Development of a risk score to guide targeted hepatitis C testing among human immunodeficiency virus patients in Cambodia
2021-10-11AnjaDeWeggheleireJozefienBuyzeSokkabAnSopheakThaiJohanvanGriensvenSvenFrancqueLutgardeLynen
Anja De Weggheleire, Jozefien Buyze, Sokkab An, Sopheak Thai, Johan van Griensven, Sven Francque,Lutgarde Lynen
Anja De Weggheleire, Jozefien Buyze, Johan van Griensven, Lutgarde Lynen, Department of Clinical Sciences, Institute of Tropical Medicine Antwerp, Antwerp 2000, Belgium
Sokkab An, Sopheak Thai, Infectious Diseases Department, Sihanouk Hospital Center of Hope, Phnom Penh 12101, Cambodia
Sven Francque, Department of Gastroenterology Hepatology, Antwerp University Hospital, Antwerp 2000, Belgium
Sven Francque, Laboratory of Experimental Medicine and Paediatrics, University of Antwerp, Antwerp 2000, Belgium
Abstract BACKGROUND The World Health Organization recommends testing all human immunodeficiency virus (HIV) patients for hepatitis C virus (HCV). In resource-constrained contexts with low-to-intermediate HCV prevalence among HIV patients, as in Cambodia, targeted testing is, in the short-term, potentially more feasible and cost-effective.AIM To develop a clinical prediction score (CPS) to risk-stratify HIV patients for HCV coinfection (HCV RNA detected), and derive a decision rule to guide prioritization of HCV testing in settings where ‘testing all’ is not feasible or unaffordable in the short term.METHODS We used data of a cross-sectional HCV diagnostic study in the HIV cohort of Sihanouk Hospital Center of Hope in Phnom Penh. Key populations were very rare in this cohort. Score development relied on the Spiegelhalter and Knill-Jones method. Predictors with an adjusted likelihood ratio ≥ 1.5 or ≤ 0.67 were retained, transformed to natural logarithms, and rounded to integers as score items. CPS performance was evaluated by the area-under-the-ROC curve (AUROC) with 95% confidence intervals (CI), and diagnostic accuracy at the different cut-offs. For the decision rule, HCV coinfection probability ≥1% was agreed as test-threshold.RESULTS Among the 3045 enrolled HIV patients, 106 had an HCV coinfection. Of the 11 candidate predictors (from history-taking, laboratory testing), seven had an adjusted likelihood ratio ≥ 1.5 or ≤ 0.67: ≥ 50 years (+1 point), diabetes mellitus (+1), partner/household member with liver disease (+1), generalized pruritus (+1), platelets < 200 × 109/L (+1), aspartate transaminase (AST) < 30 IU/L (-1), AST-to-platelet ratio index (APRI) ≥ 0.45 (+1), and APRI < 0.45 (-1). The AUROC was 0.84 (95%CI: 0.80-0.89), indicating good discrimination of HCV/HIV coinfection and HIV mono-infection. The CPS result ≥0 best fits the test-threshold (negative predictive value: 99.2%, 95%CI: 98.8-99.6). Applying this threshold, 30% (n = 926) would be tested. Sixteen coinfections (15%) would have been missed, none with advanced fibrosis.CONCLUSION The CPS performed well in the derivation cohort, and bears potential for other contexts of low-to-intermediate prevalence and little onward risk of transmission (i.e. cohorts without major risk factors as injecting drug use, men having sex with men), and where available resources do not allow to test all HIV patients as recommended by WHO. However, the score requires external validation in other patient cohorts before any wider use can be considered.
Key Words: Hepatitis C virus; Hepatitis C/human immunodeficiency virus coinfection; Clinical prediction rule; Targeted screening; Cambodia; Development prediction model
INTRODUCTION
Interferon-free antiviral treatment has replaced the combination of pegylated interferon and ribavirin as standard-of-care for chronic hepatitis C[1]. These new treatments are highly efficacious, short in duration, well-tolerated and hold, as becoming increasingly affordable, real promise of worldwide scalability[2]. On the other hand, less than 5% of people living with hepatitis C virus (HCV) in low and middle income countries (LMIC) were aware of their status end of 2016[3]. To boost identification of HCV infected individuals, particularly in LMIC, the World Health Organization (WHO) launched a first set of HCV testing guidelines in 2017[4]. Routine testing throughout the whole population is recommended where HCV seroprevalence is of intermediate (≥ 2%) or high (≥ 5%) level, and targeted testing in all other settings. Clinical suspects, people who inject drugs (PWID), men having sex with men (MSM), people in prisons, birth cohorts, and people living with human immunodeficiency virus (HIV) (PLWH) are the main targets for this latter.
