Bacterial communities in paddy soils changed by milk vetch as green manure:A study conducted across six provinces in South China
2021-10-15SongjuanGAOWeidongCAOGuopengZHOUandRobertREES3CollegeofResourcesandEnvironmentalSciencesNanjingAgriculturalUniversityNanjing0095China
Songjuan GAO,Weidong CAO,Guopeng ZHOU and Robert M.REES3College of Resources and Environmental Sciences,Nanjing Agricultural University,Nanjing 0095(China)
2Key Laboratory of Plant Nutrition and Fertilizer,Ministry of Agriculture and Rural Affairs/Institute of Agricultural Resources and Regional Planning,Chinese Academy of Agricultural Sciences,Beijing 100081(China)
3Scotland’s Rural College(SRUC),West Mains Road,Edinburgh EH9 3JG(UK)
ABSTRACT The use of green manures contributes to sustainable soil and nutrient management in agriculture;however,the responses of soil microbial communities to different fertilization regimes at the regional scale are uncertain.A study was undertaken across multiple sites and years in Hunan,Jiangxi,Anhui,Henan,Hubei,and Fujian provinces of South China to investigate the effects of green manuring on the structure and function of soil bacterial communities in rice-green manure cropping systems.The study included four treatments:winter fallow with no chemical fertilizer as a control(NF),milk vetch as green manure without chemical fertilizer(GM),winter fallow and chemical fertilizer(CF),and a combination of chemical fertilizer and milk vetch(GMCF).Significant differences were found in the responses of soil microbial communities at different sites,with sampling sites explaining 72.33%(F=36.59,P=0.001)of the community composition variation.The bacterial communities in the soils from Anhui,Henan,and Hubei were broadly similar,while those from Hunan were distinctly different from other locations.The analysis of Weighted UniFrac distances showed that milk vetch changed soil microbial communities compared with winter fallow.Proteobacteria and Chloroflexi predominated in these paddy soils;however,the application of green manures increased the relative abundance of Actinobacteria.There was evidence showing that the functional microbes which play important roles in the cycling of soil carbon,nitrogen(N),and sulfur(S)changed after several years of milk vetch utilization(linear discriminant analysis score>2).The abundance of methane-oxidizing bacteria and S-reducing bacteria increased,and microbes involved in N fixation,nitrification,and denitrification also increased in some provinces.We concluded that the application of milk vetch changed the bacterial community structure and affected the functional groups related to nutrient transformation in soils at a regional scale.
Key Words:alpha diversity,bacterial composition,functional group,operational taxonomic unit,soil fertility,Weighted UniFrac distance
INTRODUCTION
The application of green manures in rice cropping system is known to be an effective practice for maintaining high yields of rice while at the same time providing a wide range of environmental benefits(Gaoet al.,2013;Xieet al.,2016;Couëdelet al.,2018).Milk vetch(Astragalus sinicusL.)is widely used in South China as a winter green manure.Previous studies have shown that the utilization of milk vetch can improve the productivity and sustainability of paddy soils(Hwanget al.,2015;Xieet al.,2016).The incorporation of green manures changes microbial processes,thereby affecting microbial community structures and nutrient cyclings,especially soil carbon(C),nitrogen(N),and sulfur(S)(Wanget al.,2013;Gaoet al.,2015).However,the nature of these changes and their consequences for nutrient cycling are poorly understood.
Soil microorganisms play pivotal roles in nutrient cycling processes in agro-ecosystems,and changes in microbial communities can be an indicator of soil environment and nutrient turnover.The types and chemical compositions of organic substrates affect the diversity,composition,and functional group of soil microbes(Panet al.,2016).Climatic conditions(Zhanget al.,2013;Guoet al.,2015),cropping systems(Zhaoet al.,2014),field management practices,and fertilization regimes(Lagomarsinoet al.,2016;Zhaoet al.,2016;Zhang X Xet al.,2017)can also interact with organic matter and affect the composition and function of soil microbes.Previous studies reported that the use of mineral fertilizers may cause a decrease in soil microbial diversity,resulting in a decline in soil fertility(Zhang Y Tet al.,2017;Schmidet al.,2018).Most organic soil amendments introduce complex organic substrates into soil,which are metabolized by specialized microorganisms,and thus this would be expected to cause changes in community composition and functional groups(Schmidet al.,2018).The long-term combined utilization of organic and inorganic fertilizers also therefore increases soil fertility and bacterial community structure with implications for the decomposition of native soil organic matter(Liet al.,2017).
