Hybrid lipopolymer vesicle drug delivery and release systems
2021-10-19ErikReimhultMudassarMumtazVirk
Erik Reimhult, Mudassar Mumtaz Virk
Department of Nanobiotechnology, Institute for Biologically Inspired Materials, University of Natural Resources and Life Sciences, Vienna, 1190 Vienna, Austria.
Abstract Hybrid lipopolymer vesicles are membrane vesicles that can be self-assembled on both the micro- and nanoscale. On the nanoscale, they are potential novel smart materials for drug delivery systems that could combine the relative strengths of liposome and polymersome drug delivery systems without their respective weaknesses.However, little is known about their properties and how they could be tailored. Currently, most methods of investigation are limited to the microscale. Here we provide a brief review on hybrid vesicle systems with a specific focus on recent developments demonstrating that nanoscale hybrid vesicles have different properties from their macroscale counterparts.
Keywords: hybrid vesicles, lipopolymersomes, polymersomes, drug delivery, lipid domains, triggered release
Introduction
Encapsulating drugs and vaccines is highly beneficial for multiple reasons. In simple drug delivery systems, polymer modification or polymer micelles are used to protect the drug from the environment, increase solubility, and improve bioavailability. Micellar polymer drug delivery systems have a minimal size with a limited carrier capacity, and they can suffer from low stability due to the high critical micelle concentration of micelleforming polymer surfactants.
Vesicular delivery systems, therefore, are important as they have large but still nanoscopic sizes, low critical aggregation concentration of the surfactants,and a significantly high cargo capacity. Due to the amphiphilic membrane, they can transport hydrophobic and amphiphilic compounds as well as watersoluble compounds in their large lumen[1]. As all encapsulating drug delivery systems, a vesicle protects cargo from enzymatic degradation[2–3]. Furthermore,the delivery vehicle's surface functionality can be modified to target them to certain locations[4–10].Decreased degradation and a high degree of localization of released cargo allow for much lower overall concentrations of the drug than those required for systemic release. With the decrease in the injected and freely available drug dose, the risk of adverse side effects,e.g., toxicity or immune system reaction, is lowered[11–12].
Targeted release can be further improved by a triggered release that increases the release rate only when and where a compound should be active. In addition to drug delivery, several biotechnological applications also benefit from vesicles with an externally controlled triggered release. Delivery of compounds, more efficient transfection mechanisms,poration of cells, and even artificial organelles incorporated into cells are all possible applications that go hand in hand with a greater understanding of membrane release mechanisms. Triggers can be of many kinds,e.g., induced by changes in the local environment such as pH changes upon cell internalization, enzymatic degradation, light, or other electromagnetic irradiation that also can be actively controlled by external means and independent of the local environment[13–15].
Due to their inherent biocompatibility and significant success in clinical use, polymer-functionalized liposome drug delivery systems have attracted a lot of attention. However, analogous systems have been considered based on polymersomes, with the hope of overcoming some of the disadvantages of liposomes,e.g., low stability, high cost, and limited chemical and physical versatility. Polymersomes use synthetic block copolymers to predictably achieve vesicular structures with defined dimensions and a greater spectrum of mechanical and chemical properties than liposomes.
One of the significant drawbacks of liposomes as drug delivery vehicles is their low mechanical stability, mainly caused by their low membrane thickness and high permeability to many encapsulated compounds, especially under stress. As a result, the controlled release of encapsulated content only at a target tissue in drug delivery applications remains a challenge. Polymersomes, on the other hand, display higher mechanical stability due to their comparatively thicker amphiphilic membranes. However, polymersomes have the drawback of relatively lower biocompatibility because of the use of synthetic polymers that have slow or no biodegradability and higher toxicity. The high mechanical stability and low permeability of polymersomes are useful for storage.Still, they also make it difficult for low molar mass species to diffuse into or out of polymersomes membranes, making fast or triggered release challenging to achieve. A way to tackle this challenge consists of constructing stimuli-responsive membranes that become permeable upon exposure to a specific trigger[16–20]. A vesicle that mixes the polymers and lipids could combine the desired benefits of each. At the very least, investigating such hybrid vesicles formed from lipids and block copolymers increases the range of material properties important for biomedical applications that can be realized.
This focused review discusses the intersection of research on liposomes and polymersomes as drug delivery and triggered release systems. In particular, it highlights hybrid membrane systems, in which lipids and polymer amphiphiles are combined. Though there are some investigations of the properties of such systems, they are notably missing for nanoscale small and large unilamellar vesicles that can be used for drug delivery and biotechnological applications.However, we will discuss some general insights and a few examples of ingenious release mechanisms applicable to hybrid vesicle systems already in the literature.
