Degradation of the fungicide metalaxyl and its non-extractable residue formation in soil clay and silt fractions
2021-10-15RoschniKALATHOORJensBOTTERWECKAndreasSCHFFERBurkhardSCHMIDTandJanSCHWARZBAUER
Roschni KALATHOORJens BOTTERWECKAndreas SCHÄFFERBurkhard SCHMIDT and Jan SCHWARZBAUER*
1Institute of Geology and Geochemistry of Petroleum and Coal,RWTH Aachen University,Aachen 52062(Germany)
2Institute for Environmental Research,RWTH Aachen University,Aachen 52062(Germany)
3School of the Environment,Nanjing University,Nanjing 210023(China)
ABSTRACT The proportion of organic matter and mineral composition are important factors determining the formation and type of non-extractable residues(NERs)of pesticides in soil.In this study,we investigated the enantioselectivity in degradation and NER formation of the chiral fungicide metalaxyl in soil particle size fractions(silt and clay).Microbial and extracellular enzyme activities during these processes were monitored in incubation of silt and clay samples isolated from sterilized and non-sterilized soil samples collected from a long-term agricultural field experimental site in Ultuna,Sweden.The temporal influence on the fate of the fungicide was noted by short-term(10-d)and long-term(92-d)incubations.Besides the acquisition of quantitative data with gas chromatography-mass spectrometry(GC/MS),stereoselective analyses were performed with chiral GC/MS.Quantitative results pointed to a higher metabolism rate of the pesticide through microbial activity than through extracellular enzyme activity.This was also confirmed by the enantioselective depletion of R-metalaxyl and the subsequent formation of R-metalaxyl acid in microbially active samples from non-sterilized soil.The silt fraction containing a high amount of organic matter exhibited a significant hydrolyzable proportion of metalaxyl NERs that was releasable under alkaline conditions.On the contrary,the clay fraction showed an enhanced affinity for covalently bound residues.Based on our results,we recommend differentiating between reversibly and irreversibly bound proportions of pesticides in persistence and environmental risk assessment because the reversible fraction contained potentially bioavailable amounts of residues that may be released under natural conditions.
Key Words:chiral fungicide,enantioselectivity,metalaxyl enantiomer,non-extractable pesticide residues,organo-mineral complex,pesticide degradation,extracellular enzymes,soil particle size fractions
INTRODUCTION
Controlling excessive contamination of the environment because of the usage of pesticides is a huge challenge facing our future.These xenobiotics can be persistent and highly resistant to natural degradation processes without certainty of their re-accessibility for exposed organisms.Thus far,nonextractable residues(NERs)of pesticides are considered to be degraded and persistent(Gevaoet al.,2000;Barracloughet al.,2005,Barriusoet al.,2008).Recently,a classification scheme of NERs has been described,differentiating them into three types.Type I NERs are sequestered and entrapped residues that contain either the parent substance or transformation products or both and have the potential to be released.Type II NERs are those residues that are covalently bound to soil/sediment organic matter and are considered to be strongly bound at very low remobilization rates.It is noteworthy that harsh environmental and extraction conditions may release both types of xenobiotic NERs,but for type II,this will rarely happen under normal physiological conditions.Type III NERs comprise biogenic NERs that cause no environmental concern because they are formed after degradation of the xenobiotics and anabolic formation of natural biomolecules.The mode of binding to soil and sediment can determine the extent of persistency and remobilization potential of these chemicals(Schäfferet al.,2018).
Adsorbed proportions can be extracted by organic solvent-water mixtures,whereas covalent bonds need chemical degradation procedures to cleave the linkage(Ortega-Calvoet al.,2015).Depending upon the strength of the degradation reagents,these methods differentiate between reversible covalent bonds(ester,amide,and ether)and very strong covalent bonds.In general,covalent bonds are considered to have very low remobilization rates,such as those of humic matter degradation(half-life of years to decades)(Dec and Bollag,1997;Gevaoet al.,2000;Northcott and Jones,2000;Conantet al.,2011).
The incorporation processes of pesticides are also greatly influenced by soil organic matter(SOM)content(Bollaget al.,1992).Similar to the humification process,organic contaminants are transformed and retained in soil geopolymers through abiotic and biotic reactions.Later,the amount of SOM influences the activity of free and immobilized extracellular enzymes and microorganisms with viable enzymes in the soil as SOM is their source of energy(Nannipieriet al.,1980;Edwardset al.,1992;Stevenson,1994;Saviozziet al.,2001;Nannipieri,2006;Sinsabaughet al.,2008;Leinweberet al.,2008).This leads to the assumption of higher biotic activity in soil fractions containing high organic carbon(Schnüreret al.,1985;Gianfreda and Rao,2008;Botterwecket al.,2014;Schaefferet al.,2015).
Considering abiotic reactions,clay minerals are also associated with the formation of humic substances through different polymerization reactions involving free radicals engaged in oxidative coupling of phenolic or anilinic substructure(Bollag and Loll,1983;Senesi,1992).Accordingly,clay minerals,exhibiting large surface areas and predominantly forming complexes with SOM,are also responsible for catalyzing the incorporation and thus the accumulation of xenobiotics into the soil matrices.Here,not only the interlayer regions of minerals,but also the regions of clay that are coated with organic matter,provide microporous areas for possible pesticide retention(Stevenson,1994;Liet al.,2003).
Besides abiotic binding processes,biotic incorporation reactions depend mainly on the activities of microorganisms(involving intracellular enzymatic proteins)and extracellular enzymes(Gianfreda and Rao,2008).Previous studies have shown the impacts of microbial activity and extracellular enzyme activity during pesticide degradation and formation of non-extractable metalaxyl residues(Botterwecket al.,2014;Kalathooret al.,2015a).However,stereochemical aspects have not been followed although stereoselective processes are often directly influenced by enzyme activities(Hühnerfusset al.,1992;Pickelet al.,2010).Thus,very little information exists regarding their effects on xenobiotic retention processes,especially with regard to soil total organic carbon and mineral contents.The fungicide metalaxyl consists ofS-andR-enantiomers,with the latter being an active ingredient.Researchers have noticed favorable adsorption of metalaxyl on soil mineral surfaces,especially in soils with less organic matter(Sukop and Cogger,1992).Kalathooret al.(2015a)studied the enantiomeric distribution of metalaxyl residues during the formation of NERs in clay loam bulk soil from an agricultural field.