The incorporation of green manures introduces large amounts of fresh organic matter into soils,which may cause dramatic changes in soil nutrient transformations and associated microorganisms.A distinguishing feature of green manures in contrast to other organic materials is that their growth stage is also crucial in influencing soil processes.Zhang X Xet al.(2017)showed that the utilization of different green manures for 31 years in red paddy soil shaped the microbial communities of rice rhizosphere.Other studies revealed that the utilization of green manure can improve soil microbial characteristics,including increases in soil microbial biomass and soil extracellular enzyme activity(Elfstrandet al.,2007a;Yeet al.,2014).The changes in soil microbial communities may partly explain the mechanisms by which green manures improve soil fertility and increase grain yields.However,many uncertainties remain regarding the relationship between organic amendments and specific changes to individual microbial groups,especially at large regional scales.The responses of microbial communities at different sites with varying soil conditions and climate are particularly difficult to predict.A network of experiments in six provinces was therefore established to investigate the effects of green manure on soil properties and microbial communities in different paddy soils,and to explore the differences and relationships amongst different sites.The hypotheses were:i)the soil bacterial communities would be changed by application of milk vetch for 4 or 7 years and would differ between sites and ii)occurrence of some specific bacterial groups would be related to milk vetch application.
MATERIALS AND METHODS
Field description and experiment design
The study sites were located at experimental stations in six provinces in South China(Table I).All sites were located on paddy soils,classified as stagnant Anthrosols(FAO,2015)(http://www.fao.org/faostat/en/#data/QC).The experiments were set up in a completely randomized block design with three(Hunan,Jiangxi,Hubei,Fujian,and Anhui)or four(Henan)replicates of each treatment in 2008(Hunan,Jiangxi,Henan,Hubei,and Fujian)and 2011(Anhui).Each plot was 20 m2in area(4 m×5 m),including four treatments:winter fallow and no chemical fertilizer as a control(NF),milk vetch as green manure(GM),winter fallow and chemical fertilizer(CF),and a combination of chemical fertilizer and milk vetch(GMCF).Different rates of chemical fertilizer,which are based on local farmer practices,were used at different sites(Table II).The incorporation rate of milk vetch was 22 500 kg fresh weight ha-1each year.The managementof the field experiments were described in our previous study(van Maarschalkerweerdet al.,2016).The cropping rotation in Henan and Fujian was single rice-green manure and double rice-green manure in Hunan,Jiangxi,Anhui,and Hubei.

TABLE ILocation and climate of the six experimental sites in South China

TABLE IIFertilizera)application rates at the six experimental sites in South China
Soil sampling
All soils at the six sites were sampled after the harvest of late rice or single rice in November or December of 2014.The soil samples were collected from five points in each plot,and the top-soil(0–20 cm)was sampled.Plant residues and stones in the collected soil samples were removed,and then the samples were mixed well and sieved(<2 mm).Parts of the soil sample were stored at-80°C for molecular analysis,and the others were stored at 4°C or air dried for physical and chemical analyses.
Chemical analysis
Soil physical and chemical analyses wer e conducted according to the methods described by Lu(2000).Soil pH was tested using a pH meter with a soil to water ratio of 1:2.5(weight:volume).Soil organic matter(SOM)was tested using potassium dichromate oxidation method.Soil total nitrogen(TN)was measured using the Kjeldahl method.Available phosphorus(AP)was extracted by 0.5 mol L-1NaHCO3,and available potassium(AK)were extracted by 1 mol L-1CH3COONH4.Soil mineral N(NH+4-N and NO-3-N)was extracted using 2 mol L-1KCl,and then determined using a continuous flow analyzer(AA3,SEAL,Germany).Soil texture was measured using the pipette method,which followed Stoke’s law.Chemical properties and soil texture are listed in Tables SI and SII,respectively(see Supplementary Material for Tables SI and SII).