Though feasibility in resource-limited settings was considered when formulating the WHO recommendations, it is unlikely that many LMIC will be able to implement them at full-scale in the short-term, due to operational (human resources, diagnostic capacity, stigma), but also financial constraints[5]. There are no large global financing initiatives in the pipeline for viral hepatitis at the short-to-medium term, and countries are in the meantime left to find their own financial solutions[6]. This seriously impacts the scale of what can be implemented.
In this regard, and based on the prevalence we registered in Cambodia, and even lower rates of HCV/HIV coinfection found in several HIV cohorts in Sub-Saharan Africa[7-10], we anticipate that some LMIC with large, primarily heterosexuallyinfected, HIV cohorts (and little forward transmission risk) may opt not to offer HCV testing to all HIV patients, at least in the short-to-medium term. Applying ‘screen all’ strategies in such cohorts is resource demanding and yields low positivity. To preserve resources, countries may rather choose to prioritize testing, in first instance, only for those at higher risk.
With the possibility of very successful treatment and growing availability of cheap WHO prequalified screening tests[11], the threshold to offer testing should, however, be low enough, to avoid maximally that HCV/HIV coinfected are denied treatment because of restrictive testing strategies. The critical question is thus whether it is possible to identify accurately, and in a simple manner, a subgroup of HIV patients in which the ‘probability of being HCV infected and having to be treated in the shortterm’ is so low that it would be reasonable not to offer them HCV testing or postpone it until more resources become available. Or phrased differently, to preserve the limited budget for testing and treating those with a higher risk of being HCV coinfected.
Easy-to-use tools to guide such targeted HCV testing in HIV populations, other than prioritization of key populations or older birth cohorts, do not exist. Though many LMIC have some birth cohort effect in their epidemics, it is generally less neat than in North-America and Europe, as drivers of generalized HCV exposure were removed at much later date or only partially[12-14]. Birth-cohort testing might thus be too restrictive. In our previous study in Cambodia, 55% of HCV/HIV coinfections would have been missed if only PLWH older than 50 years would have been tested[7].
As for other pathologies and conditions[15-18], diagnostic prediction models combining several readily available elements from patient history, physical examination, and lab tests may more accurately risk- stratify HIV patients and support clinical decisions regarding the need to prioritize HCV testing.
Using data from our HCV diagnostic study in Cambodia, we developed and internally validated a clinical prediction score (CPS) to risk-stratify HIV patients for HCV coinfection, and derived a decision rule to guide prioritization of HCV testing. In addition to the full CPS, we also explored alternative risk scores, one with only sociodemographic/clinical predictors and another primarily lab-based.
MATERIALS AND METHODS
Source of data, study site and participants
For developing the score, we used data of a cross-sectional HCV diagnostic study conducted in the HIV cohort of Sihanouk Hospital Center of Hope (SHCH) in Phnom Penh, Cambodia (clinical trials.gov NCT02361541). It is one of the largest primary care HIV cohorts in Cambodia with, as most other Cambodian HIV cohorts, primarily heterosexually-infected HIV patients. Key populations (history/current injecting drug use: 0.2%, history/currently engaged in sex work: 0.2%, self-identified MSM: 0.6%) were rare. Data were prospectively collected following a pre-specified protocol for HCV diagnostic work-up and predictors. The information on predictors (by historytaking, physical examination and laboratory tests) was collected without knowledge of the results of HCV diagnostic testing. Details of the study and diagnostic results have been published previously[7].
In brief, all consecutive adult HIV patients without history of HCV treatment and visiting the HIV clinic of SHCH between November 2014 and May 2016 underwent, if consenting, a structured health and HCV risk factor screening immediately followed by lab testing (hepatitis C, hepatitis B, CD4, platelets and liver tests (transaminases). HCV testing was done according to the classic two-test algorithm; initial testing for HCV antibodies followed by confirmatory HCV-RNA testing in case of HCV antibody positive or borderline results. In total, 3045 (out of 3562 in the cohort) adult HIV patients were enrolled, of whom 106 had a current HCV infection (i.e.HCV-RNA detected).
Approval for this study was provided by the Institutional Review Board of the Institute of Tropical Medicine Antwerp, the Ethics Committee of the Antwerp University Hospital (Belgium), and the Cambodian National Ethics Committee for Health Research. All enrolled participants provided written informed consent. The statistical methods and analysis of this study were reviewed by Jozefien Buyze from the Institute of Tropical Medicine, Antwerp, Belgium.