We studied the effects of microbial and extracellular enzyme activities on the stereoselective degradation of metalaxyl,including the formation of its NERs,in silt and fractions isolated prior to incubation from sterilized and non-sterilized bulk soil samples.The effects of(free)water-extractable enzymes were also studied as the size fractionation was conducted prior to incubation.In a wider context,we addressed the consequences of certain agricultural management practices,such as tillage,resulting in a partial breakdown of soil particle aggregates and potential uncovering of releasable pesticide residues.Furthermore,understanding the interdependence of microbial activity and extracellular enzymatic activity can be related to the effects of climatic conditions,such as drought,on the degradation potential of soils.
MATERIALS AND METHODS
Chemicals
The metalaxyl used was a non-labeledrac-metalaxyl(methyl-N-(methoxyacetyl)-N-(2,6-dimethylphenyl)-DLalaninate,chemical purity>99.3%)obtained from Dr.Ehrenstorfer GmbH,Germany.Metalaxyl acid was synthesized as described by Kalathooret al.(2015b).Metaxalyl stock solutions of 2 μg mL-1were used.The surrogate standardsd10-benzophenone andd4-bis(2-ethylhexyl)phthalate(d4-DEHP)(concentrations:5 ng μL-1)were provided by Sigma Aldrich,USA.All other chemicals and solvents(ethylacetate,dichloromethane,pentane,anhydrous sodium sulfate,and mercuric chloride(HgCl2),the analytical purity grade)were purchased from Sigma Aldrich.
Soil samples used and soil sterilization
The soil samples used were taken in 2010 from a longterm agricultural field experimental site in Ultuna,Sweden,from depths of 0–17 cm(Ah horizon).The samples were dried at room temperature and sieved(2-mm mesh)immediately after sampling and kept at 4°C in the dark.Shortly before experimental usage,the soil was allowed to warm to 20°C,and sterilization was conducted according to Alef and Nannipieri(1995)with mercuric chloride treatment followed by chloroform fumigation.Sterilization allows inhibition of microbial activity,but does not significantly reduce extracellular activity(Botterwecket al.,2014;Kalathooret al.,2015b).This method allowed us to obtain soil exclusively with extracellular enzyme activity after inhibiting microbial activity.A detailed description of soil sterilization is published elsewhere(Kalathooret al.,2015b).Some relevant soil characteristics are given in Table I.All soil samples were checked for any metalaxyl residues before use for particle size fractionation and incubation.
Silt and clay fraction isolation and incubation
A schematic overview of the experimental setup and relevant fractionation is given in Fig.1.Particle size fractionation of the sterilized and non-sterilized bulk soil sampleswas conducted to isolate silt and clay fractions as formerly described(Kalathooret al.,2015b).Low-energy sonication(0.17 kJ g-1output energy)allowed for the dispersion of the soil-water suspension,yet preserved the microaggregates of the silt and clay fractions as well as enzymatic activities(Stemmeret al.,1998).Metalaxyl-free sea sand was added into each sample for better handling of the silt and clay fractions.Metalaxyl(10 μg)was dissolved in deionized water(5 mL)and added to 20 g of air-dried silt with 30 g annealed sea sand(resulting in approximately 0.5 mg metalaxyl per kg silt)or 10 g of air-dried clay with 40 g metalaxyl-free sea sand(approximately 1 mg metalaxyl per kg clay).The moisture content was adjusted to 60% of the maximum water-holding capacity by adding deionized water.Next,the spiked samples were homogenized by extensively mixing with a glass rod for 10 min.All experiments were conducted only in duplicates because of the extensive experimental setup as well as the substantial effort in sample treatment and analytical methods,and spiked samples were kept in the dark to avoid photodegradation(at 15°C).To provide insight into short-and long-term processes,duplicates of each clay or silt sample were taken after 10 and 92 d of incubation.These periods of incubation were designed in accordance with our previous study on short-and long-term reactions of metalaxyl on individual soil constituents and differential biotic influences.Because a detailed statistical analysis of this low number of replicates is not suitable,all data presented here were only calculated as an average of the duplicates and the corresponding standard deviation.
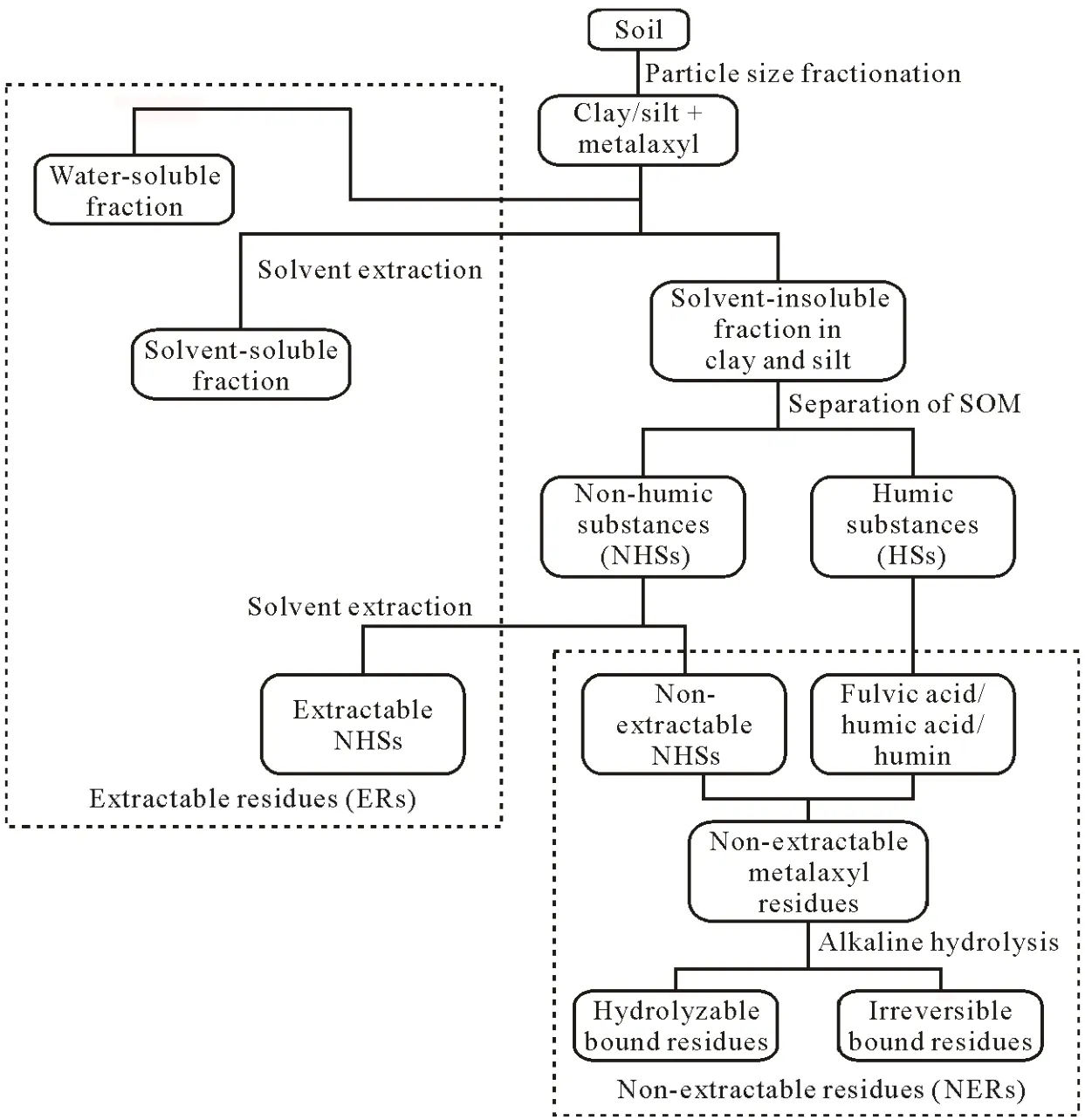
Fig.1 Experimental setup and relevant fractionation and differentiation of extractable and non-extractable residues of the metalaxyl used,rac-metalaxyl,in this study.SOM=soil organic matter.