DNA extraction and sequencing
Soil DNA was extracted using the FastDNA Spin Kit for Soil(MP Bio,Santa Ana,USA).The extracted DNA samples were quantifeid using a Nanodrop 2000 spectrophotometer(Thermo Fisher,Waltham,USA)and then stored at-80°C for further analysis.The primers of 16S rDNA sequence were 338F/806R(ACTCCTACGGGAGGCAGCA/GGACTACHVGGGTWTCTAAT)(Chuet al.,2015).The length of the primer was 468 bp.Barcode oligonucleotides were ligated to the primer to distinguish the amplicons from different samples.The PCR amplification was conducted using a GeneAmp PCR System 9700(Life Technologies,Carlsbad,USA).Then,the purified PCR products were quantified and pooled at equal concentration.Sequencing of the PCR amplicon was performed on the Illumina MiSeq PE300 platform(Caporasoet al.,2012).
Bioinformatics and statistical analyses
The sequences obtained were trimmed and screened using Trimmomatic and FLASH(version 1.2.7,http://ccb.jhu.edu/software/FLASH/).Briefly,according to their unique barcodes,sequencing reads were assigned to each sample,and then low-quality sequences(average quality score lower than 20,and length less than 50 bp)were removed.The remaining reads were then spliced based on the overlap sequence(length>10 bp),and no ambiguous base was permitted in the overlap region.The same number of sequences in each pyrosequencing library was subsampled randomly by Mothur software(Schlosset al.,2009)(http://www.mothur.org/wiki/Schloss_SOP)to eliminate the bias of the libraries’alpha diversity comparison,based on the minimum reads number of all samples(each of 23 650 reads,after quality control).
The remaining high-quality reads were then aligned and clustered into operational taxonomic units(OTUs)using Usearch(version 7.1,http://drive5.com/uparse/)(Edgar,2013).The sequence identity threshold is 97%.The fastx_unique command was used to remove the duplicated sequences,and the sortbysize derep.fasta command was used to discard singletons.The representative sequences of the OTUs were compared to the Sliva database using RDA classifier(Wanget al.,2007)to obtain the taxa of each sample.The OTU number,Good’s coverage(Good,1953),Chao 1 richness(Chao,1984),and Shannon index(Chao and Shen,2003)were calculated to estimate the alpha diversity of the samples.Approximately-maximum likelihood phylogenetic trees were conducted using FastTree(version 2.1.3,http://www.microbesonline.org/fasttree/).Then,the Weighted UniFrac distances were calculated using Fastunifrac(Hamadyet al.,2010)(http://unifrac.colorado.edu/).The heatmaps based on the Weighted UniFrac distances and the principle coordinate analysis(PCoA)based on Bray-Curtis distances were analyzed in R software(R development Core Team,2008).Linear discriminant analysis(LDA)effect size(LEfSe)was employed to identify the specific phylotypes responding to the green manure treatments.A nonparametric factorial Kruskal-Wallis(KW)sum-rank test was performed to estimate the differences between green manure and winter fallow treatments and to determine phylotypes with significant differences.The groups with logarithmic LDA scores larger than 2 were evaluated as significantly different,and cladogram was constructed using the LDA score.A nonparametric multivariate analysis of variance(Adonis)was conducted using the“vegan”package in R software and a mantel test using PASSaGE software.The analysis of variance(ANOVA)and two-way ANOVA were conducted using SAS 8.1.
The pyrosequencing data were deposited into the NCBI Sequence Read Archive(SRA)database,and the accession number is SRP140602.
RESULTS
Alpha diversity
The values of Good’s coverage of all samples were higher than 95%(Table SIII,see Supplementary Material for Table SIII),suggesting that the bacterial communities could be well reflected using the obtained libraries.The OTU number,Chao 1 richness,and Shannon index were selected to represent the alpha diversity of soil bacterial communities(Table SIV,see Supplementary Material for Table SIV).The OTU number,Chao 1 richness,and Shannon index differed among sites,and the alpha diversity was the highest in Anhui and the lowest in Jiangxi(Fig.1).The OTU number of all samples ranged from 1 690 to 3 493.Compared with NF,the GM and GMCF treatments increased OTU number and the Shannon index(P<0.05),and the GM treatment increased Chao 1 richness in Jiangxi(P<0.05).In terms of OTU number,Chao 1 richness,and Shannon index,there was no significant difference between winter fallow and green manure treatments of the other sites(Table SIV).

