B[46]andE[47]reported by different groups of authors are mutually consistent, showing the same set of EPR signals at close proportions. A comparison of spectra inFig. 1EandF(from the same group[47]) demonstrates dramatic changes of the blood EPR spectrum that take place when the animal inhales NO gas before giving blood: the level of metHb is increased but more notable is the appearance of a new EPR signal, which is caused by the complex of NO with ferrous Hb, Hb-NO and shown in greater details in spectrumG.
The EPR spectra similar to the one of the Hb-NO complexes[53](Fig. 1G) have been reported in many systems when physiologically released or externally added NO interacts with haem proteins in the ferrous state. In many cases, it takes place in pathological processes. This EPR signal was first reported in 1969 in three different types of malignant tissues[21]although the representation of the EPR spectra in the publication was not conventional (with a reverse direction of the magnetic field (thus showing thegfactors increasing from the left to the right), probably reflecting the diversity in the EPR instruments at the time. The new EPR signal was explained to be caused by a hyperfine interaction of the electron spin of haem with an N atom but was only later demonstrated to originate from an NO complex with ferrous haems[54].
Spectrum inFig. 1His of whole human blood and should be similar to spectrum inFig. 1A, but it is rather different. The reason for it is the temperature of EPR spectrum detection. Once the temperature is decreased from 77 K to 10 K, the intensity of the high spin ferric haem signal (g=5.86) is significantly increased with respect to the other signals. At the same time, two more lines from a high spin ferric haem enzyme are clearly visible. Instead of the single line atg=5.86 (which is the perpendicular component of theg-value in metHb—almost of the same value along the two directions in the haem's plane), the two lines in the EPR spectrum of this haem enzyme reflect non-equivalence of the EPR absorption along the xand y-directions in the haem's plane—the haem is not axial any more with theg-values being different along the x- and y-directions—6.39 and 5.31. This EPR signal has been well characterised—it is caused by the high spin ferric haem state of catalase[52,55]. Linked to this in-plane anisotropy, the thirdg-value, along to the haem's plane perpendicular direction z (g||) is shifted from theg=2 to a lower value of 1.98. Interestingly,once we see these two components of ferric catalase in the 10 K spectrum (Fig. 1H), we can discern them in the 77 K spectrum of human blood as well, as faint absorbance at the shoulders around the maing=5.86 line (Fig. 1A).
It is difficult to comment on the white cells isolated from human blood spectrum (Fig. 1I), and more spectra from other sources should be helpful. The spectrum is too narrow to be assigned to a copper complex. Part of the spectrum might be associated with free radicals but the origin of the main EPR line is not clear. Possibly, a part of the overall line shape originates from a non-haem iron complex with NO(dinitrosyl complexes)[56–57]. It is known that a similar EPR signal was observed during activation of macrophages[58].
The last three spectra inFig. 1J–Lpublished by the same group of authors bring an important message.The three EPR spectra are from the same kind of samples—from blood of three female breast cancer patients—of 68, 51 and 51 years old. The worrying thing is that the three spectra differ significantly from each other, although obviously of the same pathology kind. The spectra differ in the line shape of the high spin ferric haemoglobin EPR signal as well as of that of the 4.3 signal from transferrin. The strong feature close tog=2.05 signal is not from ceruloplasmin since theg-value is slightly but statistically significantly different. It still can be caused from Cu2+ions, occasionally seen as a contaminant (which might be coming, to our experience, from the copper parts of the syringe needles used). The authors conclude that the EPR spectroscopy is not giving a clear signature of the tumour tissue which poses questions on whether it could be used for diagnostics of this pathology. The spectra registration temperature of 170 K is likely set by a liquid nitrogen variable temperature unit. At that value of the temperature, a significant variance might occur, potentially producing a variability in the EPR spectra detected.
We would like to emphasise that the variability of the EPR spectra of biological and particularly blood samples is caused by two factors: individual physiological/biochemical status of the subject (the patient) and the way the blood sample is prepared. We strongly believe that once the sample making methodology is standardised to the degree that a number of samples from the same subject provide virtually identical EPR spectra, we might be in the position to draw conclusions about the differences, at a statistically significant level, between normal and cancerous blood (or blood from the patients with another pathological condition). The issue of the variability of the blood EPR spectra of different subjects will persist, but it will be easier to address once we have a firm methodology of preparing blood samples and analysing them by the EPR spectroscopy.
Conclusions
Continuous wave X-band EPR spectra of blood exhibit intimate details of the paramagnetic status of the donor's blood. Statistical significance of the difference between EPR data from normal subjects and those with a pathology depends on two factors,one being much more manageable that the other—the methodology of sample preparation. While blood from different patients of the same cohort might differ as well (and that constitutes a challenge imposed by the other factor!), the ground zero approach should be in developing a methodology of taking blood samples from patients which would ensure identical EPR spectra from a number of samples taken from the same person.
杂志排行
THE JOURNAL OF BIOMEDICAL RESEARCH的其它文章
- Editorial commentary on the special issue of Advances in Nanomedicine
- Superior in vitro anticancer effect of biomimetic paclitaxel and triptolide co-delivery system in gastric cancer
- Nanobody-based immunosensing methods for safeguarding public health
- Bacterial nanocellulose production and biomedical applications
- Hybrid lipopolymer vesicle drug delivery and release systems
- Mechanotransduction, nanotechnology, and nanomedicine