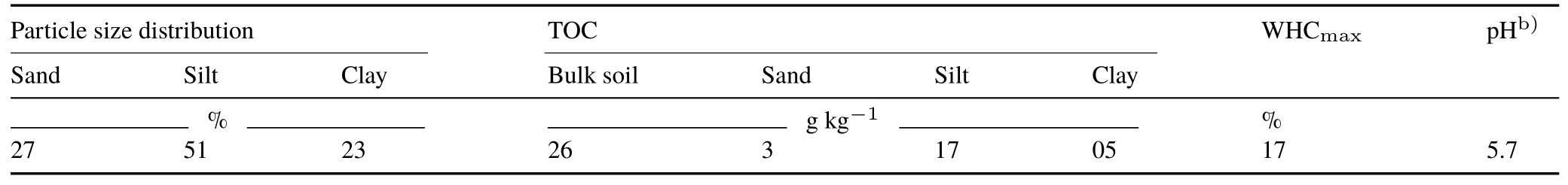
TABLE ISelected propertiesa)of the soil used(0–17 cm,Ah horizon),a soil taken from a long-term agricultural field experimental site in Ultuna,Sweden,in this study(Kirchmann et al.,1994;Gerzabek et al.,2006)
Extractable fractions of metalaxyl residues
Millipore water(approximately 200 mL)was added to each clay or silt sample taken after incubation,and the suspensions were dispersed using an ultrasonic disaggregator(Sonopuls HD200,Bandelin Electronic,Germany)at 0.17 kJ g-1output energy for 6 min as a pre-extraction procedure with water.The additional sea sand was separated through wet sieving and centrifugation.The water-soluble proportion of clay and silt samples was obtained after liquid-liquid extraction of this fractionation water with organic solvents.Metalaxyl was sequentially extracted with ethyl acetate(3×40 mL);although with a pH slightly above 7,metalaxyl acid was subsequently extracted also with ethyl acetate(3×40 mL)with acidified water(pH 2).Each combined solvent fraction was dried over anhydrous sodium sulfate and concentrated to a final volume of 1 mL.The recovery rate for the total procedure was 83.2%±0.5%of the applied radioactivity.
Sokolet al.(1988)verified the complexation of metalaxyl with HgCl2.When using HgCl2as a sterilization agent for soil,this should be taken into account.The formation of a secondary complex with the metabolite metalaxyl acid as the ligand for HgCl2inhibits the extractability of the acid metabolite with dichloromethane.In this study,we used ethyl acetate as the extraction solvent because of its ability to form coordination complexes with metal ions.
The solvent-soluble proportion of metalaxyl residues was obtained by exhaustive extraction of the freeze-dried particulate matter with 200 mL methanol for 6 h by means of a Soxhlet apparatus.Extractable proportions of metalaxyl residues were also obtained from non-humic subfractions after dilute HCl treatment and subsequent liquid-liquid extraction,as the non-humic extractable residue(NH-ER)fraction of metalaxyl residues.Thereafter,all pre-extracted silt and clay samples were fractionated into fulvic acid,humic acid,and humin-mineral complexes using an alkaline separation method according to Stevenson(1994).A detailed extraction procedure for the non-humic and humic matter fractionation has been formerly described(Kalathooret al.,2015b).All solvent-soluble extracts and extractable nonhumic matter fractions were dried over anhydrous sodium sulfate(Na2SO4)and concentrated into a volume of 100 μL.
Non-extractable fractions of metalaxyl residues
Alkaline hydrolysis.A variable volume of 5–30 mL of a 2 mol L-1solution of potassium hydroxide(KOH)was added to the neutralized and dried clay and silt humic acid,fulvic acid,humin-mineral complex and non-humic NER fractions and heated at 105°C for 24 h in a closed vessel.The cooled aqueous phases were acidified(pH 2),and after sonication for 15 min,they were sequentially extracted with ethyl acetate(3×15 mL).Combined organic phases were dried over anhydrous Na2SO4and reduced to a volume of 200 μL.The hydrolysates of each sample were fractionated into five fractions by micro-column chromatography,as formerly described(Kalathooret al.,2015b).The fourth and fifth fractions of the micro-column chromatography were combined and concentrated into a volume of 100 μL.Recovery rates for micro-column fractionation of metalaxyl were 98.5%±0.3%.
High performance liquid chromatography(HPLC)fractionation.The combined polar fractions containing meta
laxyl and metalaxyl acid were further fractionated on a Nucleosil 100-5 C18 AB column(5 μm×4.6 mm×250 mm)with a Nucleosil C8 EC pre-column(Macherey-Nagel GmbH& Co.,Germany)using an HPLC-UV device at 254 nm(Knauer,Germany).The chromatographic conditions and further sample preparation are described in detail elsewhere(Kalathooret al.,2015a).Fractions containing metalaxyl acid were derivatized with diazomethane and concentrated into a volume of 25 μL.Unhydrolyzed metalaxyl-containing fractions were also reduced to a final volume of 25 μL.Recovery rates for HPLC fractionation were 70.5%±0.5%.