Fig.1 Operational taxonomic unit(OTU)number,Chao 1 richness,and Shannon index at the six experimental sites in South China.Different letters indicate significant differences at P<0.05.Boxes show the 25%–75%quartiles.Solid lines in boxes indicate the mean values.The top and bottom error bars of the box indicate the 95%and 5%percentiles,respectively.The dots indicate values out of 5%–95%quartiles.
Bacterial composition
In total,10 phyla(relative abundance>1%)were chosen to evaluate bacterial composition(Fig.2).Proteobacteria were predominant in Hunan,Jiangxi,Anhui,Henan,and Hubei,and the relative abundances were 30.10%,28.30%,30.76%,27.95%,and 27.51%,respectively.This was followed by Chloroflexi(the relative abundances were 30.06%,22.41%,20.70%,25.09%,and 16.51%,respectively)(Fig.2).In Fujian,the relative abundance of Chloroflexi(31.75%)was higher than Proteobacteria(22.52%).The two-way ANOVA(Table III)showed that the sampling site and the interaction between sampling site and treatment had significant effects on the abundances of Proteobacteria and Chloroflexi.The abundances of Acidobacteria ranged from 5.29%to 29.12%and were affected significantly by both sampling site and green manure treatment(Fig.2,Table III).Treatments with winter green manuring increased the abundances of Actinobacteria in Jiangxi,Henan,and Fujian,and decreased the abundance of Nitrospirae in Hunan compared with the winter fallow.The relative abundances of Nitrospirae ranged from 2.11%to 15.74%,although this varied between sites.In Hunan,Jiangxi,Anhui,and Henan provinces,the relative abundances of Firmicutes were higher in the treatments with milk vetch than the winter fallow treatments,suggesting that the abundances of Firmicutes were also significantly affected by green manuring treatment.The relative abundances of Bacteroidetes and Gemmatimonadetes ranged from 0.21% to 4.52% and from 0.69% to 2.98%,respectively,and significant differences were found between sites.The relative abundances of Chlorobi and Planctomycetes ranged from 0.35% to 3.72% and from 0.13% to 5.63%,respectively.Compared with winter fallow,the relative abundance of Chlorobi in Jiangxi under the milk vetch treatment decreased,while the relative abundance of Planctomycetes in Hubei increased.

Fig.2 Relative abundances of major phyla(>1%)with or without winter green manure at the six experimental sites in South China.Vertical bars indicate the standard errors of the means(n=6 for Hunan,Jiangxi,Anhui,Hubei,and Fujian;n=8 for Henan).The asterisks*and**represent significant differences at P<0.05 and P<0.01,respectively,between groups.NGs indicates NF(winter fallow and no chemical fertilizer as a control)and CF(winter fallow and chemical fertilizer);TGs indicates GM(milk vetch as green manure)and GMCF(the combination of chemical fertilizer and milk vetch).
Community difference
Weighted UniFrac distances were calculated to evaluate the community differences between experimental sites and green manure treatments.In Hunan,Henan,and Fujian provinces,the distances between NF and GMCF were the largest,while the largest distances occurred between NF and GM treatments in Jiangxi,Anhui,and Hubei provinces(Fig.S1,see Supplementary Material for Fig.S1).Results of the Adonis showed that the green manure treatment explained 35.68%(F=36.53,P=0.028)of the microflora variations in Hunan and explained 35.30%(F=1.45,P=0.058)and 24.12%(F=1.27,P=0.074)of the community variations in Jiangxi and Henan,respectively.
The community differences between sites were evaluated using both Weighted UniFrac distances(Fig.3a)and Bray-Curtis distances(Fig.3b).The Weighted UniFrac distances showed that there was little difference among microbial communities in Anhui,Henan,and Hubei,and the samples of Hunan were far away from those of other sites(Fig.3a).The PCoA based on the Bray-Curtis distances showed similar results(Fig.3b).The samples of Hunan were separately in the second quadrant,Jiangxi and Fujian were clustered together in the first quadrant,and the other three sites were clustered in the third and fourth quadrants.Results of Adonis showed that sampling sites explained 72.33%(F=36.5 9,P=0.001)of the community variation.
