Atezolizumab and bevacizumab as first line therapy in advanced hepatocellular carcinoma: Practical considerations in routine clinical practice
2021-10-11AnkitJainShivakumarChitturiGeoffreyPetersDesmondYip
Ankit Jain, Shivakumar Chitturi, Geoffrey Peters, Desmond Yip
Ankit Jain, Geoffrey Peters, Desmond Yip, Department of Medical Oncology, The Canberra Hospital, Garran 2605, ACT, Australia
Ankit Jain, Shivakumar Chitturi, Geoffrey Peters, Desmond Yip, ANU Medical School, Australian National University, Canberra 0200, ACT, Australia
Shivakumar Chitturi, Gastroenterology and Hepatology Unit, Canberra Hospital, Canberra 2605, ACT, Australia
Abstract Hepatocellular carcinoma (HCC) is the most common primary liver cancer. For advanced HCC, sorafenib was considered the standard of care for more than ten years. Recently the atezolizumab and bevacizumab combination has become standard of care for these patients without contraindications to either immune checkpoint inhibitors or antiangiogenic therapy. We now review the practical aspects of the atezolizumab and bevacizumab combination, including current evidence, indications, contraindications, management of adverse events, sequencing of this combination, areas of current knowledge gaps and future areas of active clinical research of this combination for busy clinicians in clinical practice.
Key Words: Hepatocellular carcinoma; Atezolizumab; Bevacizumab; Immunotherapy; Child Pugh cirrhosis; Anti-angiogenic therapy
INTRODUCTION
Hepatocellular carcinoma (HCC) is the sixth most common cancer worldwide and leading cause of cancer related death[1]. Early-stage HCC can be treated by resection, liver transplantation or ablation. Unfortunately, most patients present with an intermediate or advanced-stage disease with limited systemic options and a dismal prognosis. The multikinase inhibitor sorafenib was initially approved more than a decade ago for the management of advanced HCC[2]. Recently, four additional targeted therapies were approved for advanced HCC based on positive phase III randomised controlled trials (RCTs): Lenvatinib in the first-line setting and regorafenib, cabozantinib and ramucirumab, all in the second line after the failure of sorafenib therapy[2-5].
The recent publication of successful results of Phase III RCT IMbrave 150 has established the combination of atezolizumab and bevacizumab (Atezo and Beva) as first line therapy for advanced treatment naïve HCC with Child Pugh A cirrhosis[6]. We now review the pharmacological rationale, evolution, results, practical issues in clinical practice, current knowledge gaps and future possibilities of this combination therapy. This is an expert review based on our current clinical knowledge of this combination.
PHARMACOLOGICAL RATIONALE OF THIS COMBINATION
Atezolizumab is a monoclonal antibody against programmed cell death ligand 1 (PDL1). PD-L1 receptors are expressed on tumour cells. The programmed cell death protein 1 (PD-1) is present on cytotoxic T lymphocytes (CTLs) and tumour cells. The interaction of PD-1 and PD-L1 is an immune inhibitory pathway. Atezolizumab reverses T cell suppression by preventing interaction between the inhibitory immune checkpoint molecules PD-1 and PD-L1. Vascular endothelial growth factor (VEGF) induces tumour angiogenesis. In addition to inducting tumour angiogenesis, VEGF also mediates immunosuppression within the tumour microenvironment by promoting immunosuppressive cells such as regulatory T cells (Treg), myeloidderived suppressor cells (MDSCs) and tumour associated macrophages. VEGF also suppresses antigen-presenting cells and CTLs. In summary, bevacizumab not only inhibits tumour growth by inhibiting angiogenesis but also augments the immune agonistic effects of atezolizumab by reversing the immune suppressive mechanisms of VEGF pathways[7].