Gas chromatography-mass spectrometry(GC/MS)analysis.The GC/MS analyses were performed on a Trace MS mass spectrometer linked to a Trace GC(Thermo Fisher Scientific,USA)equipped with a ZB-5 capillary column(30 m×0.25 mm×0.25 μm,Agilent Technologies,USA).An example of GC/MS measurements is given in Fig.2.Chiral GC/MS analyses were performed on the same instrument with an enantioselective BGB-172 chiral column(30 m×0.25 mm×0.25 μm,20%tert-butyldimethylsilyl-βcyclodextrin dissolved in 15%phenylpolysiloxane and 85%methylpolysiloxane,BGB-Analytik,Switzerland).Chromatographic and mass spectrometric conditions were as formerly described(Kalathooret al.,2015a).Prior to GC/MS analysis,all samples were dried by evaporation at room temperature under ambient pressure and reconstituted to their previous volume in a surrogate standard solution containing 10 ng μL-1ofd10-benzophenone and 9 ng μL-1ofd4-DEHP.
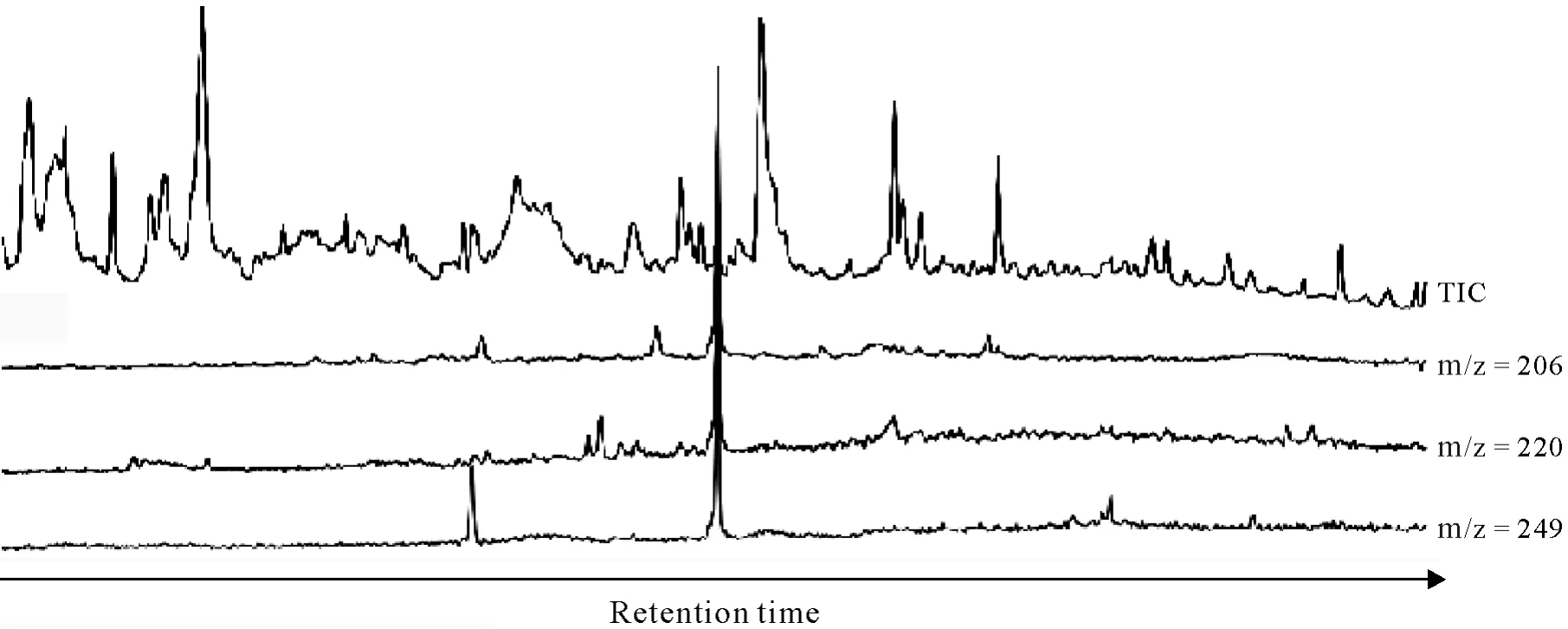
Fig.2 Exemplifying gas chromatography-mass spectrometry(GC/MS)measurement of metalaxyl in an extract obtained after 10-d incubation of a sample of soil silt fraction isolated prior to incubation.Besides the total ion chromatogram(TIC),the specific ion traces with mass-to-charge ratio(m/z)of 206,220,and 249 are illustrated.
The elution order of the chiral GC/MS analyses showedS-prior toR-metalaxyl,with the degree of separation by their resolution(Rs)=0.95.The peak areas of the enantiomers were corrected using the tailing factor at a 5%peak height.The enantiomeric fraction(EF)was calculated using the corrected peak areas:

where AS and AR are the peak areas ofS-andR-metalaxyl,respectively.
RESULTS
In this study,the microbially and predominantly extracellular enzyme-assisted processes were differentiated using sterilized and non-sterilized soil samples,respectively,according to Botterwecket al.(2014),where a particle size fractionation was performed after a short-incubation period(10 d)and a long-term incubation period(92 d)to examine the distribution of the pesticide and its metabolites in the silt and clay fractions.The focus of the present study was to investigate the enantioselectivity of the degradation and NER formation of metalaxyl in the pre-separated soil silt and clay fractions from the sterilized and non-sterilized soil samples.Kalathooret al.(2015a)gave a detailed description of incubation withrac-metalaxyl on bulk soil samples from the same sampling area as used in this experiment.
Extractable metalaxyl residues
Three subsequent extraction steps revealed three different fractions of extractable residues as shown in Fig.1:i)extractable residues from water-soluble fraction,ii)a particle-associated extractable fraction,and iii)NH-ERs.
In the short-term incubation(10 d),both water-soluble and solvent-soluble residues in the clay and silt fractions contained high concentrations of metalaxyl(approximately 30%of the total applied metalaxyl),except the solvent-soluble silt extract of the non-sterilized soil sample,which contained only 11% of the total applied metalaxyl(Table II).The missing proportions were detected in the NH-ER fraction(approximately 21%of the total applied metalaxyl.The silt and clay fractions of the sterilized soil sample delivered higher solvent-extractable proportions than the corresponding fractions of the non-sterilized soil sample,whereas the latter yielded higher amounts of the NH-ER fraction(Fig.3).Such residues of the non-ionic fungicide metalaxyl are considered to be rapidly adsorbed to soil organic surfaces.On the other hand,they have a high affinity to the polar non-humic substances(Rieferet al.,2011a).