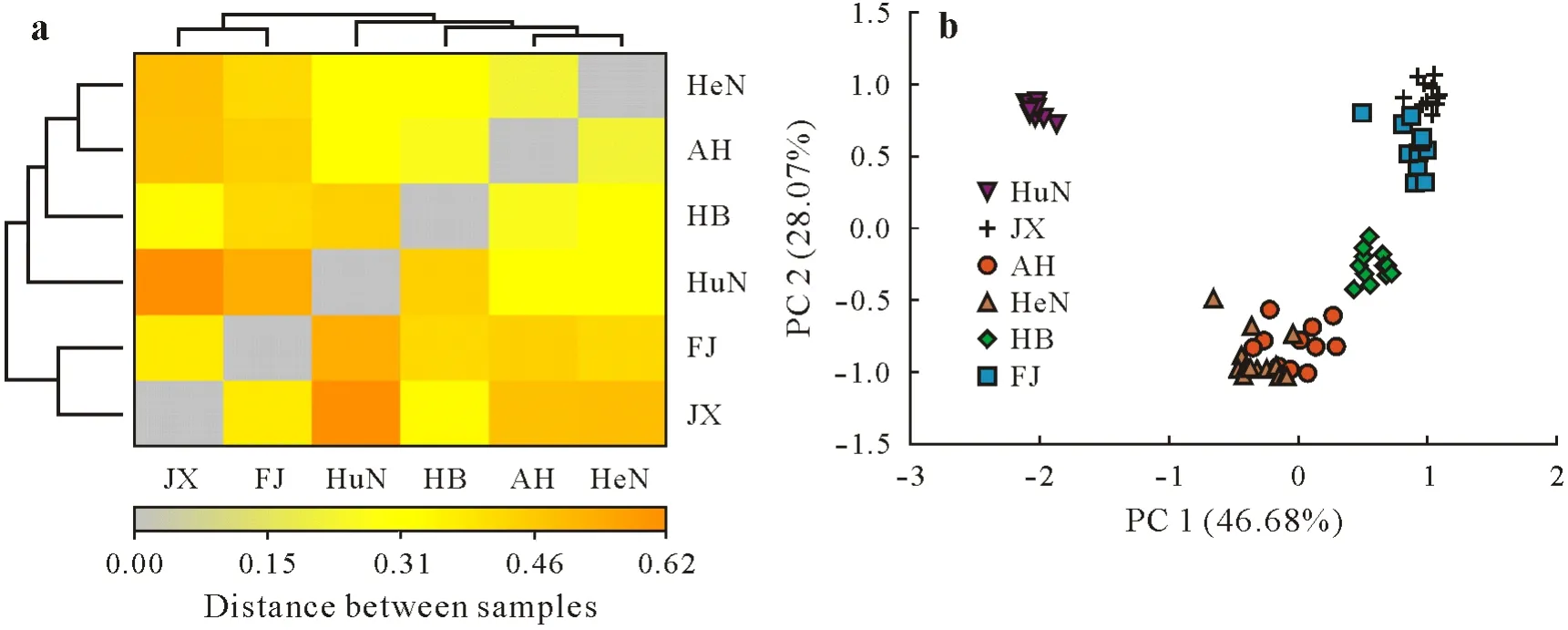
Fig.3 Weighted UniFrac distance(a)and principal coordinate analysis based on the Bray-Curtis distance(b)among the six experimental sites in South China.The darker yellow color represents the larger distance between treatments.PC=principal component.HeN,AH,HB,HuN,FJ,and JX=Henan,Anhui,Hubei,Hunan,Fujian,and Jiangxi,respectively.