Development of the clinical prediction score
Outcome of interest: The outcome event was having a current HCV infection, which was defined as having a detectable HCV-RNA viral load as measured by the quantitative COBAS®AmpliPrep/COBAS®TaqMan®HCV PCR Test, v2.0, on the COBAS®TaqMan®48 Analyzer (Roche Diagnostics Ltd, Mannheim, Germany). The lower limit of detection was 15 IU/mL. Further in this paper, we refer to ‘current HCV infection’ as ‘HCV infection or coinfection’.
Candidate predictor variables:The clinical variables we explored as predictors were selected based on the distribution of the variables in our study data[7], reported associations in the literature and clinical plausibility, with preference for readily available and objective parameters. Potential predictors considered were: age (years), gender (female/male), platelet count (× 109cells/L), aspartate aminotransferase (AST, IU/L), alanine aminotransferase (ALT, IU/L), AST-to-platelet ratio index (APRI), having diabetes mellitus (yes/no), any of the following symptoms: fatigue, myalgia/arthralgia, anorexia/weight loss (yes/no), presenting generalized pruritus without obvious skin lesions (yes/no), having a household member and/or partner with liver disease (yes/no), and poor CD4 recovery on antiretroviral treatment (ART),i.e.CD4 below 200 after 3 years or more on ART (yes/no). Known major risk factors for HCV infection (history/current injecting drug use, sex work, being homosexual) were not considered as they were very uncommon in this cohort[7]. As we were mainly interested in the joint effects of the different variables to predict the probability of HCV infection and less to get an idea of the individual contribution of each variable, we did not exclude potentially correlated variables as long as they validly contributed to improving the predictive ability of the model[19,20].
Derivation cohort and sample size:We did not calculate a formal sample size for this CPS development study. We included the data of all 3,045 adult HIV patients enrolled in the cross-sectional study in the data set for derivation of the score to allow an adequate assessment of the potential predictors following the rule of thumb to have 10 outcome events per explored predictor variable[21].
Score development:We used the Spiegelhalter and Knill-Jones method adapted by Berkleyet al[22] and Stéphanet al[23] to develop the score. The continuous candidate predictors (age, platelets, AST, ALT, APRI) were dichotomized guided by Receiver Operating Characteristic (ROC) curves at the point with the highest sum of sensitivity and specificity, and rounded to values that are easy to use in clinical practice. Crude likelihood ratios (LHR) were calculated for all candidate predictors. Candidate predictors with a crude LHR ≥ 2 or ≤ 0.5 were, in a next step, used in a multivariable logistic regression model to calculate adjusted LHRs. The predictors with an adjusted LHR ≥ 1.5 or ≤ 0.67 were selected for the CPS. The adjusted LHRs were transformed to their natural logarithm, and rounded to the nearest integer to calculate the score (relative weight) of each predictor. By summing the scores of all risk factors presented by a patient the total predictor score for each patient was obtained. A value of 0 was assigned to missing data.
Score performance:The CPS’s performance to differentiate patients with HCV coinfectionvsthose without HCV coinfection (discrimination) was evaluated by the area-under-the-ROC curve (AUROC) with 95% confidence intervals (CI). AUROCs of 0.7-0.79, 0.8–0.89, ≥ 0.9 were respectively considered acceptable, good, and outstanding in terms of discrimination[24]. In addition, diagnostic accuracy (sensitivity, specificity, positive predictive value, negative predictive value) was calculated at the different cutoffs of the score. Statistical analysis was done using Stata 14 and R 3.4.2 software.
Derivation and performance of the decision rule to guide prioritization of HCV testing
As clinically useful decision threshold (test-threshold in our case), we opted for the CPS cut-off which dichotomizes the HIV patients in a subgroup with probability of HCV coinfection < 1% and a subgroup with probability ≥ 1% (Figure 1). This latter group could be prioritized for HCV testing, while for those with probability below 1% testing could be postponed if ‘testing all’ is not feasible or not affordable in the shortterm.
We considered the harm/benefit of ‘testing and not testing’ at patient (access to treatment) and public health level (onward transmission, cost) (Table 1). Generally, due to the introduction of nearly 100% curative, well-tolerated generic DAA treatment options the potential harm of not testing has become much more important in recent years. In addition, HCV coinfected HIV populations in resource-constrained settings might be at higher risk of advanced HCV disease as they have often started ART late or with less optimal regimens. Pondering this, but also the possibility to repeat the risk scoring regularly (as HIV patients are in chronic care follow-up), we opted for a 1% probability threshold for the decision rule (i.e., giving false negatives much more weight than false positives). Logically, this threshold is lower than the WHO recommended threshold range (2%-5%) for HCV testing in the general population[4].
