Effect of long-term fertilization on bacterial communities in wheat endosphere
2021-10-15YuyingMAPamelaWEISENHORNXishengGUODaozhongWANGTengYANGYuSHIHuanchaoZHANGandHaiyanCHU
Yuying MAPamela WEISENHORNXisheng GUODaozhong WANGTeng YANGYu SHIHuanchao ZHANG*and Haiyan CHU*
1Co-Innovation Center for the Sustainable Forestry in Southern China,College of Forestry,Nanjing Forestry University,Nanjing 210037(China)
2State Key Laboratory of Soil and Sustainable Agriculture,Institute of Soil Science,Chinese Academy of Sciences,Nanjing 210008(China)
3Biosciences Division,Argonne National Laboratory,Argonne,IL 60439(USA)
4Key Laboratory of Nutrient Cycling and Resources Environment of Anhui Province,Soil and Fertilizer Research Institute,Anhui Academy of Agricultural Sciences,Hefei 230031(China)
5University of Chinese Academy of Sciences,Beijing 100049(China)
ABSTRACT Fertilization has been shown to exert a significant influence on soil microorganisms and directly and indirectly influences plant growth and survival in agroecosystems.However,it is unknown whether fertilization affects endophytic microbial communities,which are ubiquitous and intimately associated with plant growth and health.Herein,we investigated endophytic bacterial communities in wheat leaves and roots under different long-term fertilization regimes,including NPK chemical fertilizer and NPK chemical fertilizer combined with wheat straw,pig manure,or cow manure.Endophytic bacterial community composition considerably differed in leaves and roots.Although different fertilization treatments did not affect the endophytic bacterial species richness or phylogenetic diversity in either leaves or roots,the community composition was significantly altered,particularly in roots.The endophytic bacterial co-occurrence network in leaves was more complex and stable than that in roots.Furthermore,many of the keystone species that were identified by their topological positions in the co-occurrence networks of leaves and roots were involved in plant growth and fitness.The total relative abundance of keystone species was the highest in the NPK plus cow manure treatment in both leaves and roots.Overall,our results suggest that different fertilization regimes can strongly affect endophytic bacterial communities,and the combination of NPK fertilizer and cow manure promoted the relative abundance of the key endophytic bacterial microbiota in both leaves and roots,which might be beneficial for plants in agroecosystems.
Key Words:co-occurrence network,endophytic bacterial community,key microbiota,long-term fertilization,organic matter
INTRODUCTION
Plants can be viewed as complex systems with an intimately associated microbiome,much like the relationship between humans and our own microbiomes(Gordon,2012).Plant-dwelling microorganisms are crucial for plant growth and health;moreover,they play a fundamental role in the adaptation of the plant to diverse environments(Vandenkoornhuyseet al.,2015).Plant endosphere and ectosphere of both aboveground and belowground compartments provide diverse habitats for microbial communities,and distinctive microbiota become established within each plant tissue(Vandenkoornhuyseet al.,2015).Endophytic bacteria,which commonly occur within plants without causing disease symptoms,can be involved in major physiologic functions,such as nutrient acquisition(Matoset al.,2017),pathogen defense(Verma and White,2018),and abiotic stress tolerance(Abd_Allahet al.,2018).Hence,these organisms may promote host plant growth and productivity in agricultural ecosystems(Rehmanet al.,2018).To effectively utilize microbiota to influence plant growth and productivity in agricultural systems,it is imperative for us to examine the driving factors controlling endophytic communities.To date,studies have explored microbial communities associated with some crop plant species under agricultural environments.For example,the phyllosphere and rhizosphere of rice were colonized by particular bacterial communities with physiological traits of differential importance,such as transport processes and stress responses were more conspicuous in the phyllosphere,whereas dinitrogenase reductase was exclusively identified in the rhizosphere(Kniefet al.,2012).Individual microbial populations in the maize rhizosphere were strongly modified by the crop genotype(Airaet al.,2010).The dominant factors influencing microbial community composition in the wheat rhizosphere included both plant age and site(Donnet al.,2015).However,in contrast to the well-studied rhizosphere and phyllosphere microbiome,the more intimate associations of plants with their endophytic microbiota are less well understood.Improving our knowledge of plant endophytes is critical because these organisms are likely to have a strong influence on plant physiology.
Interspecific interactions can influence the composition and development of the microbiome and the interconnected microbiome found within plant tissues can critically affect plant physiology directly and indirectly(Van Der Heijden and Hartmann,2016).Co-occurrence networks are a helpful approach to better understand the potential direct or indirect interactions between microbial species.These networks are constructed by calculating pairwise correlations based on the relative abundance of individual taxa(Van Der Heijden and Hartmann,2016).Microbial taxa that are highly connected within the network potentially exert a considerable influence on the microbiome,irrespective of their abundance,and are proposed to be keystone taxa(Banerjeeet al.,2018).These species may have a strong and unique influence on microbial communities;therefore,they may have a greater contribution to the system overall(Banerjeeet al.,2018).For example,by examining the effect of the two keystone microbes(isolates ofAlbugoandDioszegia)on other phyllosphere microbiota,it was found that the presence or absence of these keystone microbes could have disproportionately large effects on phyllosphere microbiota(Agleret al.,2016).In the rhizosphere,a keystone species in the genusMesorhizobiumwas responsible for the production of alkaline phosphomonoesterase and was positively correlated with the most dominant bacterivores in the nematode genusProtorhabditis(Jianget al.,2017).However,interspecific interactions and potential keystone microbial species of plant endophytes remain largely unexamined.