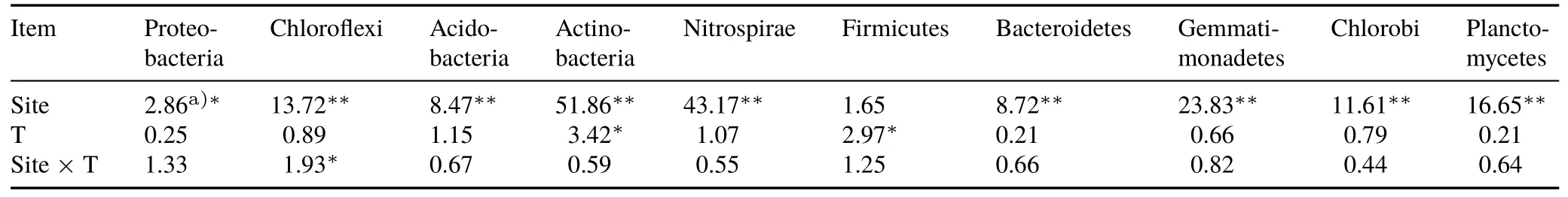
TABLE IIIEffects of experimental site and green manure treatment(T)on the relative abundances of major phylum(>1%)and their interaction effects
Effects of milk vetch on functional genus
The LDA effect size analysis was conducted to find out the effects of green manure utilization on soil bacteria.The cladogram showed that both the number and species of enriched groups changed between sites(Fig.S2,see Supplementary Material for Fig.S2).At the six sites,the most enriched groups affected by green manure treatment were found in Anhui and Hubei(Fig.S2).We further investigated the functional groups involving soil C,N,and S transformations and found that winter green manuring enriched some of these functional groups(Table IV).In Hunan,the incorporation of milk vetch increased the abundance ofMethylocystis(Type II Methanotrophs),while in Anhui and Hubei,Type I methanotrophs(Methylobacterium,Methylococcus,Methylomonas,andMethylosarcinain Anhui andMethylocaldumin Hubei)were significantly enhanced by green manuring.The N-fixing bacteriaBradyrhizobiumandRhizobiumwereenhanced by the milk vetch treatment in Jiangxi.Nitrospira,which contributes to the nitrite oxidization process,was enhanced by winter green manuring in Hunan and Anhui.DenitrifiersThiobacillus,Bacillus,andHyphomicrobiumwere significantly enhanced by the utilization of milk vetch in Jiangxi,Henan,and Hubei.Sulfur-reducing bacteria were also enhanced by the utilization of milk vetch.Among them,
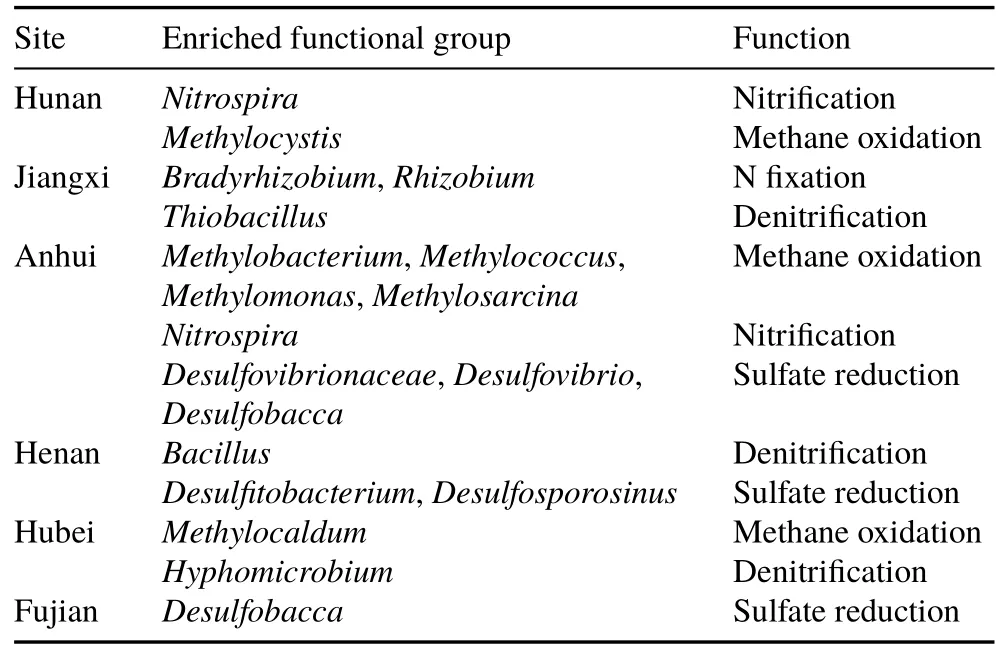
TABLE IVFunctional groups of bacteria enriched by milk vetch and their roles in soil nutrient transformations at the six experimental sites in South China based on the results of linear discriminant analysis(LDA)effect size analysis(LDA score>2)
Desulfovibrionaceae,Desulfovibrio,andDesulfobaccain Anhui,DesulfitobacteriumandDesulfosporosinusin Henan,andDesulfobaccain Fujian were increased after the application of milk vetch.
Correlations between soil properties and bacterial communities
Mantel tests were conducted to evaluate the correlations between soil properties and bacterial communities(Table V).In Hunan,soil microbial communities showed significant correlations with soil organic matter(P=0.011)and soil AK(P=0.013).In Anhui,soil pH and bacterial communities were significantly correlated(P=0.044).No significant correlation was found in other sites.