The proportion of missed HCV coinfections, and the number of patients needed to test (NNT) to identify one HCV/HIV coinfection were calculated as measures of performance (clinical usefulness) of the decision rule in the derivation cohort.
Internal validation of the CPS
Finally, in order to correct for over-optimism (over-fitting) caused by the use of the same data set for both the derivation of the score and the evaluation of its predictive ability, we assessed internal validity of the CPS performance with a bootstrapping procedure (0.632+ estimator)[25]. We determined the performance (proportion of missed coinfections) of the CPS and the decision rule derived from each bootstrap sample in the original derivation set. This bootstrap-derived performance provides a more realistic estimate of the CPS performance in similar new patient cohorts.
Development of alternative scores
We explored two reduced models: (1) using only the six clinical and sociodemographic candidate predictors (clinical CPS); and (2) starting from lab-based (ALT, AST, platelets, APRI) and socio-demographic (gender, age) candidate predictors (lab CPS). Both were developed and assessed in the same way as the full CPS. The clinical model was explored with the intention to provide a feasible alternative for HIV programs where ALT, AST and platelet count results are not routinely available. The lab model might be easier to use in large programs equipped with electronic databases which can flag patients to be prioritized for HCV testing.
RESULTS
Description of the HIV derivation cohort
A total of 3,045 ambulatory HIV patients of Sihanouk Hospital Center of Hope were included. Their median age was 43 years (interquartile range - IQR: 36-48), 43% were male patients, and 98% were on antiretroviral therapy (ART) for a duration ranging from 2 mo to 13 years. Most were on nevirapine- (n= 1189) or efavirenz-based (n= 1539) ART. HIV virological failure was rare (3.4%). The cohort counted only few people (n= 31) who reported a history or current engagement in sex work, being homosexual, or past or current injecting drug use.
In this cohort, 230 patients tested positive for HCV antibodies, two had a borderline result. Of these 232, 106 had a detectable HCV-RNA, our outcome of interest. None of the coinfected reported past/current sex work, being MSM, or injecting drug use. Distribution of the candidate predictors in the cohort and the missing values are further specified in Table 2.
Prediction score for HCV/HIV coinfection
In Table 3, we list the 11 candidate predictors, all in dichotomous format, as taken forward in the score building. We report the unadjusted associations (crude positive and negative likelihood ratios) between the candidate predictors and having a HCV coinfection. After univariable analysis, two potential predictors (poor CD4 recovery on ART, gender) were dropped as the crude LHRs were not ≥ 2 or ≤ 0.5. From the remaining candidate predictors, seven with adjusted LHR ≥ 1.5 or ≤ 0.67 were retained in the final multivariable score model. The adjusted LHRs are shown in the last two columns. Among the retained predictors, three rely on laboratory testing results(platelet count, AST, APRI).
The relative weight (further called score) of the retained predictors is detailed in Table 4. Only APRI (whether ≥ 0.45 or < 0.45) contributed in both directions, and none of the predictors weighed more than + 1 or -1. The total score for each individual patient can range from -2 to + 6.

Table 1 Harm and benefit of hepatitis C virus testing and not testing
The distribution of the total individual scores in the HIV cohort, by coinfection status, and probability of HCV coinfection by each final score is presented in Figure 2. None of the patients in the derivation cohort had a score above 5. The majority (n= 2,219, 70%) had -2 or -1 as score. The probability of HCV coinfection ranged from 0.6% when the score was -2, to 75% for those with the highest score. A score ≥ 0 seems to fit best as test-threshold by dichotomizing in a large sub-group with predictive probability of HCV coinfection < 1%vsa smaller group with probability ≥ 1%.
Performance of the full CPS and derived decision rule for targeted HCV testing
The CPS yielded an AUROC of 0.84 (95%CI: 0.80-0.89), indicating good discrimination between HCV/HIV coinfection and HIV mono-infection. Diagnostic accuracy for different cut-offs of the risk score is detailed in Table 5.
The score ≥ 0, identified above as meeting our pre-defined criteria of clinically useful threshold to guide prioritization of HCV testing, had a negative predictive value (NPV) of 99.2% (95%CI: 98.8%-99.6%) or differently put, the probability of HCV coinfection among those with score < 0 was 0.8%.
Applying this test-threshold, only 30% (n= 926) of the HIV patients would have been prioritized for HCV testing. In this subgroup, 90 HCV coinfections (85%) would have been diagnosed decreasing the number needed to test (NNT) from 29 to 10. Sixteen HCV coinfections would have been missed, but none of these missed HCV diagnoses had advanced fibrosis (i.e., ≥ 9.5 kPa as measured by transient elastography). In line with international guidelines, triple HBV/HCV/HIV coinfections should also be prioritized for testing and treatment. In this derivation cohort, they were rare (n= 2), but not missed by the prioritization rule.