In agroecosystems,fertilization has long been a primary focus of research as it is an extensive and efficacious management measure.Long-term fertilization is known to alter the soil environment and can thereby exert a profound influence on the native microbial communities on which crops depend(Shenet al.,2010).In a study of long-term fertilization,the variation in soil bacterial community composition was mainly driven by soil pH(Sunet al.,2015).In addition,bacterial community composition in the maize rhizosphere has been shown to be most strongly influenced by soil pH,soil organic matter,and available phosphorus(Wanget al.,2018).However,in comparison to soil and rhizosphere communities,crop endophytic populations may play a more direct role in helping a host plant adapt quickly to changing environmental conditions(Doty,2017).Despite the abundance of research on the effects of fertilization in agroecosystems and the importance of endophytes to crop physiologic function,the responses of endophytic microbial taxa to fertilization remain unexplored.Herein,we proposed that long-term applications of fertilizer could have an influence on crop endophytic communities.We compared bacterial endophytic assemblages within winter wheat leaves and roots subjected to 35 years of either chemical fertilizer only or supplemented with organic matter inputs,including wheat straw,pig manure,and cow manure.We also identified putative keystone species among the endophytic bacterial communities through co-occurrence network analysis.Three specific questions are addressed.i)How do different plant tissues and long-term fertilization regimes affect endophytic bacterial communities?ii)Are the patterns of interactions within endophytic bacterial communities in leaves and roots different?iii)Does fertilization affect the relative abundance of keystone species in the endophytic bacterial communities of leaves and roots?
MATERIALS AND METHODS
Study site and experimental design
The long-term field experimental site has been in wheatsoybean crop rotation since 1982.The site was located in Mengcheng,Anhui Province,China(33°13′N,116°35′E).The soil in this region is classified as a typical lime concretion black soil.Five treatments with four replicate plots for each(a total of 20 plots)were included in this experiment:no fertilization(control),NPK chemical fertilizers only(NPK),NPK combined with wheat straw(NPK+WS),NPK combined with pig manure(NPK+PM),and NPK combined with cow manure(NPK+CM).The NPK chemical fertilizers were composed of urea(180 kg N ha-1year-1),superphosphate(90 kg P2O5ha-1year-1),and potassium chloride(135 kg K2O ha-1year-1).Wheat straw(straw pieces approximately 10 cm),pig manure,and cow manure additions were 7 500,15 000(fresh weight),and 30 000(fresh weight)kg ha-1year-1,respectively.All fertilizers were applied once a year in October,prior to the sowing of winter wheat(Yannong 19).
Sample collection and analysis
Wheat leaf and root samples were collected on April 21,2017 during the booting of winter wheat.In each plot,all leaves below the flag leaf were picked from 30 randomly selected healthy wheat tillers.The root systems of plants selected for leaf collection were dug up in the field and nearly complete roots were collected from each of these wheat tillers.All leaf and root samples from the same plot were pooled into sealed polyethylene bags for leaves and roots,respectively,and stored at 4°C in coolers for transport.The samples were brought to the laboratory within 12 h where each sample was divided into two subsamples.One subsample was used for the determination of plant tissue element concentration and the other was used for DNA extraction.
Concentration determinations of 13 elements of leaves and roots in each plot were quantified to establish the nutrient status of wheat crops under fertilizer treatments(Table SI,see Supplementary Material for Table SI).Plant tissue for nutrient determinations was ground into a fine powder using a clean ball grinder(TL-2010S,DHS,China).Total carbon(TC)and nitrogen(TN)were measured using a C/N elemental analyzer(Vario MAX,Elementar,Germany).The remaining powder from each plant sample was digested in a concentrated HNO3and HClO4solution(4:1,volume/volume)for determinations of total phosphorus(TP),total potassium(TK),Ca,Mg,S,and microelements,including Na,Fe,B,Mn,Zn,and Cu,with an inductive coupled plasma emission spectrometer(Optima 8000,PerkinElmer,USA).
Surface sterilization
All plant tissues(leaves and roots)from the other subsample were surface sterilized according to a modification of the ethanol-sodium hypochlorite method reported by Sunet al.(2008).Samples were rinsed with sterile water to remove all attached soil,then soaked in 70%ethanol(2 min),20 g L-1NaOCl(5 min),and 70% ethanol(30 s),and finally washed with sterile deionized water five times.Excess moisture was subsequently removed with sterile filter paper.Water from the final rinse of each sample was spread on a tryptic soy agar plate,which was then incubated in an aseptic room.Because no bacteria were found on the plates after incubation,surface sterilization was confirmed effective.Thus,the bacterial communities remaining after surface sterilization in this study were referred to as endophytic bacterial communities.Following sterilization,samples were ground and homogenized with liquid nitrogen using sterile mortars and pestles in a sterile room,placed in airtight aseptic tubes,and stored at-40°C until further processing.