Polymer-lipid hybrid vesicles
Hybrid vesicles are vesicles assembled from a blend of phospholipids and block copolymers,i.e.vesicles formed with membranes constituted of both components. In this way, the mechanical stability and tunability of polymersomes are combined in a single system with the biocompatibility and biofunctionality of liposomes.
Although not yet a field as established as the study of liposomes and polymersomes, several systems of hybrid vesicles have been investigated. Most of these studies have been performed on polymers based on poly(dimethylsiloxane) (PDMS)[21–22], poly(isobutylene) (PIB)[23–24], or poly(butadiene) (PBD)[25–28]as hydrophobic blocks and poly(ethylene oxide) (PEO)or poly(2-methyl-2-oxazoline) (PMOXA) as hydrophilic blocks. These polymers exhibit a low glass transition temperature allowing sufficient flexibility to the polymer chains during their formation. Concerning the choice of lipids, most studies were performed with phosphatidylethanolamine[22,25]or phosphatidylcholine[21,23,26–28]head groups with either saturated or unsaturated tails.Table 1summarizes different block copolymers and lipid compositions used to assemble hybrid vesicles[21,24–25,27–32].
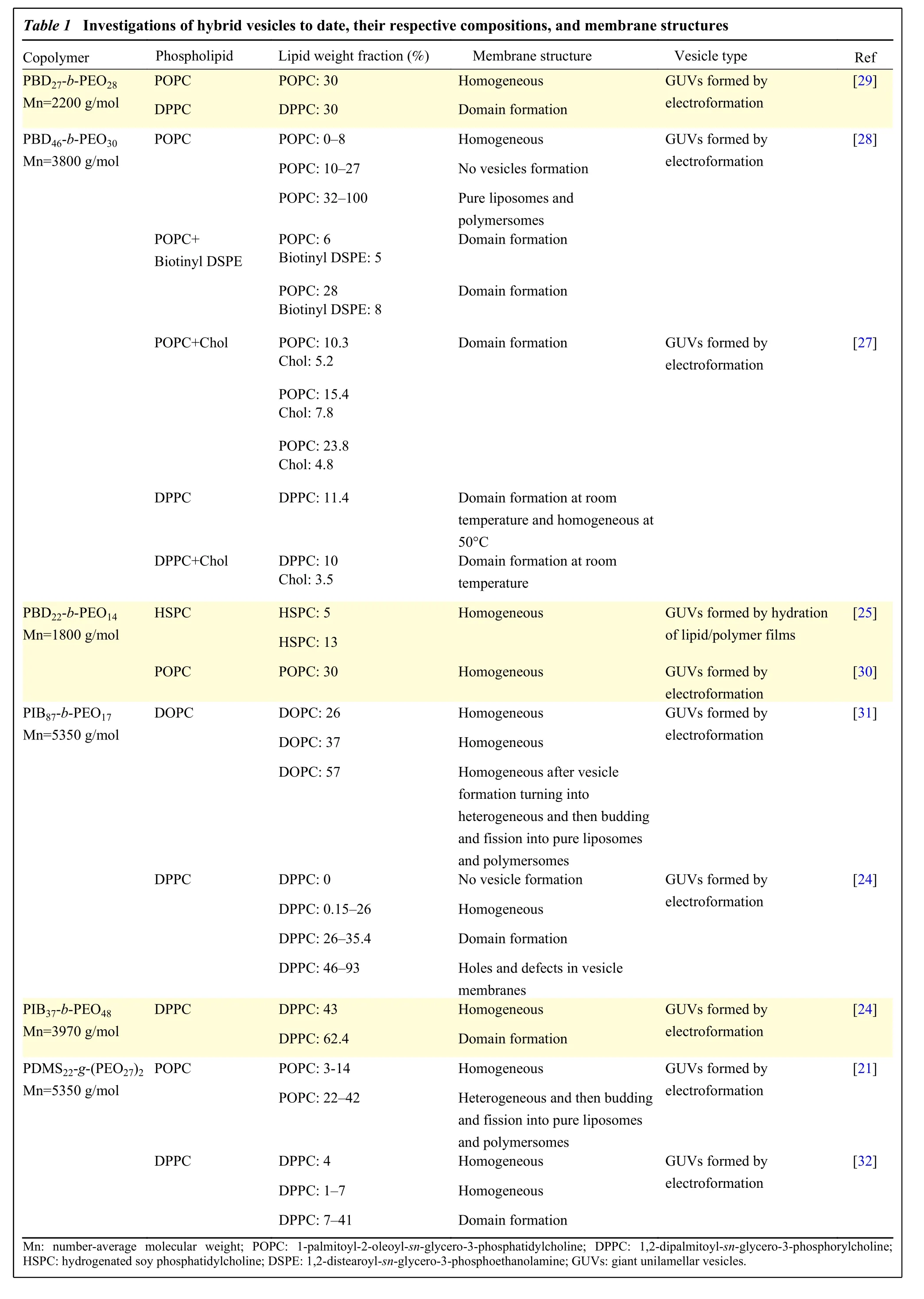
Table 1 Investigations of hybrid vesicles to date, their respective compositions, and membrane structures Copolymer Phospholipid Lipid weight fraction (%) Membrane structure Vesicle type Ref PBD27-b-PEO28 Mn=2200 g/mol POPC POPC: 30 Homogeneous GUVs formed by electroformation[29]DPPC DPPC: 30 Domain formation PBD46-b-PEO30 Mn=3800 g/mol POPC POPC: 0–8 Homogeneous GUVs formed by electroformation[28]POPC: 10–27 No vesicles formation POPC: 32–100 Pure liposomes and polymersomes POPC+Biotinyl DSPE POPC: 6 Biotinyl DSPE: 5 Domain formation POPC: 28 Biotinyl DSPE: 8 Domain formation POPC+Chol POPC: 10.3 Chol: 5.2 Domain formation GUVs formed by electroformation[27]POPC: 15.4 Chol: 7.8 POPC: 23.8 Chol: 4.8 DPPC DPPC: 11.4 Domain formation at room temperature and homogeneous at 50°C DPPC+Chol DPPC: 10 Chol: 3.5 Domain formation at room temperature PBD22-b-PEO14 Mn=1800 g/mol HSPC HSPC: 5 Homogeneous GUVs formed by hydration of lipid/polymer films[25]HSPC: 13 POPC POPC: 30 Homogeneous GUVs formed by electroformation[30]PIB87-b-PEO17 Mn=5350 g/mol DOPC DOPC: 26 Homogeneous GUVs formed by electroformation[31]DOPC: 37 Homogeneous DOPC: 57 Homogeneous after vesicle formation turning into heterogeneous and then budding and fission into pure liposomes and polymersomes DPPC DPPC: 0 No vesicle formation GUVs formed by electroformation[24]DPPC: 0.15–26 Homogeneous DPPC: 26–35.4 Domain formation DPPC: 46–93 Holes and defects in vesicle membranes PIB37-b-PEO48 Mn=3970 g/mol DPPC DPPC: 43 Homogeneous GUVs formed by electroformation[24]DPPC: 62.4 Domain formation PDMS22-g-(PEO27)2 Mn=5350 g/mol