Adjusting for over-optimism (over-fitting), the bootstrap 0.632+ estimate of proportion of missed HCV coinfections was 18%, compared to 15% in the original derivation set.
Development of alternative scores (clinical CPS, lab CPS)
In the alternative ‘clinical’ model, five predictors (age ≥ 50 years, diabetes mellitus, partner/household member with liver disease, generalized pruritus, fatigue/myalgiaarthralgia/anorexia-weight loss) were retained in the final model, each with a relative weight of +1 point. Gender was dropped after univariable analysis. The AUROC was 0.69 (95%CI: 0.64-0.74), indicative of poor discrimination of HCV/HIV coinfection and HIV mono-infection. Figure 3 further illustrates the poor discrimination of the clinical score, which moreover did not allow to identify a sub-group with predicted HCV infection probability below 1%.

Table 2 Characteristics of the derivation cohort, including the candidate predictors
For the primarily laboratory test based model, four predictors were retained in the final model (age ≥ 50 years: + 1 point, APRI ≥ 0.45: + 1, APRI < 0.45: - 1, platelets < 200 109/L: + 1, AST < 40 IU/L: -1). Gender and ALT were dropped. The AUROC of the lab CPS showed good discrimination of HCV/HIV coinfection and HIV mono-infection, and was 0.83 (95%CI: 0.79-0.87). The best-fit cut-off for the test-threshold of ≥ 1% predicted probability was a lab CPS score ≥ 0. Applying this cut-off, 22 HCV coinfections would have been missed, including two with advanced fibrosis. The NNT was 9.5, as 800 persons would have been prioritized for testing, to identify 84 coinfections.
DISCUSSION
We developed (and internally validated) a clinical prediction score to risk-stratify, primarily heterosexually-infected HIV patients for HCV coinfection, for use as first step in the identification of HIV patients to be prioritized for HCV testing when resources are insufficient to test all.
The risk score uses elements from history taking, physical examination and laboratory test results which are readily available or easily obtainable in most HIV programs, and are a combination of age, an exposure-related factor (partner/household member with liver disease) and variables related to severity of liver disease. Its overall performance in the derivation cohort in terms of discriminating HCV/HIV coinfected and HIV mono-infected was good (AUROC 0.84, 95%CI: 0.80-0.89), and wewere able to derive a clinically useful decision rule for HCV testing prioritization along our pre-set requirements (test-threshold at ≥ 1% predicted probability of HCV coinfection, and substantially decrease the number needed to test (NNT)). In our study population, not testing those with predicted probability < 1% would have decreased the NNT from 29 to 10, while missing 15% of the HCV/HIV coinfections, and thus outperforming birth cohort testing[7]. If externally validated, our score and decision rule may thus be a practical way forward for countries not able or not opting to fully implement the WHO recommendation to test all HIV patients for hepatitis C[4]. Resource-constrained countries carry the largest burden of HCV/HIV coinfection.

Table 3 Crude and adjusted likelihood ratios of the candidate predictors for hepatitis C virus coinfection
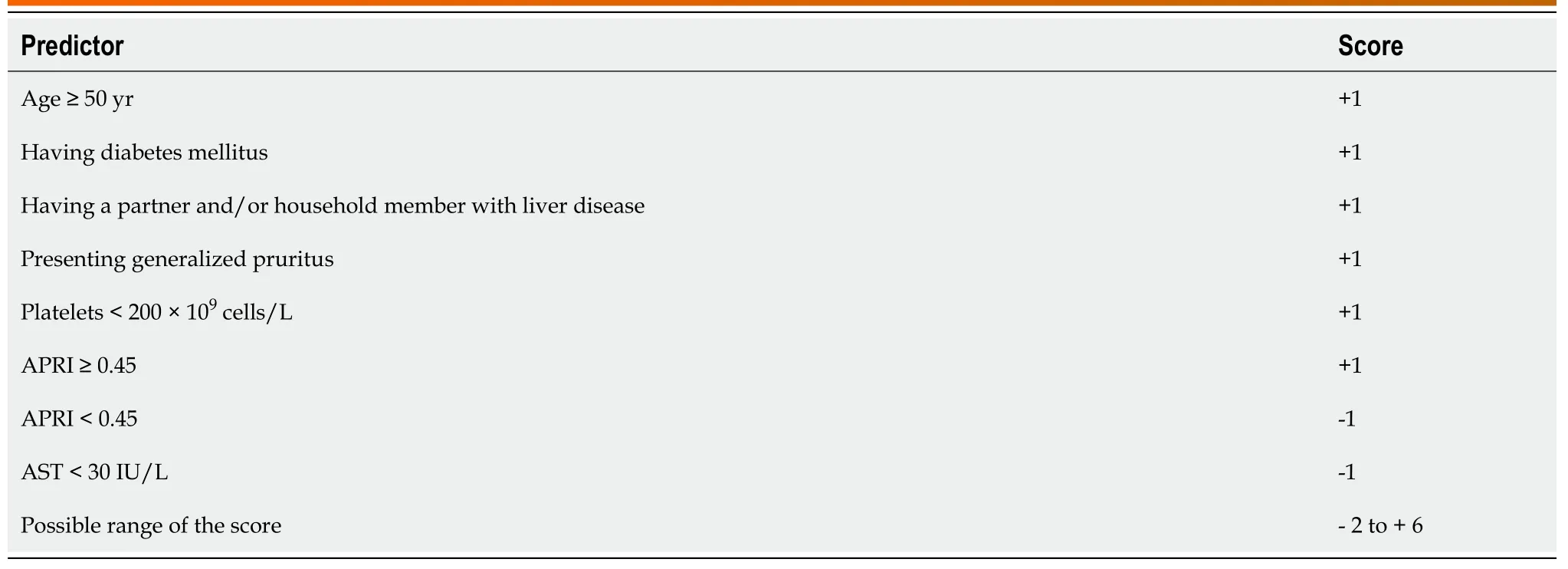
Table 4 Predictors and their weight in the clinical prediction score