DISCUSSION
Our results suggested that the application of milk vetch in paddy soils strongly affects the diversity,composition,and functional groups of soil bacteria,in ways that are broadly consistent with previously published studies(Elfstrandet al.,2007a,b;Gaoet al.,2015;Zhang X Xet al.,2017).Changes in the composition of soil bacterial communities were found at all six sites after milk vetch incorporation.Previous studies reported that long-term application of manure enhanced soil microbial diversity and changed the interactive relationship between plants and soil microorganisms(Aiet al.,2015).Zhaoet al.(2016)demonstrated that the low amounts of strawincorporation had no obvious effect on soil microorganisms,but high amounts of straw input could significantly change soil microbial community structure.Application of straw was particularly important in stimulating the copiotrophic bacteria and contributing to soil sustainability and productivity(Zhaoet al.,2017).Green manure incorporation in this study also had significant impacts on soil properties and soil microorganisms.The utilization of milk vetch increased the abundances of Actinobacteria and Firmicutes at some of our study sites.Actinobacteria could promote the decomposition of plant residues,and therefore play important roles in soil nutrient cycling.The incorporation of green manures provides additional substrate for Actinobacteria and thus enhances their growth.This phenomenon was observed at more than one site,indicating a broader relevance at the regional scale.Wanget al.(2015)found that the communities and functions of soil microorganisms showed a large variation that could be linked to the alternating wetting and drying of the paddy soil.The rice-winter green manure cropping system is also strongly influenced by wetting-drying cycles.However,the underlying mechanism by which changes in soil microbial communities structure take place after green manure application needs further study.

TABLE VMantel test of the relationship between soil properties and bacterial communities at the six experimental sites in South China
Soil environment and its interaction with management play a critical role in shaping soil microbial community structure.The growth and decomposition of green manure plants had different influences on soil conditions and caused various responses of microorganism at different sites.Results showed that the community structure of soil bacteria in Hunan was significantly different to that at other sites.This may be caused by differences in soil conditions,especially soil pH.Paddy soils in Hunan are typically alkaline purple alluvial soils,which differ from the neutral or acidic paddy soils at the other five sites.Previous studies proved that soil pH is a pivotal indicator in shaping the bacterial communities(Osborneet al.,2011;Hendriksenet al.,2016;Mirandaet al.,2018).A significant correlation between soil pH and bacterial communities was found in Anhui,and it demonstrated that the differences in soil pH may be one of the main factors that impact bacterial communities.Possibly because of the nearby proximity of sites,bacterial communities showed considerable similarity in Jiangxi and Fujian,and in Henan and Anhui.We have shown that the soil type and climate are important drivers influencing soil bacterial communities.Guoet al.(2015)reported that the community structure of soil autotrophic bacteria could be affected by elevation,soil temperature,soil water content,soil nutrient status,vegetation type,etc.Based on a five-year experiment,Zhanget al.(2013)reported that climatic conditions,such as precipitation,temperature,etc.,had direct effects,while soil total N and soil pH had indirect effects on soil bacterial communities.The synthetic action of these direct and indirect factors contributed to the various responses of soil microbial communities at the different sites.Soil bacterial communities in Hunan and Anhui were correlated with soil pH,organic matter,and some available nutrients in our study,illustrating the interaction between soil properties and microbial communities.The availability of soil nutrients(particularly available C and N)can restrict the abundance of Proteobacteria with increasing soil depth(Zhang B Zet al.,2017).Other studies also showed that Proteobacteria were more abundant in a higher C environment(Fiereret al.,2007;Eilerset al.,2010;Goldfarbet al.,2011)and demonstrated enormous metabolic diversity(Donget al.,2017).In this study,both the abundance of Proteobacteria and the quantity of soil C content in Hunan and Anhui were higher than other sites,indicating that soil nutrient condition was a major cause of the differences in Proteobacteria distribution.
The transformations of soil C,N,S,etc.are important processes in soil nutrient cycling affecting both soil fertility and plant nutrition.Soil microbes drive the biogeochemical cycles of these elements and can have positive or negative interactions with crops(Somanet al.,2017).In this study,many bacterial genera showed significant differences in abundance between milk vetch and winter fallow treatments.Such differences would be expected to alter both the rates and pathways of soil nutrient cycling in ways that we do not yet fully understand.