POPC POPC: 3-14 Homogeneous GUVs formed by electroformation[21]POPC: 22–42 Heterogeneous and then budding and fission into pure liposomes and polymersomes DPPC DPPC: 4 Homogeneous GUVs formed by electroformation[32]DPPC: 1–7 Homogeneous DPPC: 7–41 Domain formation Mn: number-average molecular weight; POPC: 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphatidylcholine; DPPC: 1,2-dipalmitoyl-sn-glycero-3-phosphorylcholine;HSPC: hydrogenated soy phosphatidylcholine; DSPE: 1,2-distearoyl-sn-glycero-3-phosphoethanolamine; GUVs: giant unilamellar vesicles.
The crucial parameters controlling the formation of stable hybrid vesicles are the size of the hydrophobic mismatch of lipids and polymers and their weight or mole fraction. The effects of size mismatch of hydrophobic segments and lipid fluidity on giant vesicles have been investigated thoroughly for PDMSbased copolymers. It has been shown that hybrid vesicles formed in a broad range of amphiphilic compositions are stable at low lipid fraction for block copolymers with high molecular weight[33–34]. Phaseseparated vesicles formed at high lipid fraction are not stable; budding and fission are observed. This process eventually leads to the formation of independent populations of pure liposomes and polymersomes. In another study, it was demonstrated that a minimum of 65 mol% of PBD46-b-PEO30block copolymer was required to form hybrid giant vesicles with 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphatidylcholine(POPC)[28]. However, stable hybrid giant vesicles based on PDMS-g-PEO copolymers were observed even at higher lipid contents when the lipids were in the gel state[21]. When the lipids are in the gel state,phase separation instead occurs within the membrane of giant vesicles, without the process of budding and fission. The result is distinct domains of lipids and polymers within the membrane on the micron scale.The molar ratio determines which component is the continuous phase[21].Fig. 1shows a schematic overview of different morphologies of hybrid vesicles at room temperature according to their compositions of PDMS-g-PEO blended with various molar ratios of POPC and 1,2-dipalmitoyl-sn-glycero-3-phosphorylcholine (DPPC). However, as other compositions were investigated lately, it is clear that the choice of parameters controlling the production of hybrid vesicles is not trivial. Further investigations must be undertaken for a better understanding and improved predictability. For example, kinetic factors and thereby method of formation could play an important role in the observed results. Additionally, it is not clear how micron-scale observations for giant vesicles translate to biomedical vesicles formed on length scales much smaller than the phase separation events of domain formation, budding, and fission observed in giant vesicles.