The elevated amounts of metalaxyl residues in the clay and silt particulate matter of the sterilized soil sample(especially the latter)in comparison to those of the non-sterilized soil sample pointed to microbially and enzymatically mediated binding.Under all conditions,metalaxyl acid was only detected in the water-soluble fraction in small amounts(<0.5%of the applied amount).The extractable fractions(water-soluble,solvent-soluble,and NH-ER),in total,accounted for approximately 60%–70%of the initially applied metalaxyl.The non-sterilized and sterilized soil silt fractions contained higher residue concentrations than their clay counterparts.All quantitative values with standard deviations are shown in Table II.
The EF values of the three extractable fractions(watersoluble,solvent-soluble,and NH-ER)showed an unchanged racemic mixture of metalaxyl identical to that of the appliedrac-metalaxyl withRsvalues of approximately 0.48.However,analysis of the main metabolite metalaxyl acid revealed stereoselective degradation.In the water-soluble fractions of the non-sterilized soil sample,R-metalaxyl acid had an EF value of approximately 0.23.On the contrary,the sterilized soil subfractions still contained the racemic mixture of the metabolite.This accorded with findings of the previous study in which stereoselective degradation of metalaxyl was associated with microbial activity(Kalathooret al.,2015a).However,because there was no corresponding depletion notable in the extractable proportions of the parent compound and because of the low concentrations of the acid,this enrichment only marginally affected the overall enantiomeric distribution.
Comparing the short-term incubation(10 d)to longterm incubation(92 d),the total extractable proportions of the fungicide were similar(50%–70%)with slightly lower amounts for the clay fraction than for the silt fraction(Table II).The formation of the main metabolite metalaxylacid increased after 92 d of incubation to 26%and 30%of the applied amount in the clay and silt fractions from the non-sterilized soil sample,respectively.Only very low concentrations of metalaxyl acid were detected in the fractions from the sterilized soil sample(1%–3%).Solvent-soluble metalaxyl,as well as the NH-ER fraction,was clearly reduced after 3 months of incubation(1%–13%).The higher particle-associated extractable proportions were obtained in the clay(7%)and silt(18%)fractions of the sterilized soil sample,pointing to increased microbially assisted retention of the fungicide on organo-mineral complexes,especially the organo-clay mineral matter(Fig.3).

Fig.3 Relative distributions of different fractions of extractable residues(ERs)of metalaxyl(MX)and its main metabolite,metalaxyl acid(MXA),after the short-term(10-d,a)and long-term(92-d,b)incubations of clay and silt soil fractions isolated from sterilized and non-sterilized soil samples(SS and NSS,respectively).Values for each fraction are means with standard deviations shown by vertical bars(n=2).NH-ERs=non-humin ERs;SERs=ERs by solvent;WERs=ERs by water.
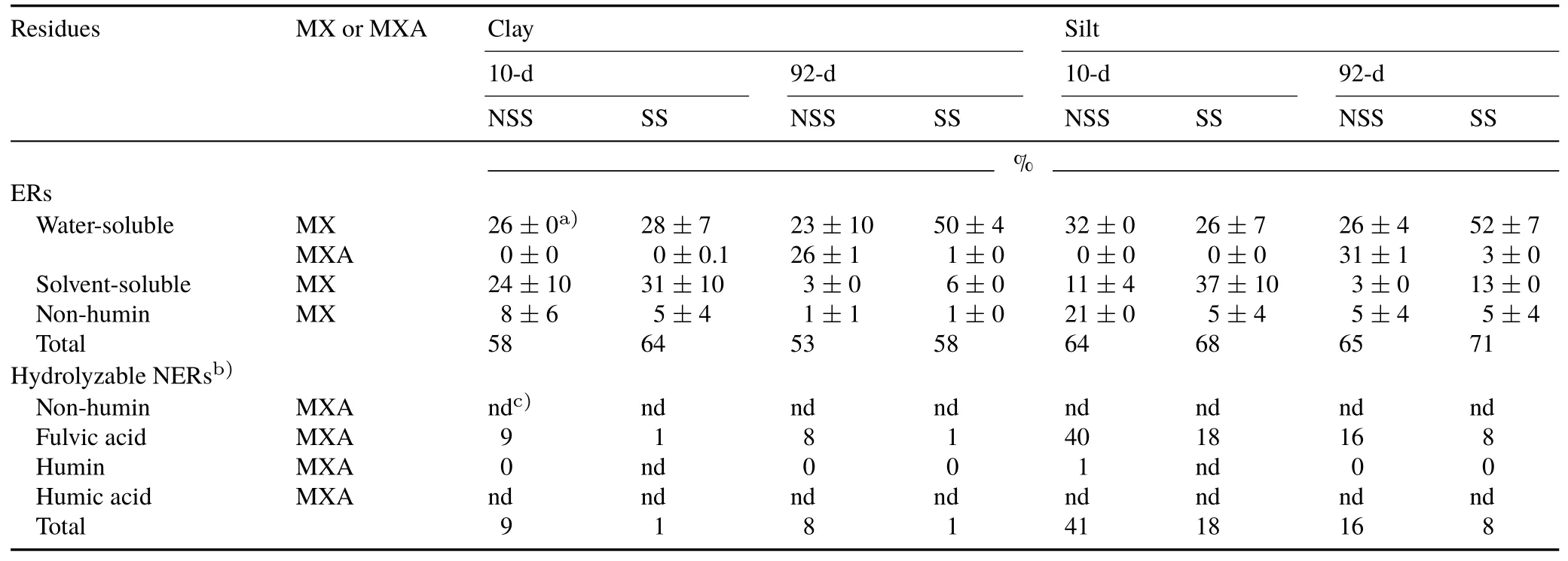
TABLE IIExtractable residues(ERs)and alkaline hydrolysis-releasable non-extractable residues(NERs)of metalaxyl(MX)and its main metabolite,metalaxyl acid(MXA),expressed as percentages of the total applied MX,after the short-term(10-d)and long-term(90-d)incubations of clay and silt fractions isolated from sterilized and non-sterilized soil samples(SS and NSS,respectively)
After 92 d of incubation,slightly higher amounts of metalaxyl residues were also extracted from the silt fraction rather than from the clay fraction.The soil used predominantly contained illite as its main clay mineral(Kirchmannetal.,1994;Gerzabeket al.,2006).Illite is a nonexpanding 2:1 layered clay.Therefore,the extractable metalaxyl residues were likely sequestered in the micropores of the organo-clay complexes and/or in voids of humic matter.The presence of expanding clay minerals,such as montmorillonite,may also allow intercalation of the fungicide or its metabolite in the interlamellar spaces of the clay minerals(Stevenson,1994).