DNA extraction and amplicon sequencing
Total DNA was extracted from the surface-sterilized sample powder(0.10 g)using a DNeasy plant mini kit(Qiagen,Germany),with a modified standard extraction protocol as described by Zimmerman and Vitousek(2012).Briefly,the recommended amounts of buffer AP1 and P3 were doubled,ten 2.3-mm zirconia/silica beads were used to improve vortexing,and the incubation step was extended to 60 min.The DNA concentrations were quantified using a NanoDrop 1000 spectrophotometer(NanoDrop Technologies,USA).The hypervariable regions V5–V7 were amplified in triplicate from the DNA extracts using the bacterial primers 799F(5′-AACMGGATTAGATACCCKG-3′)and 1193R(5′-ACGTCATCCCCACCTTCC-3′)(Bulgarelliet al.,2015).In brief,polymerase chain reactions(PCRs)were performed with the TransStart Fastpfu DNA polymerase system(AP221-02,TransGen,China)in a total volume of 20 μL:3 μL template DNA(10 ng μL-1),4 μL FastPfu buffer,2 μL 2.5 μmol L-1deoxy-ribonucleoside triphosphates(dNTPs),0.8 μL each of 5 μmol L-1forward and reverse primers,0.4 μL FastPfu polymerase,and 0.2 μL bovine serum albumin.Temperature cycling was:95°C for 3 min,13 cycles of 95°C for 30 s,55°C for 30 s,and 72°C for 45 s,72°C for 10 min,and then 4°C until use.The resulting PCR products were run on a 2%agarose gel,purified using the AxyPrep DNA gel extraction kit(Axygen Biosciences,USA),and subsequently quantified using a QuantiFluor™-ST fluorometer(Promega,USA)according to the manufacturer’s protocol.The purified amplicons were pooled in equimolar concentrations for 2×250 bp paired-end sequencing on an Illumina MiSeq platform,according to the standard protocol by Majorbio Bio-Pharm Technology Co.,Ltd.,China.The raw data were submitted to the Sequence Read Archive(SRA)at The National Center for Biotechnology Information(NCBI)with accession number SRP143450.
Data processing and statistical analysis
Raw data were subjected to quality filtering(quality score>30),length filtering(length>380 bp),chimera checking,and operational taxonomic unit(OTU)clustering using the QIIME pipeline(http://qiime.sourceforge.net/)(Caporasoet al.,2010).In brief,chimeras were identified using UCHIME and the“gold”reference database(http://drive5.com/uchime/gold.fa).The OTUs were clustered with a 97%similarity threshold using the UCLUST method with default parameters(pick_otus.by script)(Edgar,2010).Phylogenetic trees were constructed with Fast-Tree after aligning representative sequences with PyNast(http://qiime.org/pynast/).The taxonomic identity of each OTU was assigned by the UCLUST algorithm against the SILVA v128 database(Quastet al.,2013).In total,291 631 high-quality sequences across 40 samples were clustered into 3 127 OTUs after further removing singletons and unassigned taxa(filter_taxa_from_otu_table.py script).Because the minimum sequence depth in the samples was 4 767,all samples were resampled at a sequence depth of 4 700(single_rarefaction.py script);finally,2 994 OTUs were obtained.Two alpha diversity metrics,observed_species(species richness)and PD_whole_tree(phylogenetic diversity),were calculated using the command alpha_diversity.py rarefied at 4 700 counts per sample.
Differences in plant traits and alpha diversity were tested to determine significance with the independent t-test and analysis of variance analysis(ANOVA)(Tukey’s honestly significant difference)in SPSS 20.0 for Windows.To examine patterns in beta diversity,principal coordinate analyses were conducted on the Bray-Curtis dissimilarity in the R package vegan(https://CRAN.R-project.org/package=vegan).Significant differences in community composition were determined using analysis of similarities(ANOSIM)in the vegan package.Mantel tests were performed to examine the significant correlations between endophytic bacterial communities and plant traits in leaves and roots,respectively.Figures were generated in R with package ggplot2(Ginestet,2011).
The construction of co-occurrence networks used Spearman correlation coefficients with the“WGCNA”package(Langfelder and Horvath,2012),and the properties of these networks were calculated with the“igraph”package in R(http://igraph.org).Any OTU with a relative abundance of less than 0.01% were removed,andPvalues were corrected(adjustedP<0.001)for multiple testing through the Benjamini-Hochberg method(Benjaminiet al.,2006)in the“multtest”package(http://www.bioconductor.org/packages/release/bioc/html/multtest.html).The network of significant correlations(ρ>0.6,P<0.001)were visualized in Gephi(http://gephi.github.io/).Putative keystone taxa were identified statistically through microbial network analyses.Each node was assigned a role according to its module features(within-module degreezand among-module connectivityc)(Guimer and Nunes Amaral,2005).Four roles were characterized,namely:peripherals(z≤2.5 andc≤0.62),module hubs(z≥2.5 andc≤0.62),network hubs(z≥2.5 andc≥0.62),and connectors(z≤2.5 andc≥0.62).Peripheral species have few links with others in the microbial network;module hubs(with a highzvalue)have only high links inside their own modules and are important to their own module coherence;network hubs have both highzandcvalues and thus have importance to the coherence of both their own module and the whole network;and connectors that have only a highcvalue are critical for the maintenance of network coherence(Poudelet al.,2016).Because they have many connections in the network,OTUs identified as module hubs,network hubs,and connectors are referred to here as putative keystone taxa.
RESULTS
Dominant endophytic bacterial taxa
In total,2 994 OTUs were obtained after resampling at a sequence depth of 4 700.Among these,the OTU richness in roots(2 713)was substantially greater than that in leaves(700),with an overlap of 419 OTUs(Fig.S1,see Supplementary Material for Fig.S1).Only 2.2%of OTUs could be classified at the species level.The top five phyla in the endophytic bacterial community were Actinobacteria(43.5%),Proteobacteria(33.3%),Bacteroidetes(13.5%),Firmicutes(7.4%),and Fusobacteria(1.9%).The endophytic bacterial communities in leaves were strongly dominated by Actinobacteria,which accounted for 70.6% of the sequences,whereas the communities in roots were dominated by Proteobacteria(49.1%)(Fig.1).