Structure of membranes of hybrid vesicles
As mentioned for the domain formation and illustrated inFig. 1, lipids are homogeneously distributed throughout the membranes or form domains. Which case happens depends upon physical properties such as stiffness of polymer chains, melting temperature of lipids, and volume fractions of lipids and polymers[35].Table 1tabulates for which systems homogeneous distributions or domain formation have been reported.
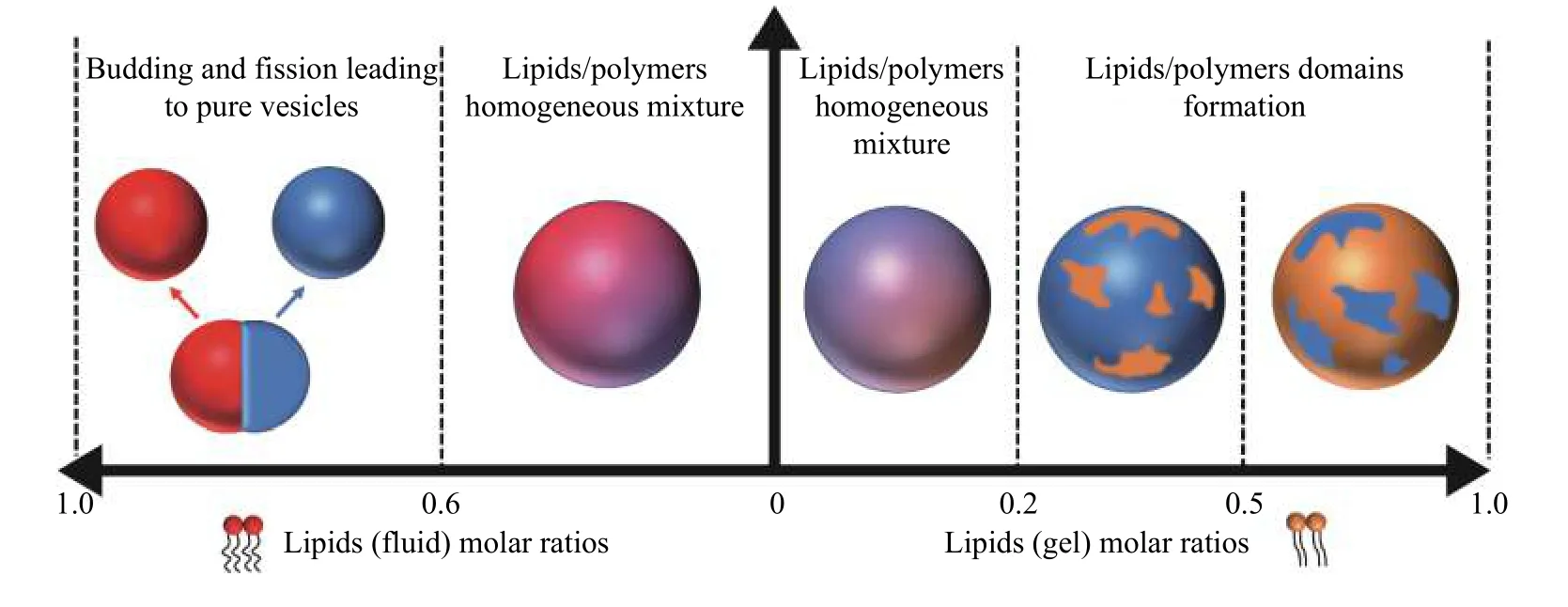
Fig. 1 Overview of different hybrid structures observed for various molar ratios of PDMS-g-PEO blended with POPC and DPPC.PDMS: poly(dimethylsiloxane); PEO: poly(ethylene oxide).