With this paper, we do not intend to advocate in a general manner for targeted HCV testing in all HIV populations. We agree with the WHO guidelines that HIV populations are a convenient population sub-group to be targeted as a whole, as they often have a higher HCV prevalence than the general population, and are easy to reach[4,26]. ‘Testing all repeatedly for HCV, accompanied by appropriate preventivecounselling’ should be aimed for whenever feasible as part of a comprehensive package of care for people living with HIV (including timely initiation of ART and treatment of comorbidities as HCV), especially as nearly 100% curative HCV treatment options are now available. However, lack of resources, and low in-country HCV coinfection prevalence in large HIV cohorts with little ongoing transmission risk, are valid contextual arguments that countries may use to opt differently[8-10,27]. As also the argument that HIV coinfection leads to faster HCV disease progression (and therefore priority) has become debatable in the early ART era[8-10,27,28], some countries may indeed opt for a more restricted HCV testing approach combined with early initiation of ART. Anticipating this, it seemed to us timely to develop this score for targeted HCV testing.

Table 5 Diagnostic accuracy at different cut-offs of the clinical prediction score

Figure 1 Threshold for the decision rule for targeted hepatitis C virus testing.
The study and the resulting risk score have a number of strengths. The study was conducted and reported in accordance with the methodological standards for development of clinical prediction rules, as outlined in the TRIPOD statement and detailed in the S1 TRIPOD checklist[29]. Data collection was done prospectively, and blinded from the HCV diagnostic results. Missing data were rare. The model was built following the Spiegelhalter Knill-Jones (SKJ) approach, a statistical method that combines elements of the Bayes theorem and logistic regression. While combining, it also sidesteps disadvantages of both conventional methods (i.e., the Bayes’ assumption of independence of predictors; and the mathematical, user-unfriendly output of logistic regression). SKJ allows and adjusts for dependency between predictors, and provides output in adjusted LHRs which are more easily understood and interpreted by clinicians[22,23,30]. The model we developed is clinically sensible as all predictors retained in the final score are plausibly related to infection risk (older age and having a household member/partner) or severity of liver disease (increased APRI, low platelets, diabetes, generalized pruritus without skin abnormalities)[7,31,32]. This, as well as the fact that the score can be repeated at regular intervals and that initially missed cases can be picked up later, may favor acceptability by clinicians. The score has a good discriminative ability and performed particularly well to identify a large subgroup of HIV patients that can be considered as a very low-risk group for HCV coinfection (probability < 1%). From a program perspective, this opens perspectives of substantial optimization of resource utilization for HCV testing.

Figure 2 Patient distribution by coinfection status, and probability of hepatitis C virus coinfection by score of the full clinical prediction score.