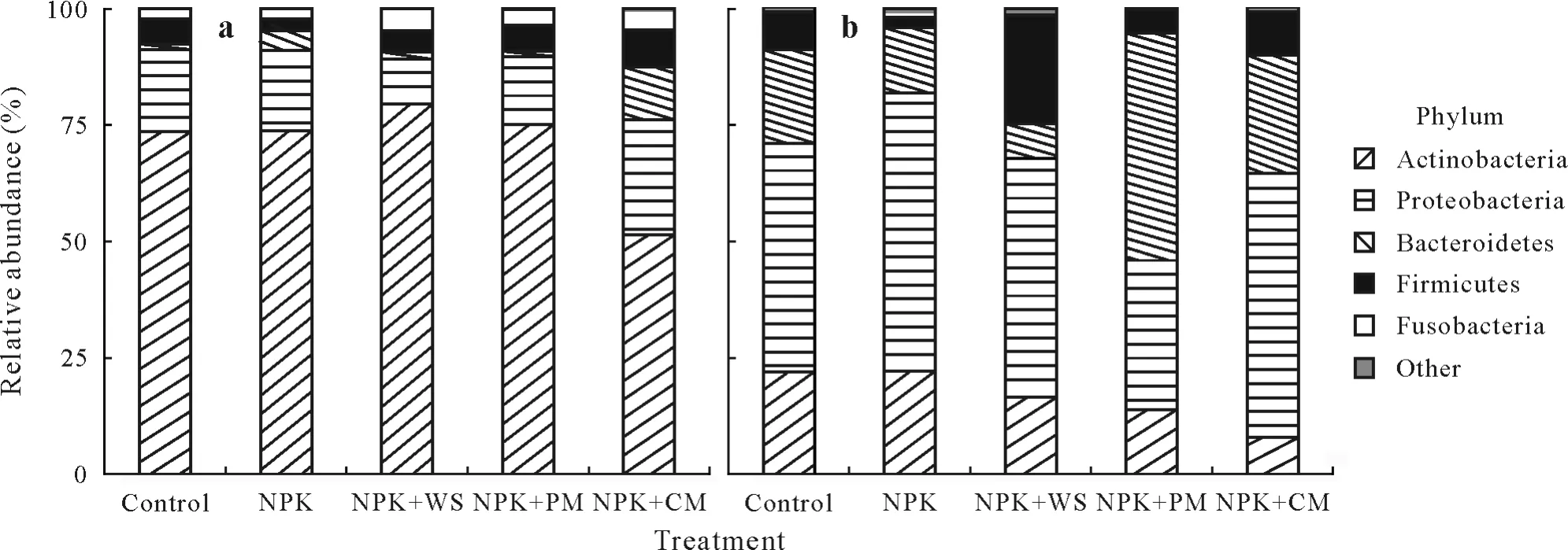
Fig.1 Relative abundance of the dominant endophytic bacterial phyla in wheat leaves(a)and roots(b)under different fertilization treatments,including no fertilization(control),NPK chemical fertilizers only(NPK),NPK combined with wheat straw(NPK+WS),NPK combined with pig manure(NPK+PM),and NPK combined with cow manure(NPK+CM).Phyla with a total relative abundance of<1%are grouped in“other”.
At the genus level,the top five genera identified in the leaf endophytic bacterial community wereNocardia(66.5%),Achromobacter(6.6%),Sneathia(3.4%),Bacillus(1.5%),andPseudomonas(1.4%).Fertilization management did not change the relative abundance of these genera compared to that in the control(Fig.S2,see Supplementary Material for Fig.S2).In the root endophytic bacterial community,Flavobacterium(20.2%),Pseudomonas(19.9%),Bacillus(8.6%),Rhodococcus(6.3%),andJanthinobacterium(4.4%)were the most abundant genera.In comparison to the control,the relative abundance ofFlavobacteriumwas significantly higher in the NPK+PM treatment(Fig.S3,see Supplementary Material for Fig.S3).The generaPseudomonas,Bacillus,Rhodococcus,andJanthinobacteriumexhibited no significant change in their relative abundance.Furthermore,we regarded all the OTUs with relative abundance over 1.0%together as the abundant microbiota,which accounted for 78.5%and 54.5%of the total community in leaves and roots,respectively.Fertilizer management did not have a significant influence on the total relative abundance of the abundant microbiota as a proportion of the total community in either leaves or roots(Fig.S4,see Supplementary Material for Fig.S4).
Endophytic bacterial diversity and community composition
The alpha diversity of bacterial endophytes was much higher in roots than in leaves,but fertilization treatment did not significantly affect the species richness and phylogenetic diversity in either leaves or roots as compared to those in the control(Fig.S5,see Supplementary Material for Fig.S5).However,although permutational multivariate ANOVA(PERMANOVA)revealed that plant tissues contributed to the largest proportion of variation(42%of the variation,P=0.000 1)in beta diversity of endophytic bacterial community composition(Table I),fertilizer treatment contributed to 12%of this variation(P=0.001 9).For each plant tissue considered individually,endophytic bacterial community composition was influenced by fertilization treatment(Fig.2).Meanwhile,PERMANOVA showed that the explained variances of fertilization to the bacterial community structure in roots and leaves were 47.1%(P=0.001)and 31.3%(P=0.008),respectively.The ANOSIM revealed that all the fertilization treatments caused significant differences in endophytic community structure comparedwith the control,and that there were greater effects on the community in roots(R=0.75,P=0.001)than that in leaves(R=0.42,P=0.001)(Table SII,see Supplementary Material for Table SII).Additionally,the communities in roots were affected by all treatments with the supplementation of organic matter compared to NPK treatment;whereas in leaves,significant differences existed between the addition of livestock manure(pig or cow manure)and NPK treatments,but not between supplemental wheat straw addition and NPK treatments.Mantel tests showed that the variation in the leaf endophytic bacterial community was primarily driven by TC,and TP,TN,Ca,Na,and Zn were significantly correlated with the root endophytic bacterial community(Table SIII,see Supplementary Material for Table SIII).