In most cases, the lipids are homogeneously distributed when they are above their melting temperature. Domains form when the lipids are in the gel phase. For example, lipid domains formed in copolymer-rich membranes with lipids in the gel phase, such as hydrogenated soy phosphatidylcholine(HSPC)[25]or DPPC[21]. Homogeneous distribution of lipids throughout the membrane was observed when the lipids were above their melting temperatures[22–23,27–28]. However, it is also possible to create lipid domains above theTmof the lipids by introducing an external constraint that separates the lipids. Namet alachieved this for hybrid vesicles composed of POPC and copolymer polybutadiene-bpoly(ethylene oxide) (PBD-b-PEO) by biotinylation of one of the constituents and crosslinking with the protein NeutrAvidin[28]. Hydrophobic mismatch between lipids and polymers also plays an essential role in controlling membrane structure. If there is a sizeable hydrophobic mismatch between lipids and polymers,the high line tension at the interface between lipids and polymers drives the domain formation. One plausible scenario to reduce the line tension is the adaption through elastic deformation of polymer chains at the interface between the lipid and polymer domains, as shown inFig. 2A. Stiff polymer chains cannot adapt in this way. Then, homogeneous distribution of lipids is expected instead, as shown inFig. 2B. This shows that the chain length and stiffness of the hydrophobic polymer backbone also play a vital role in the lateral structure of membranes of hybrid vesicles[35].
Adding cholesterol to lipid/polymer mixtures controls the shape of domains. For instance, hybrid vesicles with circular lipid domains in the fluid phase were obtained with the addition of cholesterol[27]. In the same work, Namet alproposed a method, which consists of forming hybrid vesicles comprising PBDb-PEO, blended with DPPC above the melting temperature of DPPC and cooling the system below the melting temperature at different cooling rates[27].This procedure could control the size and number of domains in the hybrid vesicle membranes. When the cooling is fast, a large number of small domains form,while slower cooling leads to fewer and larger domains. It is interesting to note that the surface fraction of lipids decreases with increasing cooling rate. A plausible reason for this observation is that some domains are too small to be detected by optical microscopy. The work by Namet alshows that one should be careful to construct phase diagrams with current methods of membrane formation and characterization, as kinetically trapped morphologies can be mistaken for equilibrium structures.
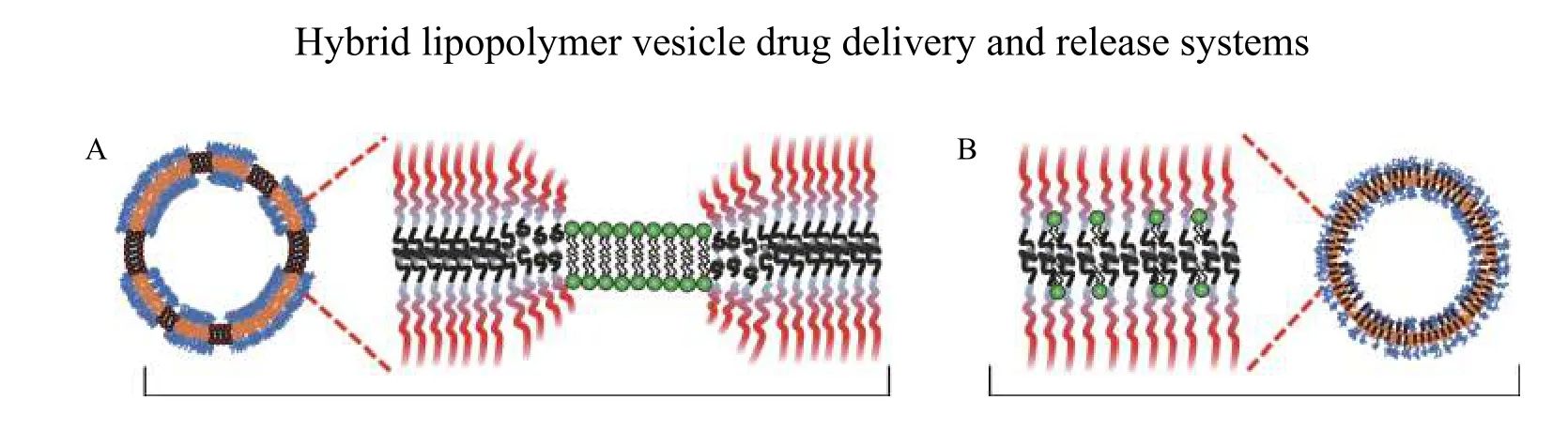
Fig. 2 Lipid mixing in block copolymer membranes. A: Elastic deformation of polymers at the phase domain boundary in case of a sizeable hydrophobic mismatch between lipids and block copolymers can favor domain formation to minimize the line tension. B: The absence of adaption of the chain conformation due to a stiff polymer backbone could favor homogeneous distribution of lipids and polymers in the membrane.