EVOLUTION OF ATEZOLIZUMAB AND BEVACIZUMAB COMBINATION IN THE MANA-GEMENT OF ADVANCED HCC
Phase Ib GO30140 study
In this phase I B study, there were four cohorts of various malignancies. In the HCC cohort, arm A received the combination of atezolizumab and bevacizumab in patients with unresectable HCC. The primary endpoint for this arm was overall response rates (ORR). Arm F of the same study randomised patients with unresectable HCC to atezolizumab and bevacizumab combinationvsatezolizumab monotherapy arm. The primary endpoint of Arm F was progression-free survival in the intention-to-treat population. The dose of atezolizumab in both the arms with or without combination was 1200 mg I.V. every three weeks. In the combination arm, the bevacizumab dose was 15 mg/kg. The critical results of the trial are summarized in Table 1[8].
Kudo[7] have comprehensively reviewed these results. As per Kudo[7], the 12% C.R. rates in arm A is very impressive as this group had patients with advanced HCC with poor prognostic factors such as α-fetoprotein (AFP) ≥ 400 ng/mL, extrahepatic spread (EHS), major vascular invasion. These results were never achieved in the tyrosine kinase inhibitors (TKI) era. The other important finding was the ORR of 62% (8/13) in intermediate stage disease with a high tumour burden.
As per Kudo[7], the Arm F is an essential proof of concept study that demonstrates the favourable results obtained in Arm A are not solely due to the efficacy of atezolizumab monotherapy but precisely due to a combination of atezolizumab and bevacizumab. The Arm F scientifically reinforces the synergistic combination of antiangiogenic therapy and immunotherapy.
In Arm A, The most common grade 3-4 treatment-related adverse events were hypertension (13%) and proteinuria (7%). Treatment-related adverse events occurred in 25 (24%) patients. There were three (3%) treatment-related deaths due to abnormal hepatic function, hepatic cirrhosis and pneumonitis.
IMBrave 150 trial
IMbrave 150 was a global, open-label, randomised phase III trial comparing atezolizumab plus bevacizumabvssorafenib in systemic treatment-naive unresectable HCC[6]. Patients were randomly assigned in a 2:1 ratio either to atezolizumab plus bevacizumab or sorafenib until unacceptable toxic effects occurred or loss of clinical benefit[7]. The coprimary endpoints were overall survival and progression free survival in the intent to treat population, as assessed at an independent review facility according to Response Evaluation Criteria in Solid tumours, version 1.1 (RECIST 1.1).
The main inclusion criteria for the study were unresectable or metastatic HCC patients with ECOG-PS (Eastern Cooperative Oncology Group-Performance Status) of 0 or 1, Child-Pugh A cirrhosis. Patients with disease not amenable to curative surgical and or locoregional therapies or progressive disease after surgical or locoregional therapies were eligible. For patients with active hepatitis B virus (HBV), the trial requirement was quantitative HBV DNA < 500 IU/mL obtained within 28 d before initiation of therapy, and patients who have taken at least two weeks of anti-HBV treatment and willing to continue throughout the study duration.
The key exclusion criteria were a history of autoimmune disease and untreated or incompletely treated oesophageal or gastric varices (assessed with esophagogastroduodenoscopy) with bleeding or higher risk of bleeding. The trial required mandatory assessment of oesophageal or gastric varices within six months of initiation of trial therapy.
The most important autoimmune diseases in the exclusion criteria were myasthenia gravis, myositis, autoimmune hepatitis, systemic lupus erythematosus, rheumatoid arthritis, inflammatory bowel disease, antiphospholipid antibody syndrome, Wegener granulomatosis, Sjogren’s syndrome, Guillain-Barre syndrome or multiple sclerosis. Patient with known fibrolamellar variant, sarcomatoid HCC or mixed cholangiocarcinoma and HCC were excluded from the study.
The patients were stratified by geographical region (Asia excluding Japanvsthe rest of the world), macrovascular invasion or EHS of disease (presencevsabsence), baseline alfa fetoprotein levels of (< 400 ng/mLvs> 400 ng/mL), ECOG of 0 or 1.