Methane(CH4)is one of the main greenhouse gases,and paddy soil is an important source(Nazarieset al.,2013).In paddy fields,CH4is produced by methanogens(a broad spectrum of heterotrophs)and consumed by methanotrophs(autotrophs),which use CH4as their only C and energy sources.The balance between these processes is regarded important in the mitigation of CH4emission(Nazarieset al.,2013).Methanotrophs are traditionally clustered into Type I(Gammaproteobacteria)and Type II(Alphaproteobacteria)(Semrauet al.,1995;Fanget al.,2000;Choiet al.,2008).In our study,either Type I or Type II methanotrophs were enhanced by green manuring in Hunan,Anhui,and Hubei.Some studies indicated that the quantity of methanotrophs is negatively correlated with CH4emission(Liuet al.,2017).Leeet al.(2010)reported that when 10 Mg ha-1milk vetch was applied as green manure,it was possible to sustain rice productivity without increasing CH4emission.This contrasted with an NPK fertilizer treatment in a mono-rice cropping system which had higher CH4emission.As a leguminous plant,milk vetch has a relatively low C/N ratio,and this might be one of the reasons that milk vetch induced the lower CH4emission than other organic amendment,such as straw(Zhouet al.,2019).These findings indicate the importance of understanding underlying microbial dynamics when developing CH4mitigation strategies.
Because milk vetch is a legume,its effects on rhizobial populations in paddy soils deserve special attention.Nitrification and denitrification processes are controlled by nitrifiers and denitrifiers,and are critical in determining the rates of loss and turnover of soil N in cropland ecosystems(Ishiiet al.,2011).In this study,the genera which are crucial in N cycling(N fixation,nitrification,and denitrification)were enriched by milk vetch.These results indicated that the application of milk vetch in paddy soils have profound influences on soil N transformations.Liet al.(2015)reported that the combination of inorganic N fertilizer and plant residues may be an effective way to increase N use efficiency.The N transformation processes affected by green manuring may be one of the immanent causes of the improvement of N use efficiency.
Soil acidification is an important issue in cropland ecosystems(Guoet al.,2010).The main causes of soil acidification in China are excessive use of N fertilizers,S oxidation(following the drainage of rice paddies),and the acidic deposition containing nitric and sulfuric acids(Larssen and Carmichael,2000;Guoet al.,2010).Sulfur-reducing prokaryotes play important roles in neutralizing soil acidity within the S biogeochemical cycle.In our study,S-reducing bacteria were enhanced by the utilization of milk vetch.The increase of S-reducing bacteria may indicate that the utilization of milk vetch in paddy soil could be a valuable tool for alleviating soil acidification.
CONCLUSIONS
Green manures used within rice cropping systems can fundamentally change soil microbial community structures.This research also revealed that environmental factors such as soil conditions are important in regulating the balance of microbial populations.Understanding the relationships between changes in microbial composition and the functional consequences of such changes are still in their infancy,especially at the regional scale.In this study,we have clearly demonstrated that management interventions,especially the introduction of green manuring,have profound impacts on some of the most important microbial groups related to the biogeochemical cycling of C,N,and S.This information will be critical to the design of new approaches aimed at mitigating greenhouse gas emission,ensuring better nutrient use efficiency,and achieving improved sustainability in rice production.
ACKNOWLEDGEMENT
This work was supported by the earmarked fund for Modern Agro-industry Technology Research System-Green Manure,China(No.CARS-22)and the National Natural Science Foundation of China(No.42007071).
SUPPLEMENTARY MATERIAL
Supplementary material for this article can be found in the online version.
杂志排行
Pedosphere的其它文章
- Rice productivity and profitability with slow-release urea containing organic-inorganic matrix materials
- Efficiency of soil-applied 67Zn-enriched fertiliser across three consecutive crops
- Effect of long-term fertilization on bacterial communities in wheat endosphere
- Degradation of the fungicide metalaxyl and its non-extractable residue formation in soil clay and silt fractions
- Soil micromorphological and physical properties after application of composts with polyethylene and biocomponent-derived polymers added during composting
- Effect of sewage sludge and sugarcane bagasse biochar on soil properties and sugar beet production