Structure of membranes of nanoscale hybrid vesicles
Investigations on formulation and mixing behavior of lipids and block copolymers are mostly done on giant unilamellar vesicles (GUVs) using optical microscopes as mentioned above. However, nanoscale vesicles required for encapsulation and release applications such as drug delivery are much smaller than the micron-sized domains observed in GUVs.The thermodynamic balance between line and surface tension in the membrane as well as the kinetics of formation are very different. Hence, it is rational to debate whether the same phase separation is present within individual nano-sized hybrid vesicles as observed in the phase diagrams for GUVs.
The characterization of membrane morphology at the nanoscale requires much more complicated and indirect methods. There are very few studies in which the phase behavior of nanoscale hybrid vesicles was studied[22,30,33,36–37]. Recently, Daoetalstudied the mixing of PEO-PDMS-PEO with a molecular weight of 3000 g/mol and lipids in nanoscale hybrid vesicles using small-angle neutron scattering and timeresolved Förster resonance energy transfer (FRET)[37].Their observations confirmed the presence of nanodomains of lipids even when the lipids were in the fluid phase. Homogeneous distribution of lipids and polymers was previously shown for the same membrane composition in GUVs[21]. However,microscopy studies on GUVs cannot resolve nanoscale domains and are not subject to the limited membrane size and high curvature of nanoscale vesicles. The same group also studied the mixing behavior of PDMS-b-PEO with different molar masses combined with POPC in the membranes of giant hybrid vesicles using FRET and fluorescence lifetime imaging microscopy. They confirmed the presence of nanodomains in the membrane, which were not visible in confocal images for the same compositions of polymer and POPC[33].
Virket alinvestigated PBD-b-PEO and phosphocholine hybrid vesicles and supported bilayers[29,38]. GUVs showed the previously reported behavior of domain formation for gel phase phosphocholine lipids and homogeneous GUVs for liquid phase phosphocholine lipids. Large unilamellar vesicles in the 100-nm size range of the same compositions showed a different behavior. At a roughly even weight ratio of lipid to polymer, the vesicle composition appeared inhomogeneous.Polymer-rich and lipid-rich vesicles formed instead of a uniform population. These might have corresponded to vesicle compositions that formed stable homogeneous hybrid vesicles. Interestingly,the triggered release of encapsulated dye from the 100-nm vesicles by enzymatic (e.g., PLA2) degradation indicated a rapid release from vesicles with lipids in the fluid phase. In contrast, the homogeneous distribution of lipids in the GUVs led to a slow release on that scale. The reversal of release rates on the nanoscale indicated the formation of nanoscale lipid domains in large fluid vesicles absent in giant vesicles[29]. Similarly, a follow-up study showed that the presence of the interaction with a solid substrate prevented the demixing of lipids and polymers observed in equivalent GUVs[38].
These findings raise severe doubts on the general equivalence of macroscopic and nanoscale phase separation, which is of utmost importance for designing nanoscale hybrid vesicles for applications such as drug delivery.
Physical properties of hybrid vesicles
Physical properties of hybrid vesicles like permeability, fluidity, bending, and stretching elasticity depend on their composition and the mixing behavior of their components (homogeneous or domain formation). These properties are expected to be different from pure vesicles and can be of importance for applications in fields like drug delivery, cell membrane mimics, micro- or nanoreactors.
Membrane toughness
Typical parameters that characterize the membrane toughness are bending rigidity, stretching modulus,and lysis tension. Although such parameters are well studied for pure liposomes and polymers, few data are available for hybrid vesicles. In general, such parameters of hybrid vesicles are expected to be between the values of their respective pure liposome and polymersome counterparts. Chenget alreported that hybrid nanovesicles composed of a homogeneous blend of PBD22-b-PEO14mixed with 25 mol% of HSPC exhibited an intermediate elastic modulus (112 mN/m). This value is between that of pure liposomes(206 mN/m) and polymersomes (72 mN/m)[25].Similarly, Namet alshowed a gradual decrease in elastic modulus with increasing polymer content for hybrid GUVs composed of homogeneous blends of PBd46-b-PEO30and POPC[28]. Alternatively, measurements performed on heterogeneous hybrid GUVs composed of triblock PDMS22-g-(PEO27)2mixed with DPPC indicated that the elastic modulus of polymerrich domains was similar to that of pure polymersomes[32]. The lysis strain of homogeneous hybrid vesicles was always found to be between the values of pure liposomes and polymersomes.