Patients assigned to the atezolizumab -bevacizumab group received 1200mg of atezolizumab plus 15mg/kg of body weight of bevacizumab intravenously every three weeks. Dose modifications were not permitted in the atezolizumab group but were allowed in the sorafenib group. Patients who transiently or permanently discontinued either atezolizumab or bevacizumab because of an adverse event were allowed to continue taking the single-agent therapy as long as the investigator determined that there was a clinical benefit. Table 2 describes the confirmed response rates, progression-free survival, overall survival and disease control rate in the IMBrave 150 trial.

Table 1 Results of phase Ib GO30140 study
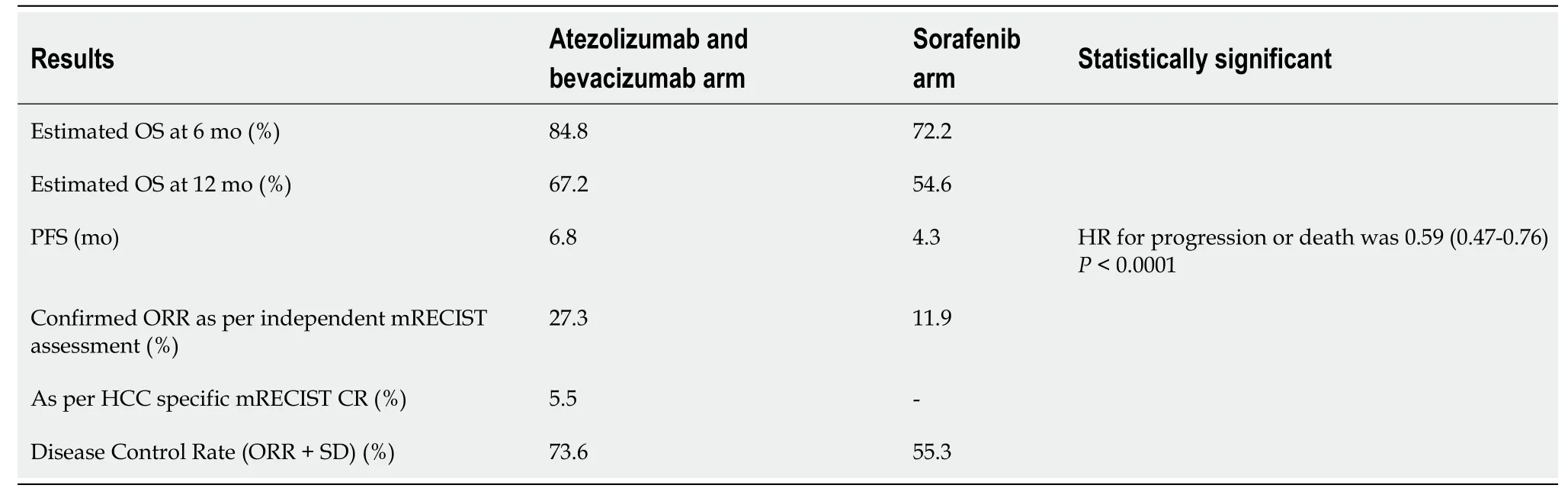
Table 2 Results of IMBrave 150 trial
Quality of life:Atezolizumab-bevacizumab delayed deterioration of patient-reported quality of life (median time to deterioration), 11.2 mo with atezolizumab- bevacizumab combinationvs3.6 mo with sorafenib arm. The deterioration in physical functioning and role functioning were also delayed in the experimental arm by an additional 8.2 mo and 5.5 mo, respectively.
IMBrave 150 investigators have recently published the patient reported outcomes (PROs) of this study. The PROs were prespecified exploratory endpoints of the study. The study showed clinically meaningful benefit in terms of patient reported quality of life, functioning and disease symptoms with atezolizumab and bevacizumab as compared to sorafenib. The patients completed the European Organisation for Research and Treatment of Cancer quality-of-life questionnaire for cancer (QLQ-30) and quality of life questionnaire for HCC (QLQ-HCC18). As compared to sorafenib, atezolizumab and bevacizumab combination reduced the risk of deterioration for appetite loss, diarrhoea, fatigue and pain. The benefits for fatigue and pain were maintained in QLQ-HCC18 scale too[9].