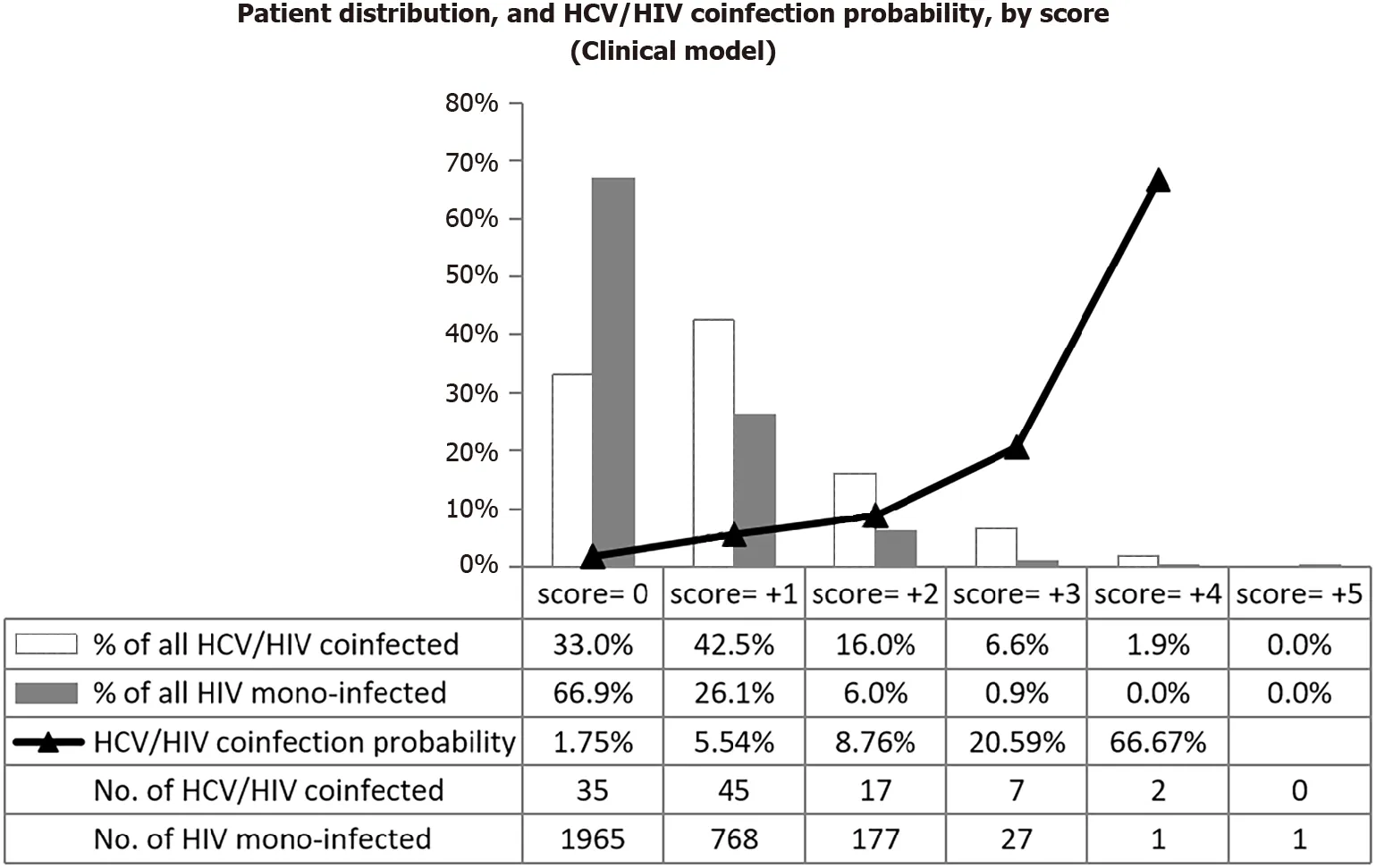
Figure 3 Patient distribution by coinfection status, and probability of hepatitis C virus coinfection by score of the clinical prediction score.
There are also several limitations. It is a model development study, with internal validation to correct for over-optimism by bootstrapping, but no external validation was done yet. Further validation in different settings will thus be crucial before decisions on generalizability can be taken[33]. Inherent to the score building method used (Spiegelhalter Knill-Jones), continuous variables had to be categorized. This may have led to information loss[34,35]. The SKJ method adjusts for dependency between predictors (confounding), but in a more restricted manner than the conventional logistic regression. Each result (present or absent) of a particular predictor/test is being shrunk to the same degree[30]. Taking into consideration these potential weaknesses, we used our dataset to compare the performance of logistic regression, CART and SKJ to predict HCV/HIV coinfection. Logistic regression missed less HCV coinfections, but would refer 98% of HIV patients for HCV testing. The SKJ method had the highest area under the ROC curve and missed less coinfections than CART. CART delivered a better positive predictive value[36]. Another potential weakness of the score is its dependence on some lab tests (mainly transaminases). Though we aimed to use information which is readily available or easily obtainable in HIV programs, these lab tests might not be done regularly anymore in some programs. The clinical score (without lab tests) did unfortunately not perform well. On the other hand, the alternative score without clinical variables did perform reasonably well, and can, if validated, be a handy alternative in certain HIV programs. Routine electronic HIV databases containing these variables could flag patients to be prioritized for HCV testing without any need for further data collection by the clinician.