Fig.2 Variations of endophytic bacterial communities in wheat leaves and roots under different fertilization treatments,including no fertilization(control),NPK chemical fertilizers only(NPK),NPK combined with wheat straw(NPK+WS),NPK combined with pig manure(NPK+PM),and NPK combined with cow manure(NPK+CM),based on principal coordinate(PCo)analyses of Bray-Curtis dissimilarity.

TABLE IResults of permutational multivariate analysis of variance testing of the effects of plant tissue and fertilization treatment on bacterial communities in wheat endosphere
Associations within endophytic bacterial communities of wheat leaves and roots
Co-occurrence networks of endophytic bacterial communities contained OTUs extensively belonging to Proteobacteria in both leaves(46.3% of taxa included in network)and roots(50.3% of taxa included in network)(Fig.3a).As a whole,the majority of correlations between OTUs were positive in both leaves(99.8%)and roots(81.2%).However,there were strong differences in endophytic bacterial co-occurrence patterns between leaves and roots(Table SIV,see Supplementary Material for Table SIV).Although microbial network had more bacterial members in roots than in leaves(491vs.285 nodes),more correlations between the members were found in leaves than in roots(2 567vs.2 466 edges),suggesting that the endophytic bacterial members were more connected to each other in leaves than in roots(average node degree of 18.0vs.10.0)(Table SIV).Moreover,compared with that in roots,the endophytic bacterial network in leaves had greater connectedness among members(clustering coefficient of 0.7vs.0.3),interacting bacterial consortia(modularity of 0.6vs.0.5),and network complexity(connectance of 0.03vs.0.01)(Table SIV).The distribution of the number of interactions for individual OTUs followed a power-law function in both leaves(P=0.024)and roots(P<0.001),indicating a non-random distribution of OTU interactions and a hub-based structure for each of these cooccurrence networks(Fig.3b).In other words,within each network,there were consortia of OTUs,which interacted with each other,with fewer OTUs interacting with OTUs outside of their own consortium.In addition,we found that the natural connectivity of the network in leaves was greater than that in roots,suggesting the leaf network was more robust(Fig.3c).
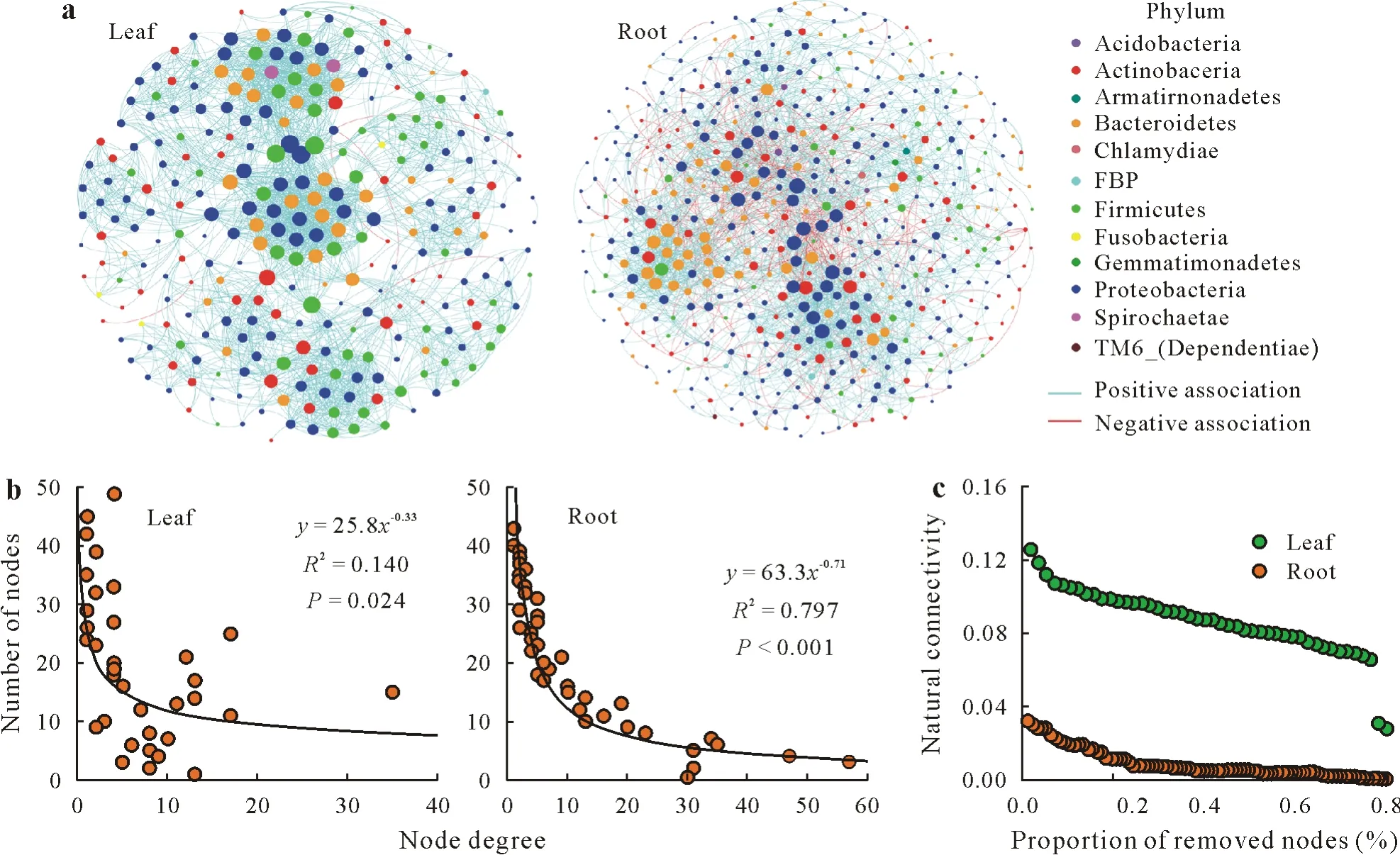
Fig.3 Endophytic bacterial co-occurrence network patterns of wheat leaves and roots:co-occurrence networks visualizing significant associations(ρ>0.6,P<0.001)between endophytic bacterial operational taxonomic units(OTUs)in leaves and roots,respectively,with a dot representing an endophytic bacterial OTU,color representing different phyla,node size representing node degree,and edges denoting significant relationships between OTUs(a);node degree distribution of the co-occurrence networks in leaves and roots,with power-law curves fitted to plots(b);and robustness of the co-occurrence networks in leaves and roots(c).
Keystone species in wheat leaves and roots