A clear stereoselective shift from the initial enantiomeric distribution(EF=0.48)was observed after metabolism of metalaxyl to metalaxyl acid in the fractions from the nonsterilized soil sample(Table III).While the EF values of the clay and silt subfractions of the sterilized soil sample(including water-soluble metalaxyl acid)remained unchanged,those from the non-sterilized soil sample showed a clear preference for one enantiomer.Water-and solvent-soluble and NH-ER fractions of the clay and silt fractions from thenon-sterilized soil sample had EF values of approximately 0.77–0.93,showing enrichment ofS-metalaxyl.Complementarily,the EF values of the metalaxyl acid showed a distinct preference for the faster degradingR-metalaxyl(0.04 and 0.06 for the clay and silt fractions of the nonsterilized soil sample,respectively).This priority for one specific enantiomer during degradation was also an indicator of microbial assistance in the degradation process.Only a weak stereochemical effect was noticed by the formation of metalaxyl acid in the water extract of clay and silt fractions from the sterilized soil sample(EF of approximately 0.45).Quantitative data for the total extractable metalaxyl proportions were combined with enantiomer fractions to demonstrate the stereochemical effect in the degradation and metabolism of metalaxyl after 92 d(Fig.4).
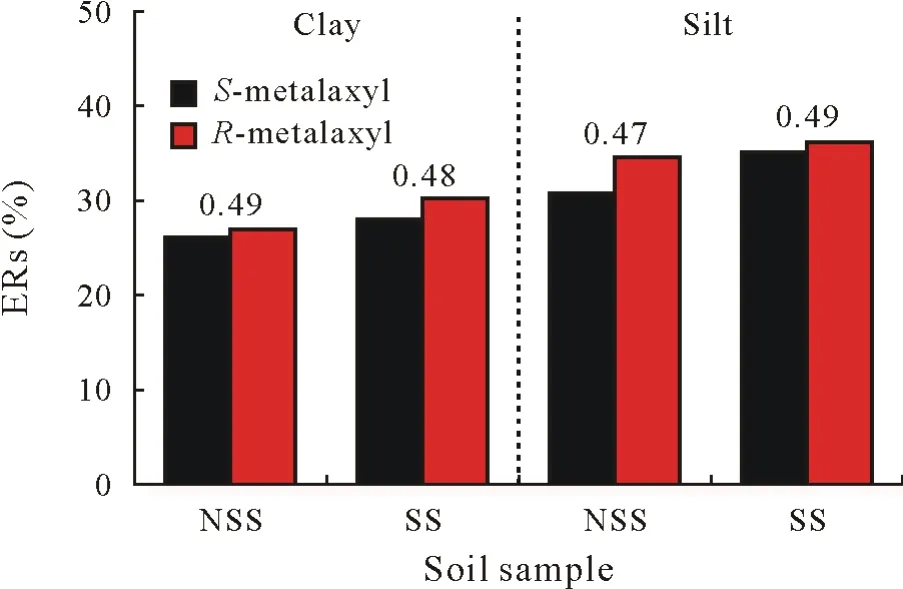
Fig.4 Total extractable residues(ERs)of metalaxyl enantiomers on day 92,i.e.,the chiral gas chromatography-mass spectrometry(GC/MS)data linked with quantitative data of metalaxyl enantiomers after 92-d incubation of clay and silt fractions isolated from sterilized and non-sterilized soil samples(SS and NSS,respectively).The total enantiomeric fraction(EF)values are given over each column pair.
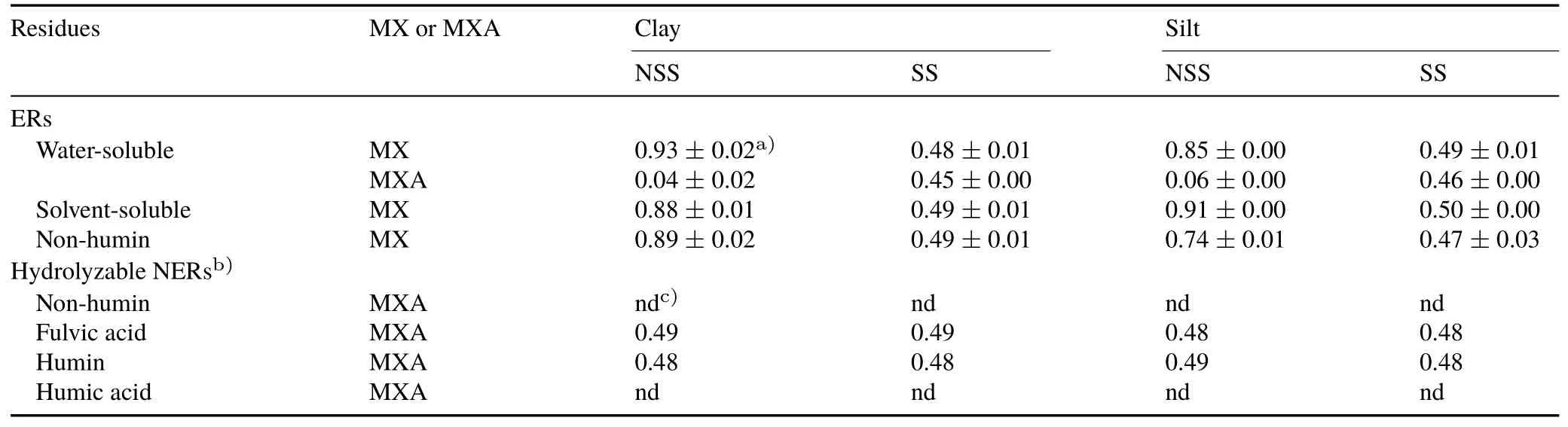
TABLE IIIEnantiomer fraction values of extractable residues(ERs)and hydrolyzable non-extractable residues(NERs)of metalaxyl(MX)and its main metabolite,metalaxyl acid(MXA),after 92-d incubation of clay and silt fractions isolated from sterilized and non-sterilized soil samples(SS and NSS,respectively)
Non-extractable metalaxyl residues hydrolyzable under alkaline conditions
For all samples,conditions,and incubation periods(silt and clay fractions from both sterilized and non-sterilized soil samples after 10 and 92 d of incubation),the highest amount of hydrolyzable NERs was found in the fulvic acid fraction(Fig.5).In the silt fraction from the sterilized soil sample,alkaline hydrolysis released the highest amount of metalaxyl residues for both time periods(40%after 10 d and 16%after 92 d).For the sterilized soil sample,higher amounts were released in the silt fraction(8%–18%)than in the clay fraction(1%).Although the hydrolyzable NER proportions halved in the silt fraction after 92-d incubation compared with those after 10-d incubation,the amounts of the corresponding clay fraction remained nearly constant.Correspondingly,after 92 d of incubation,the water-soluble fractions increased,whereas the particle-associated and strongly incorporated fractions decreased(Table II).