In addition to tension, the elastic deformation and brittleness of a membrane to local pressure or indentation are essential parameters to predict stability and release behavior. They are difficult to measure directly on vesicles. Virket alrecently used atomic force microscopy and force spectroscopy to investigate this for hybrid membranes supported on solid substrates reconstituted from nanoscale hybrid vesicles by vesicle fusion[38]. They found that for membranes blending PBD-b-PEO block copolymers with POPC, the thickness and elastic deformation increased with polymer fraction. However, the pressure at which the membrane ruptured also decreased, showing that the membranes became more brittle to local deformation.
Fluidity
The fluidity of membranes is mostly evaluated by measuring the lateral diffusion coefficients of amphiphiles, which depends on the surface shear viscosity of the membrane. Fluorescence recovery after photo-bleaching (FRAP) measurements is the most common way to measure the lateral diffusion coefficients of membranes. In the case of hybrid membranes, one can access the mobility of either the lipids or the block copolymers depending upon the localization of the fluorescent probes. Lateral diffusion coefficients of lipids are very high compared to those of block copolymers in polymersomes, and considerable differences in surface shear viscosity have been reported[39–40]. Namet almeasured the lateral diffusion coefficient of lipids in the hybrid membranes of GUVs composed of PBD46-b-PEO30and POPC for different compositions. They observed that the diffusion of lipid molecules became slower with increasing block copolymer fraction into the membrane[28]. This is rationalized as the homogeneous insertion of polymer chains into lipid membranes that act as obstacles to free lateral diffusion. In another study, rhodamine-dihexadecanoyl-sn-glycero-3-phosphoethanolamine (Rh-DHPE) lipids did not show fluorescence recovery at room temperature below a threshold polymer fraction in hybrid membranes of GUVs composed of DPPC and PIB87-b-PEO17copolymers[41]. This shows an increase in lateral mobility of lipids in hybrid membranes with the copolymer content. The authors interpreted their observations as the breaking up of rigid DPPC densely packed phase by the copolymer chains. However, it is likely that the Rh-DHPE prefer to be in polymer-rich domains, which presents higher mobility, than in the DPPC gel phase, and the FRAP recovery therefore becomes more visible above a threshold polymer fraction.
Permeability
To fully exploit the advantages of hybrid vesicles, it is crucial to evaluate and control the permeability of such hybrid systems in the presence of osmotic gradients. The permeability of pure polymersomes is far below that of pure liposomes. Therefore,varying the lipid/copolymer fractions tunes hybrid vesicle permeability. Very few studies exist on the permeability of hybrid vesicles. Shenet alinvestigated the water permeability of large unilamellar hybrid vesicles composed of DOPC mixed with different molar fractions (0 to 10 copolymer chains per 100 lipid molecules) of triblock copolymers PMOXA-b-PDMS-b-PMOXA[42]. For shorter triblock copolymers, the permeability weakly decreased with increasing polymer content. In contrast, the permeability of hybrid vesicles with longer triblock copolymers decreased up to 5 mol% of the copolymer as the incorporation of the triblock copolymer increased the membrane packing density. This made the membrane less permeable to water. Due to the large hydrophobic mismatch between the lipid chain and the hydrophobic block of longer triblock copolymers, a high fraction of copolymer led to reduced packing density. As a result, the permeability increased as the polymer content was increased above 5 mol%.
Limet alstudied the passive release of hydrophilic carboxyfluorescein (CF) through nanoscale hybrid vesicles composed of PBD22-b-PEO14blended with different mole fractions of POPC[30]. They found that the permeability of hybrid vesicles depends on the mole fractions of their respective amphiphiles. A higher polymer content significantly reduced the permeability and delayed CF release compared to pure liposomes, as shown inFig. 3A.
Research on hybrid membranes has focused on understanding their morphology and physical properties. Still, we have recently demonstrated how they might differ from polymersomes and liposomes for triggered release applications. Local change of the phase-state of the membrane by heatingviamembrane-embedded nanoparticles has emerged as a novel way to trigger the release of hydrophilic contents from vesicles remotely using,e.g.,alternating magnetic fields[43–45]. This has been demonstrated with precision using the well-described phase states of lipid membranes and superparamagnetic iron oxide nanoparticles. However, attempts to translate it into a similarly efficient release mechanism using thermoresponsive polymer membranes have largely failed[16,46]. Rapid heat diffusion through water and a poor understanding of the structural changes of thermoresponsive polymer membranes are likely to blame for that nanoparticle-triggered release from polymersomes has largely failed. The release was observed either at meager rates[47]or upon significant heating of the environment surrounding the polymersomes[16]. However, experiments showed that large, hydrophobic superparamagnetic iron oxide nanoparticles could be incorporated into hybrid vesicles of PBD-b-PEO and domain-forming phosphocholine lipids in the gel phase (Fig. 3B)[48].This configuration led to efficient release by the magnetothermal actuation of the lipid membraneviathe embedded nanoparticles, similar to pure liposomes(Fig. 3C).