Co-occurrence networks were used to explore the potential keystone species in endophytic bacterial communities of wheat leaves or roots.Namely,the co-occurrence network position of each OTU within both its own consortium(module)and with regard to other consortia(modules)was measured byzandcscores(Fig.4).The OTUs that had few interactions(i.e.,peripheral nodes)dominated both networks(97.2%and 82.3%in leaf and root networks,respectively),with some OTUs that may have had few interactions but belonged to two or more consortia(i.e.,connectors)having the only other species role(2.8%)in the leaf network and the second largest role(15.5%)in the root network(Fig.4).Potential keystone species were defined as OTUs that had a large number of interactions with other OTUs in their own consortia(i.e.,module hubs)and a large number of interactions overall(network hubs),or OTUs that played a role in connecting multiple consortia to each other(i.e.,connectors).There were 8 potential keystone taxa in wheat leaves and 87 in roots.All keystone OTUs were assigned a taxonomic name at the species level based on the NCBI BLAST(Basic Local Alignment Search Tool)(Table SV,see Supplementary Material for Table SV).Together,the keystone taxa accounted for 1.1%and 16.5%relative abundance in leaves and roots,respectively.This suggested that some low abundance species may act as keystone taxa,potentially having larger effects on community structure and interactions compared with those apparent based on their proportional abundance alone.

Fig.4 Distributions of network roles by analyzing module features,within-module degree z and among-module connectivity c,in the endophytic bacterial co-occurrence networks of wheat leaves and roots,respectively.
On the whole,there were a large proportion of keystone species in both leaves and roots belonging to the class Flavobacteriia.In addition,root bacterial communities had a large proportion of keystone species in the Betaproteobacteria,Alphaproteobacteria,Actinobacteria,Bacilli,and Gammaproteobacteria(Fig.5a).The composition of keystone species at the genus level is displayed in Table SVI(see Supplementary Material for Table SVI).The variation of the key microbiota(collection of putative keystone species in each respective network)based upon their relative abundance among different fertilization treatments was examined.In comparison with the control,the relative abundance of the key microbiota was significantly lower in NPK treatment in both leaves and roots.Supplementation with organic matter(wheat straw,pig manure,or cow manure)significantly increased the relative abundance of the key microbiota in roots compared with NPK alone,whereas only the addition of cow manure had a significant effect in leaves(Fig.5b).
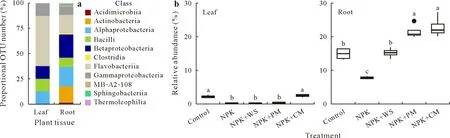
Fig.5 Taxonomic composition of keystone species of endophytic bacterial communities in wheat leaves and roots reported as proportional operational taxonomic unit(OTU)number per class(a)and relative abundance of the key microbiota(all keystone species)in wheat leaves and roots in response to different fertilization treatments,including no fertilization(control),NPK chemical fertilizers only(NPK),NPK combined with wheat straw(NPK+WS),NPK combined with pig manure(NPK+PM),and NPK combined with cow manure(NPK+CM)(b).Bars with the same letter are not significantly different at P<0.05.