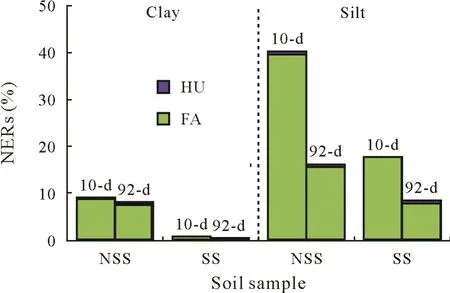
Fig.5 Hydrolyzable non-extractable residues(NERs)of metalaxyl,i.e.,the total ester/amide-bound NERs of metalaxyl after alkaline hydrolysis after the short-term(10-d)and long-term(92-d)incubations of clay and silt fractions isolated from sterilized and non-sterilized soil samples(SS and NSS,respectively).FA=fulvic acids;HU=humin.
The humin-mineral complex-associated metalaxyl residues accounted for only one tenth(92-d incubation)–even less for the 10-d incubation–of the hydrolyzable NER amounts.No metalaxyl residues were detectable in the humic acid subfractions or in the non-humic NER fractions either after 10 d or after 92 d of incubation.
Alkaline hydrolysis of the pre-extracted samples predominantly cleaved the covalently bound main metabolite metalaxyl acid from the humic matter of soil as demonstrated by Kalathooret al.(2015b).In the present study,we performed a long-term experiment to analyze the effect of ageing on the formation of covalent ester bonds between acidic metabolites of pesticides and SOM.The release data pointed to such linkages in addition to further binding interactions,such as hydrogen bonds,as well as entrapment of metalaxyl residues in the voids of the organo-mineral complexes.The amounts of residues that could be released by alkaline hydrolysis increased with incubation time,which corresponded to the kinetics of NER formation described by Barriusoet al.(2008)with rapid flash NER formation,followed by a slower maturation stage or ageing.
Any stereoselective preference in NER formation could not be shown by chiral analysis.The EF value of the residues released by alkaline hydrolysis was approximately 0.48 for the fractions from sterilized soil sample and 0.75–0.9 for the fractions from the non-sterilized soil sample,which corresponded well with the EF value of the extractable fraction(Table III).Thus,microbial activities,which were clearly responsible for the enantioselective degradation and the NER formation,especially after 92 d(Botterwecket al..2014),did not influence stereoselectivity in the incorporation process.Because the NERs releasable after alkaline hydrolysis of the fractions from the sterilized soil sample also had no enantiomeric preference,extracellular enzymes were also not accountable for the stereoselectivity in NER formation.Both microbially and enzymatically assisted reactions led to ester bond formation,which could be readily cleaved by the alkaline degradation step(Kalathooret al.,2015b).
The results for the clay fraction remained unchanged throughout the experiment,indicating fast incorporation through covalent linkage with organic matter.Overall hydrolyzable NERs and the extractable proportions after 10 d,as well as 92 d,were higher(irreversible)in the clay fraction than in the silt fraction(Fig.5).According to other studies(Sukop and Cogger,1992;Sukul and Spiteller,2001;Fernandeset al.,2003;McAllister and Semple,2010),metalaxyl has a preference for sorption to mineral surfaces.In particular,the interlamellar spaces of clay minerals,as well as diffusion of metalaxyl to cavities of microaggregates and organo-clay complexes,offer sites protected from enzymatic or microbial degradation;such residues are likely released to the environment under natural conditions.
Comparison between incubations of bulk soil and isolated silt and clay fractions
Results from a previous study(Kalathooret al.,2015a)with bulk soil differed from those of the current findings in soil particle size fractions.Short-term incubation(10 d)of bulk soil yielded approximately 60%–90%of the initially applied metalaxyl amount in the water-soluble fraction,i.e.,three to four times more than that of pre-isolated silt and clay fractions.This is in accordance with the hypothesis of rapid association of metalaxyl and its metabolites on the more exposed macromolecular and mineral surfaces of the isolated fractions.Also,the formation of metalaxyl acid was higher in the bulk soil compared to that in the individual particle size fractions.One reason for reduced metabolism in the isolated fractions may be the removal of water-extractable enzymes during the soil particle size fractionation process.
In contrast,long-term incubation(92 d)delivered similar amounts of extractable residues in both bulk soil(Kalathooret al.,2015a)and soil particle size fractions(Fig.6).However,a significant difference occurred between the amount of metalaxyl acid recovered in the fractionation water for the sterilized samples(about 1%of the applied amount of metalaxyl)and that for the non-sterilized samples(about 23%of the applied amount of metalaxyl).This,again,pointed to the importance of microbial activity,because extracellular enzyme activity only remained in the sterilized samples.The proportion of metalaxyl residues hydrolyzable under alkaline conditions was higher in the clay fraction than that in silt fraction in the bulk soil(Kalathooret al.,2015a),but the opposite occurred in the current study with a lower proportion in the clay fraction(Fig.6).

Fig.6 Total releasable residues of metalaxyl after the short-term(10-d)and long-term(92-d)incubations of clay and silt fractions isolated from sterilized and non-sterilized soil samples(SS and NSS,respectively).ERs=extractable residues;NERs=non-extractable residues.
DISCUSSION
Findings of our study accorded well with results from other studies on the sorption and degradation of metalaxyl in soil.Sorption of metalaxyl to soil is influenced both by SOM and clay minerals(Sukop and Cogger,1992).Binding constants based on Freundlich adsorption isotherms were highest in soils with high clay content with corresponding lowest mobility in such soils(Sharma and Awasthi,1997).Similar results were reported by Fernandeset al.(2003),who compared 15 agricultural soils by performing batch equilibration studies.Besides the clay content,SOM was the most important soil property for metalaxyl sorption,which could even be enhanced when SOM was increased by organic amendments,such as biochar(Gámizet al.,2016a).Organo-clay amendment of a soil reduced the bioavailability of metalaxyl enantiomers and their leaching in the soil,mitigating the particularly high leaching potential of the more persistentS-enantiomer(López-Cabezaet al.,2016).Enantioselective sorption of metalaxyl in soil was demonstrated by Gámizet al.(2016b)in column leaching tests.They found thatR-metalaxyl,with overall distribution coefficient(Kd)value of 1.73 L kg-1,was more strongly sorbed and showed less leaching compared toS-metalaxyl,withKdvalue of 1.15 L kg-1,in the studied soil.