To further improve cost-effectiveness of HCV testing, the potential of the risk score to identify subgroups best to be tested with the classical two-step algorithm (HCV antibody test followed by HCV-RNA testing), or one-step test procedure (HCV-RNA) could also be further explored.
CONCLUSION
We successfully developed and internally validated a practical score, based on readily available clinical data, to risk-stratify HIV patients for HCV coinfection. In our setting, a large cohort of primarily heterosexually-infected Cambodian HIV patients, the score has shown promising potential to substantially reduce the number needed to test (to 30% of the cohort) without compromising access to testing and treatment for HIV patients with advanced HCV disease, especially as this score can be repeated regularly. Confirmation of these promising findings through external validation is required before its use in other low-risk HIV cohorts (i.e., with few MSM or injecting drug users) in settings with limited resources can be considered.
ARTICLE HIGHLIGHTS
Research background
The advent of direct-acting antivirals has revolutionized hepatitis C (HCV) treatment and has generated interest in the global elimination of hepatitis C as a public health problem. To allow timely scale up of treatment, efficient HCV testing strategies are crucial. By the end of 2017, only about 20% of those living with hepatitis C knew their status, with significantly lower proportions in low and middle income countries(LMIC).
Research motivation
In the absence of funding initiatives dedicated to viral hepatitis, it is expected to remain difficult for LMIC to offer broad access to HCV testing. Depending on local resources and epidemiology, offering targeted HCV screening might be a more feasible option. However, easy-to-use tools to guide such targeted HCV testing, other than prioritization of key populations or older birth cohorts, do not exist.
Research objectives
To develop and internally validate a clinical prediction score for targeted HCV screening combining age and factors linked to liver disease severity, aiming to identify most of the chronic hepatitis C patients in low-risk human immunodeficiency virus(HIV) populations, but especially those in more urgent need of treatment.
Research methods
Score development relied on the Spiegelhalter and Knill-Jones method which was applied on a cross-sectional dataset from a large HIV cohort in Phnom Penh,Cambodia. Predictors independently associated with current HCV infection (HCV RNA detected) with likelihood ratio ≥ 1.5 or ≤ 0.67 were retained in the score.Performance of the score was estimated by the area-under-the-ROC curve and diagnostic accuracy at the different cut-offs. For the decision rule, HCV coinfection probability ≥ 1% was agreed as test-threshold.
Research results
We developed (and internally validated) a clinical prediction score to risk-stratify, primarily heterosexually-infected HIV patients for HCV coinfection, for use as first step in the identification of HIV patients to be prioritized for HCV testing when resources are insufficient to test all. The risk score uses elements from history taking, physical examination and laboratory test results which are readily available or easily obtainable in most HIV programs. In the Cambodian derivation cohort, the score would have enabled identifying 85% of the coinfected while reducing the need for testing by 70%. At the best-fitting threshold-to-screen (score ≥ 0), a negative predictive value of 99.2% was obtained, and no cases with advanced fibrosis were missed.
Research conclusions
The score for targeted HCV screening performed well in the derivation cohort and bears potential to substantially reduce the number needed to test without compromising access to testing and treatment for HIV patients with advanced HCV disease. Confirmation of these promising findings through external validation is required before recommendations on wider use can be made.
Research perspectives
The validity of the score should be tested in other HIV cohorts with low onward risk of transmission, starting from similar HIV cohorts in Cambodia but also in HIV populations in other settings.
ACKNOWLEDGEMENTS
The authors of this paper thank the HIV clinicians, counsellors, laboratory technicians and, data-management team of Sihanouk Hospital Center of Hope (SHCH), ITM, and the Antwerp University Hospital for their contribution to the implementation of this study.
杂志排行
World Journal of Hepatology的其它文章
- Coronavirus disease 2019 and non-alcoholic fatty liver disease
- Epigenetic mechanisms of liver tumor resistance to immunotherapy
- Advances in the management of cholangiocarcinoma
- Herbal and dietary supplement induced liver injury: Highlights from the recent literature
- Challenges in the discontinuation of chronic hepatitis B antiviral agents
- Liver Kidney Crosstalk: Hepatorenal Syndrome