Safety: Adverse events of any grade were reported in 323 patients (98.2%) who received the atezolizumab- bevacizumab and 154 patients (98.7%) who received sorafenib. Grade 5 events occurred in 15 patients (4.6%) in the experimental group and in 9 patients (5.8%) in the sorafenib group. Table 3 tabulates the number of Grade 5 events in both arms.

Table 3 Grade 5 events in both the arms IMBrave 150 trial
The most common grade 3 or 4 adverse event with atezolizumab-bevacizumab was hypertension (15.2%). Grade III HTN is defined as Stage II HTN with blood pressure (≥ 160/≥ 100 mmHg). Serious adverse events occurred more frequently with atezolizumab and bevacizumab combination 125 patients (38%) than with sorafenib 48 patients (30.8%).
SELECTING APPROPRIATE PATIENTS FOR THE ATEZOLIZUMAB AND BEVACIZUMAB COMBINATION
It will be crucial for multidisciplinary teams (MDTs) to cautiously choose the most suitable patients for this combination. Patients with locally advanced unresectable tumours not suitable for locoregional therapies such as transarterial chemoembolization (TACE) and metastatic HCC with Child-Pugh A liver disease will be the most appropriate patients provided they have no other major contraindications to immunotherapy or VEGF inhibition therapy. The patients not amenable to locoregional therapies will be patients with severely impaired main portal vein flow (resulting from occlusive thrombus, tumour invasion or hepatofugal blood flow) because of dependence on the arterial inflow to adequately supply the liver[10].
TACE has not shown any survival benefit in patients with extensive bilobar involvement, so these patient will need upfront consideration of systemic therapy[11].
Stopping rules for TACE
TACE sessions are scheduled more often performed on-demand than on a predetermined time line. Decisions to continue or cease TACE are based on repeat liver imaging and the tumour response to treatment. Many algorithms have been developed to help with these decisions but are not universally validated[12]. In general, the appearance of extrahepatic metastases, vascular invasion or worsening clinical status would usually lead to ceasing further TACE procedures. Further, the concept of TACE-‘refractoriness’ is also to be considered. First proposed by the Japanese Society of Hepatology, the primary definition includes lack of objective response to 2 sessions of TACE (viable lesion > 50% or two or more consecutive increases in tumour number), the continuous elevation of tumour markers after TACE, vascular invasion and metastasis.
Repeated TACE procedures can lead to worsening liver function due to hepatic devascularisation[13]. This can preclude effective systemic therapies.
OPTIMIS was an international prospective observational study enrolling patients with unresectable HCC who were being considered for TACE. The authors noted that over 90% of patients continued to receive TACE despite an inadequate response. Those who transitioned to sorafenib earlier at the time of TACE-‘refractoriness’ had longer overall survival rates than those who were treated later. A recent Korean retrospective study also reiterated early transitioning to systemic therapy in patients without an objective response to 2 consecutive TACE procedures[14].
These patients need discussion at MDT meetings for consideration of alternative treatment options such as the atezolizumab and bevacizumab combination if there are no contraindications for this protocol.
SYSTEMATIC REVIEW AND META-ANALYSIS SUPPORTING ATEZOLIZUMAB AND BEVACIZUMAB IN FIRST LINE SETTINGS FOR MANAGEMENT OF ADVANCED HCC
In the most recent systematic review and network meta-analysis of eight first line trials with a total of 6290 patients, the combination of atezolizumab and bevacizumab was superior to lenvatinib [hazard ratio (HR) 0.63], sorafenib (HR 0.58) and nivolumab (0.68)[15].