Masbouet al.(2018)combined compound-specific isotope analysis and enantioselective analysis to study the degradation of metalaxyl in soil:althoughR-metalaxyl degradation was fast(half-life of about 10 d),concomitant enrichment in heavy isotopes of the more persistentS-metalaxyl occurred after 200 d of incubation;in contrast,initial racemic ratios and isotopic compositions were conserved in the sterilized soil sample,indicating the predominance of microbial degradation in soil.They suggested hydroxylation as a major enantioselective degradation pathway of metalaxyl in soil.They reported that degradation of metalaxyl in soil was enhanced after repeated applications,which led to the discrimination of the two enantiomers,enhanced degradation of the biologically activeR-enantiomervs.the retarded non-activeS-enantiomer.In addition,the soil column studies of Celiset al.(2015)showed that the leachate became increasingly enriched inS-enantiomer as the number of fungicide applications increased.
Fungi have been shown to be able to degrade metalaxyl in soil(Martinset al.,2017).Two Mucorales strains,previously isolated from soil and identified asGongronellasp.andRhizopus oryzae,were able to use metalaxyl as the main carbon and energy source and to degrade metalaxyl in polluted soils.Yueet al.(2016)found that urease,invertase,and catalase activities in soil were not only related to the concentrations of the enantiomers and soil incubation time,but also to the chiral configuration.They also found that the effect on urease and catalase activities ofR-metalaxyl was stronger than that ofS-metalaxyl at the same concentration,but the opposite was observed on the activity of invertase.These showed that the effect of metalaxyl on enzymatic activities in soil was enantioselective.
In our study,the total organic carbon was approximately 5 g kg-1in the clay fraction and about 17 g kg-1in the silt fraction.Therefore,larger amounts of metalaxyl residues were reversibly chemically bound to organic macromolecules in the silt fraction than in the clay fraction(Fig.5),which indicated higher sorption affinity of the residues to clay minerals and confirmed the earlier mentioned preference for minerals with larger surface area and coated organic matter.
Stereoselective reactions in both bulk soil incubation and particle size fraction incubation showed some similarities,but also some discrepancies.Although extractable fractions of the sterilized bulk soil showed stereoselective degradation of metalaxyl in the bulk soil,this was not noticed in the particle size fractions of the current study.Interestingly,in contrast to the trend in the previous study with bulk soil,the EF values of hydrolyzable metalaxyl residues in the particle size fractions of the current study from both sterilized and non-sterilized soil samples showed no enantiomeric preference in the incorporation process.Recovery of some microbial and enzyme activities in the sterilized bulk soil sample after 92 d of incubation was reported(Botterweck,2014),which might explain the stereoselectivity and higher metalaxyl acid quantities in the sterilized bulk soil sample compared to that of the clay and silt fractions from the sterilized soil sample in our study.The only differing factor besides the total applied metalaxyl amount is the waterextractable enzyme proportion,which was removed in the current study through prior particle size fractionation.This pointed to an influence of dissolved extracellular enzymes,as described by Botterweck(2014)in stereoselectivity during the incorporation process for metalaxyl residues.Additionally,these results also supported the idea of abiotically influenced formation of non-extractable metalaxyl residues through organo-mineral-pesticide interaction.
This study provided insights into the effects of biotic and specific abiotic effects during degradation of metalaxyl and the formation of its NERs in soil silt and clay fractions incubated.Degradation and mineralization of this xenobiotic were shown to be linked to the microbial and enzymatic activities of the matrix(Botterwecket al.,2014).On the other hand,extracellular enzymes only showed a minor contribution to the metabolic formation of metalaxyl acid,as well as to the mineralization process(Botterweck,2014).Nevertheless,both activities were relevant to the incorporation of the fungicide.Hydrolyzable metalaxyl residues showed a fast association of this fungicide to the particulate humic substances,pointing not only to covalently ester-or amide-bound acid metabolites,but also to high amounts of entrapped residues.However,these proportions decreased in the long run,resulting in a temporary diffusion of the fungicide into micropores of soil aggregates,including humic substances protecting the xenobiotic from microbial and enzymatic degradation.Remobilization of the entrapped residues could become bioavailable,which indicated the significance of elucidating reversibly bound transformation products of this pesticide.
The affinity of metalaxyl and its metabolites to form irreversibly bound NERs onto organo-clay surfaces was confirmed by our study,especially in contrast to the more organic matter-containing silt fraction.This showed that organo-clay complexes were major sinks for irreversibly bound NERs.Agricultural practices that alter the aggregation of soil particles,i.e.,tillage,lead to reduced soil structural stability and increased erosion.Disaggregation of silt and clay agglomerates after such actions will uncover pesticide residues from these fractions,which we showed to have different binding strength than the silt and clay fractions.The confirmation of the potential release of pesticide NERs,especially those entrapped in the matrix,after mechanically disturbing the soil structure calls for future research.
Chiral analyses displayed an increased stereochemical effect during the metabolism of metalaxyl in the long term and supported preliminary observations(Rieferet al.,2011b).In particular,microbial activity promoted the enantioselective process at a much higher rate than extracellular enzymes.However,in contrast to our previous study with bulk soil,lack of enantioselective incorporation of the fungicide in pre-isolated soil particle size fractions indicated less biotic influence and higher abiotic binding interactions with no enantioselective preference during this process.Furthermore,our study pointed to the significance of water-extractable extracellular enzymatic activity in soil with regard to the stereoselectivity of bound metalaxyl residues.Our findings could be related to the impact of natural stressors,such as drought or water logging,on the degradation potential of soil.The effects of such conditions on the microbial and extracellular enzyme activities and thus pesticide degradation should be further investigated.
As an environmental implication,it must be considered that the biological degradation of metalaxyl proceeded enantioselectively,leading to non-racemic residues in soil shortly after application of the fungicide as a racemic mixture.The differentiation of sequestered NERs,prone to slow remobilization and thus to being included in persistence assessment,and covalently bound residues should be included in future studies on the fate of chemicals in soil and sediment.
ACKNOWLEDGEMENTS
Financial support by the German Research Foundation(DFG)(SCHW750/9)in the frame of the Priority Program SPP 1315 is greatly acknowledged.We also thank Prof.H.G.Schmalz and Prof.Andreas Adler,University of Cologne for their support with the preparative HPLC fractionation.
杂志排行
Pedosphere的其它文章
- Rice productivity and profitability with slow-release urea containing organic-inorganic matrix materials
- Bacterial communities in paddy soils changed by milk vetch as green manure:A study conducted across six provinces in South China
- Efficiency of soil-applied 67Zn-enriched fertiliser across three consecutive crops
- Effect of long-term fertilization on bacterial communities in wheat endosphere
- Soil micromorphological and physical properties after application of composts with polyethylene and biocomponent-derived polymers added during composting
- Effect of sewage sludge and sugarcane bagasse biochar on soil properties and sugar beet production