Fig. 3 Release from hybrid vesicles with or without magnetothermal trigger. A: CF release over time through hybrid vesicles consisting PBD-b-PEO (PBPEO) mixed with different mole fractions of POPC. Reprinted with permission from ref.[30]. B: Transmission electron micrograph of ultrathin sections of magnetic nanoparticles localized in PBD-b-PEO polymersomes. C: Release kinetics of encapsulated calcein from hybrid vesicles (30% w/w DPPC) and pure PBD-b-PEO vesicles loaded with 3.5 nm 5% w/w iron oxide nanoparticles. Hybrid vesicles actuated with 40-min alternating magnetic field pulses are shown as the solid black line and their passive release as the dotted black line exemplifying magnetothermally triggered release. The release from the same actuation of equivalent polymersomes is shown as the solid green line, which is indistinguishable from the passive release of the same vesicles (dotted green line). B and C reprinted from ref.[48]. CF: carboxyfluorescein; POPC: 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphatidylcholine; PBD-b-PEO:polybutadiene-b-poly(ethylene oxide).
Similarly, lipid-doped polymersomes,i.e., hybrid vesicles, are susceptible to enzymatic degradation by lipases, which provides a mechanism for triggered release by the increased permeability during membrane degradation[29]. We observed a continuous decrease in vesicle size and loss of content for giant unilamellar hybrid vesicles with homogeneous membranes until they crumpled from phospholipase degradation. If the phospholipids were in the gel state and phase-separated in the PBD-b-PEO hybrid vesicles, a sudden burst release occurred during lipase degradation, but the vesicle retained its size and shape.Interestingly, probing the same compositions as nanoscale vesicles demonstrated higher release rates for vesicles with lipids in the fluid phase and even higher rates for hybrid vesicles than for pure liposomes subjected to lipase degradation. As mentioned above, these observations support the idea of a different phase diagram for nanoscale vesicles,where nanoscale domain formation is likely, and large-scale phase separation for gel phase lipids is frustrated.
Conclusions
Hybrid vesicles are still a relatively new field of investigation in soft matter physics. However, it is already obvious that they offer more opportunities as smart materials for biomedical applications than their better investigated pure polymer and lipid counterparts.The current state of knowledge implies that the richness of phase behavior and structure might increase in nanoscale hybrid vesicles compared to that in microscale hybrid vesicles. On the one hand, this is good news, as it is nanoscale vesicles that are biomedically relevant, and we can therefore tailor them to the application. On the other hand, it sadly means that the already limited knowledge from microscale hybrid vesicles is not entirely relevant to biomedical applications. The emerging studies show that nanoscale hybrid vesicles can be used as triggered delivery vehicles using triggers both inherent to biological environments and remotely actuated. They show advantages in that higher stability can be achieved, while lipid-degrading mechanisms, such as lipases, can be used for controlled release in the tissue.It is also clear that they possess a higher potential for biomimetic interactions with cell membrane receptors because they more closely mimic the organization and interactions of biological cell membranesviathe lipid component. Finally, it is crucial to trigger rapid release at the delivery destination, which has always been an Achilles' heel for polymersomes. Severalin vitroexamples of triggered release from hybrid vesicles greatly surpass the release rates of equivalent polymersome systems. The major concern on the route forward is whether nanoscale hybrid vesicles can be reproducibly assembled with the stability advantage of polymersomes, given their complex phase behavior on the nanoscale.
Acknowledgment
We acknowledge funding for the work from the European Research Council under the European Union's Seventh Framework Program (FP/2007-2013)/ERC grant agreement No. 310034, and the Austrian Science Fund (FWF) grant No. I 3064.
杂志排行
THE JOURNAL OF BIOMEDICAL RESEARCH的其它文章
- Editorial commentary on the special issue of Advances in Nanomedicine
- Superior in vitro anticancer effect of biomimetic paclitaxel and triptolide co-delivery system in gastric cancer
- Nanobody-based immunosensing methods for safeguarding public health
- Bacterial nanocellulose production and biomedical applications
- EPR spectroscopy of whole blood and blood components: can we diagnose abnormalities?
- Mechanotransduction, nanotechnology, and nanomedicine