ROLE OF ATEZOLIZUMAB AND BEVACIZUMAB IN COMBINATION WITH LOCOREGIONAL THERAPIES
Locoregional therapies such as radiofrequency ablation, TACE and cryoablation can induce multiple immunogenic effects. These procedures have multiple mechanisms to stimulate the immune system. These mechanisms are: (1) Inhibiting immunosuppressive cells like MDSC and Tregs; (2) PD-L1 upregulation; (3) Increased effector immune cells like dendritic cells, natural killer cells and T cells; and (4) Increased release of tumour antigens like glypican 1, AFP.
Several trials are examining combinations of various locoregional modalities with different immune checkpoint inhibitors (ICI). Multiple biomarkers will be evaluated in these studies including AFP, cell death biomarkers like sRAGE and circulating GPC3 cytotoxic lymphocytes[16].
TACE-induced tissue hypoxia leads to upregulation of hypoxia-inducible factor-1a, which facilitates VEGF and platelet derived growth factor expression[17]. The latter promotes neoangiogenesis and tumour revascularisation. These diverse mechanisms provide a rationale for combining atezolizumab and bevacizumab with locoregional therapies.
Currently NCT04224636 trial is recruiting patients for treatment with TACE in combination with atezolizumab and bevacizumab. There are many unanswered questions about sequencing of locoregional therapies and various ICIs.
ROLE OF ATEZOLIZUMAB AND BEVACIZUMAB IN ADJUVANT SETTINGS
Up to 70% of patients can develop recurrence in 5 years after curative intent resection for early stage HCC[18]. There is high rate of intrahepatic recurrences in patients with large tumour size, an incomplete tumour capsule, venous or microvascular invasion. There are multiple mechanisms by which surgery or radiofrequency ablation can alter the immune microenvironment of liver[19]: (1) More MDSC accumulates, leading to the immunosuppressive microenvironment; (2) The balance of proinflammatory phenotype 1 helper T cell is altered to a more immunosuppressive T-helper 2 phenotype; and (3) Tumour macrophages are polarized to an immunosuppressive M2 phenotype during postoperative wound healing. So, there is a solid rationale for considering immunotherapy in the postoperative adjuvant setting for HCC.
The major success of atezolizumab and bevacizumab in the metastatic setting has led to new trials of this combination in the adjuvant setting and in combination with other locoregional therapies. IMbrave050 (NCT0410298) is testing atezolizumab and bevacizumabvsactive surveillance as adjuvant therapy in patients with HCC at high risk of recurrence after surgical resection or ablation. The primary outcome of the study is recurrence-free survival. The Supplementary material, Appendix 1 provides information on currently listed trials of this combination in various settings at clinical trial.gov website.
BIOMARKERS WITH THE PROGNOSTIC AND PREDICTIVE ROLE FOR THE USE OF A COMBINATION OF ATEZOLIZUMAB AND BEVACIZUMAB IN ADVANCED HCC
In the phase Ib exploratory analysis, higher expression of PD-L1 in tumour tissue, higher expression of VEGF receptor 2, and higher T-regulatory cells immunophenotype were associated with better survival[8]. Currently, this analysis is pending for the IMBrave phase III trial. In this trial, the combination showed more benefits in patients with AFP of < 400 ng/mL, viral aetiology (HBV and HCV associated HCC) had more benefits than non-viral aetiology[6]. This can be due to the immune stimulatory environment due to chronic inflammation associated with viral aetiology associated with HCC. The prevalence of microsatellite instability (MSI)-high disease and TMB is very low in HCC. In a study of 755 patients out of 542 cases assessed for MSI, only one patient (0.2%) was MSI-high and TMB-high[20].
At this stage, aetiology (viral or non-viral) should not be used in triaging the types of systemic treatments in advanced HCC. There are preclinical and clinical signals that the atezolizumab and bevacizumab combination may not be very effective in patients with HCC associated with non-alcoholic fatty liver disease (NAFLD). There is preclinical evidence that NAFLD decreases CD4+T cells and induces tumour promoting functions in CD8+T cells, natural killer cells and Th17 cells[21,22]. More than 50% of patients with NAFLD are obese, and obesity may increase the resistance to VEGF therapy[23]. In the IMbrave 150 trial, the combination of atezolizumab and bevacizumab was less effective in patients with non-viralvsviral etiology with a HR of 0.91 as compared to sorafenib[6].