DISCUSSION
Effects of plant tissue and fertilization on endophytic bacterial communities
Changes in the soil microbiome caused by fertilizer inputs can lead to subsequent changes in plant endosphere populations(Segherset al.,2004).In a previous study,within the same experimental field,fertilization treatments affected the soil bacterial community structure(Sunet al.,2015).In this study,we found that fertilization treatment also affected endophytic bacterial communities in both leaves and roots.This may be related to the shift of soil bacterial population according to the Seghers’s hypothesis that changes in plant endosphere populations resulting from fertilizer inputs may be owing to changes in the soil microbiota.In contrast,Robinsonet al.(2016)proposed that endophytic bacterial assemblages might be altered by fertilization through changes in recruitment caused by plant growth and/or exudates.Herein,we found that the endophytic bacterial community in leaves was only significantly influenced by TC.Similarly,Yanget al.(2016)also observed a strong correlation between endophytic fungal communities and foliar carbon content in a natural ecosystem,indicating a potential widespread effect of foliar carbon source on endophytic microbial communities.Furthermore,the bacterial community in roots was significantly correlated with several elements,including TP,TN,Ca,Na,and Zn,but the strength of each of these correlations was low(Table SIII).These results are in agreement with previous work that has shown only weak relationships between plant nutrient status and microbial community composition(Hamontset al.,2018).Therefore,other factors,such as the quantity and chemistry of plant exudates and secondary metabolites,should be considered to explain the responses of plant endophyte recruitment to fertilization.
The lack of an effect of fertilization treatment on endophytic bacterial alpha diversity may be a result of the strong influence of the host plant on endophytic taxa.In this study,there were no significant differences in endophytic bacterial alpha diversity among fertilization treatments(Fig.S5).Similarly,Segherset al.(2004)found that the OTU richness of the maize root endophytic microbiome did not differ between conventional and organic fertilization management.In a comparison among different root compartments,Edwardset al.(2015)found effects of conventional and organic cropping management on bacterial diversity in the rhizosphere,but not in the rhizoplane or the endosphere.It seems that the more intimately linked the bacterial microbiota is to the host plant,the less its diversity is affected by soil management strategy.
Hub-based co-occurrence networks in leaves and roots
Co-occurrence networks were built to examine the interconnections among bacterial populations within the wheat leaf and root endospheres.Patterns in the frequency,strength,and distribution of interactions among OTUs in the endophytic communities of leaves and roots varied(Table SIV).Although the size of the microbial network in leaves was smaller than that in roots,the network in leaves had more connections among taxa and higher correlations for per bacterial member with other members,indicating closer associations among the endophytic taxa in leaves.Moreover,there was stronger clustering into and within consortia in the leaf endophytic networkversusthat in roots.This stronger clustering may reflect the importance of ecological processes,such as degradation pathways that involve cooperation among multiple taxa in shaping the leaf endophytic bacterial community(Röttjers and Faust,2018).Considered together,these factors suggest a more complex set of interactions influencing the structure of the endophytic bacterial community in leaves than in roots.
The differences in interactions among OTUs within a community may be a result of niche differentiation between the plant tissues(Mülleret al.,2016).Overall,positive associations were common in both leaves and roots(Fig.3a).More negative correlations were found in the root endosphere network than in the leaf endosphere network(Table SIV),which is possibly attributable to the variance in carbon resource availability among these plant tissues.Leaves contained more carbon compared with roots(Table SI)and there was less evidence of competition(lower proportion of negative correlations)in the microbial network of the leaf endosphere,indicating that great resource availability may decrease competition in microbial communities(Costelloet al.,2012).
It was also found that both endophytic bacterial cooccurrence networks showed a hub-based structure with the distribution of connections among individual OTUs,following a power-law distribution(Bergman and Siegal,2003)(Fig.3b).Although the distribution of the OTU interactions in leaves and roots both followed a power-law function,there were fewer OTUs with many connections to other OTUs in the leaf endophytic community than in the root endophytic community(Fig.3b).Because there were fewer OTUs with many connections in the leaf community,these OTUs are(statistically)less likely to be lost at random;thus,the leaf network may be more stable to non-taxon specific perturbations(Röttjers and Faust,2018).In addition,the network in leaves had higher clustering coefficient,modularity,connectance,and natural connectivity(Table SIV,Fig.3c),suggesting that the endophytic bacterial network had a higher complexity with stronger connectivity in leaves than in roots.Complex networks with greater connectivity have been found to be more robust to environmental perturbations(Santolini and Barabasi,2018).In this sense,the leaf microbiota in this study might be more resilient to environmental stresses as different taxa or functions can complement each other.
Predicted functions and importance of key microbiota in wheat leaves and roots
We considered the traits of these putative keystone species to ascertain whether they might possess traits impacting the stability of microbial interaction networks or otherwise be crucial for plant growth and health(Agleret al.,2016).Taken together,the most dominant genus of the key microbiota in both leaves and roots belonged to the genusFlavobacterium,which also had the highest number of connections to other taxa(Table SVI).This genus has been found to be an important member of the root-and leaf-associated microbiome in multiple studies and have beneficial functions supporting plant growth and resistance to pathogens(Manteret al.,2010;Koltonet al.,2014).In addition,some genera in the key microbiota had a large number of interactions despite having relative abundances below 0.10%(Table SVI)and are known to have effects on plant growth or health.For example,in the leaf key microbiota,the generaChryseobacterium(0.05%,node degree=23)andPseudomonas(0.01%,node degree=14)were previously reported as plant growth promoting rhizobacteria(Singhet al.,2013;Dorjeyet al.,2017).In the root key microbiota,the generaMesorhizobium(0.08%,node degree=46)andPaenibacillus(0.04%,node degree=43)are known to have many species that act as nitrogen-fixing bacteria(Moscatielloet al.,2015;Gradyet al.,2016).Moreover,other genera detected in the key microbiota of leaves or roots are known to possess functional traits to promote plant fitness,includingBacillus,Rhizobium,Paraburkholderia,Thiobacillus,Sphingomonas,Mycobacterium,Microbacterium,Rhodococcus,andBradyrhizobium(Trivediet al.,2007;Ansori and Gholami,2015;Khanet al.,2017;Lemanceauet al.,2017;Cordovezet al.,2018).Thus,we propose that the remaining keystone taxa identified may also be important for host plants,although the influence of these taxa,which have not previously been recognized as plant beneficial taxa,needs further study and confirmation.
Less abundant key microbiota affected by fertilization
Many studies have focused on differences in the abundant taxa of a community,because these taxa have been shown acting as main drivers for community patterns(Shade and Handelsman,2012).However,in ecological systems most species are rare,and the importance of rare members have been highlighted as these taxa often have distinct functional traits from the abundant species in many studies(Mouillotet al.,2013;Shadeet al.,2014).The influence of keystone taxa,rare community members with a disproportionate influence on community stability or functioning,also emphasizes the potential importance of numerically inconspicuous taxa(Banerjeeet al.,2018).Most of the potential keystone species identified in our study based on their position within interaction networks had low relative abundance(Table SV).Further,the presence or loss of keystone species in certain environments may affect not only the stability of interactions within the community but also the fitness of the host(Agleret al.,2016;Van Der Heijden and Hartmann,2016;Banerjeeet al.,2018).In our study,it was found long-term fertilization affected the relative abundance of key microbiota,but not the abundant microbiota,in both leaves and roots(Figs.5b and S5).This indicates that the effects of fertilization on whole endophytic communities may be mediated by effects on keystone taxa.Compared to the other fertilization treatments,the supplement of cow manure significantly increased the relative abundance of keystone species in both leaves and roots.This suggests that NPK fertilization combined with cow manure may be a balanced fertilization approach from the perspective of maintenance of endophytic bacterial cooccurrence networks and the accommodation of a larger proportion of keystone species.However,the actual effects of keystone species on plant growth and health still need to be validated through well-designed experiments.Furthermore,considering the limitation of 16S rRNA gene taxonomic resolution at the species level(Větrovskýand Baldrian,2013),a more accurate phylogenetic marker such as gyrB should be considered in the validation of the responses of keystone microbes to fertilization at the species level.
In this study,fertilization treatments had significant influences on endophytic bacterial community composition,indicating that these special communities should be taken into consideration for the assessment of fertilization management and regulation of microbial resources in agroecosystems.Furthermore,previous studies have shown that endophytic microbial communities vary among plant genotypes,development stages,as well as seasons and years(Van Overbeek and Van Elsas,2008;Marqueset al.,2015;Campisanoet al.,2017).Therefore,the response of endophytic associations to fertilization may need to be considered in light of these factors.Examining the interactions of fertilization with these other factors is necessary to understand its effects on endophytic bacterial communities.Our work provides the first step towards the consideration of endophytic bacterial communities in the development of agricultural fertilizer management strategies.
CONCLUSIONS
The endophytic bacterial communities in wheat leaves and roots were dominated by Actinobacteria,Bacteroidetes,Firmicutes,Fusobacteria,and Proteobacteria.Plant tissue and fertilization treatment both affected bacterial community structures in the plant endosphere.The alpha diversity of bacterial endophytes in roots was much higher than that in leaves,whereas fertilization had no significant effects on the alpha diversity of the endophytic bacterial community.The endophytic bacterial co-occurrence network in leaves had a smaller network size(285 OTUs),but a greater number of positive correlations(99.8%),and a more robust,complex structure compared with that in roots,with leaves having a higher average number of connections per OTU(node degree 18.0),and stronger clustering into and within consortia(modularity 0.6 and connectance 0.03).Ninety-five unique keystone species were identified in the networks of wheat leaves and roots,which may serve as a list of potential plant beneficial endophytic bacteria and provide novel targets for the sustainable management of wheat crops in agroecosystems.In a previous study,we found that the long-term fertilization of NPK plus cow manure not only improved crop production,but also maintained bacterial diversity in soils(Sunet al.,2015).In this study,we also found that this treatment(NPK+CM)could increase the relative abundance of the putative keystone bacterial microbiota,which are potentially beneficial for plant growth and fitness.Therefore,the combination of chemical fertilizer and cow manure may be a good agricultural practice from the viewpoint of both below-and aboveground microbial communities.
ACKNOWLEDGEMENTS
We thank Mr.Guo Zhibin and Mr.Hua Keke from the Anhui Academy of Agricultural Sciences,China,and Ms.Ni Yingying,Ms.Fan Kunkun,and Ms.Wang Hongfei from the Institute of Soil Science,Chinese Academy of Sciences for their assistance in field management and soil sampling.This work was funded by the National Natural Science Foundation of China(No.31870480),the Strategic Priority Research Program of Chinese Academy of Sciences(No.XDB15010101),and the China Biodiversity Observation Networks(Sino BON).
SUPPLEMENTARY MATERIAL
Supplementary material for this article can be found in the online version.
杂志排行
Pedosphere的其它文章
- Rice productivity and profitability with slow-release urea containing organic-inorganic matrix materials
- Bacterial communities in paddy soils changed by milk vetch as green manure:A study conducted across six provinces in South China
- Efficiency of soil-applied 67Zn-enriched fertiliser across three consecutive crops
- Degradation of the fungicide metalaxyl and its non-extractable residue formation in soil clay and silt fractions
- Soil micromorphological and physical properties after application of composts with polyethylene and biocomponent-derived polymers added during composting
- Effect of sewage sludge and sugarcane bagasse biochar on soil properties and sugar beet production