There is emerging evidence that WNT/B-Catenin signaling is associated with a lack of T cell infiltrates and predict resistance to immunotherapy like atezolizumab[24]. There is a proposed immunological classification in HCC, which divides HCC into three subclasses: (1) Immune (30%); (2) Immune intermediate (45%); and (3) Immune excluded class (25%). There is preclinical and clinical data of activation of WNT/Bcatenin pathway leading to resistance to immunotherapy in immune excluded subtype of HCC.
In summary, there are currently no proven biomarkers that can be used to select patients for this particular combination.
COMMON OVERLAPPING TOXICITIES IN CIRRHOTIC PATIENTS TREATED WITH IMMUNOTHERAPY
Meriggi and Graffeo[25] have comprehensively reviewed the toxicities due to cirrhosis but overlap with immunotherapy agents and TKI. Due to the secretion of gastrin and vasoactive peptides, diarrhoea or loose stools can be a common symptom in patients with cirrhosis. Both immunotherapy and TKI can worsen diarrhoea. It is essential to adequately investigate the diarrhoea with stool culture, Clostridium difficile toxin assessment and standard biochemical tests. Diarrhoea associated with abdominal pain and signs of colonic inflammation is most likely related to immune-mediated colitis. It is helpful to do a baseline calprotectin when patients are admitted with diarrhoea to rule out immune-mediated colitis. Titrating the dose of lactulose used to prevent encephalopathy may be necessary to control the diarrhoea. Adequate doses of loperamide and steroids should be used to manage patients with possible immunemediated colitis, once the common causes of diarrhoea are ruled out. Colonoscopy should be reserved for patients with severe diarrhoea with a high index of suspicion for immune-mediated colitis or those who remain steroid refractory. For those patients with steroid-resistant or refractory colitis, the use of infliximab will be challenging, given it can cause liver injury in susceptible patients.
Cancer-related fatigue is also one of the symptoms common to cirrhotic patients and can worsen with ICI therapy. Education about exercise and physical activity is crucial at the start of treatment. According to Meriggi and Graffeo[25], profound asthenia is common in HCC patients and can be multifactorial due to electrolyte imbalance, thyroid dysfunction, increased cytokine production, serotonin imbalances and vagal response activation[25]. Baseline assessment of thyroid function can dictate the need to initiate the thyroxine therapy before starting ICIs as autoimmune thyroiditis is a common side effect in the first 3-6 mo after initiation of ICIs.
Pruritis is also an overlapping symptom in HCC patients treated with ICIs. It is a common symptom of chronic liver disease and can be exacerbated by ICIs and potentially impact the quality of life.
Adrenal insufficiency caused by ICI therapy will usually pose challenges in patients with HCC. The hemodynamic changes in cirrhosis, hyponatremia due to hemodilution and use of diuretics can pose a significant challenge will mask the diagnosis of adrenal insufficiency in these patients[26].
CHILD PUGH B CIRRHOSIS AND COMBINATION OF ATEZOLIZUMAB AND BEVACIZUMAB
The IMBrave 150 trial excluded the patients with Child-Pugh B cirrhosis. Currently, the data for the use of individualized care plans, in general, is scarce in patients with HCC. The largest retrospective series of 18 patients assessed the role of nivolumab in patients with Child-Pugh B cirrhosis after progression on sorafenib. In this study cohort, > 60% of patients had ascites, and 28% of patients had a Child-Pugh B score of 9. There were higher rates of adverse events, but the frequency of irAEs ( immunerelated adverse events) was similar to patients with Child-Pugh A cirrhosis in the CheckMate 40 trial. Interestingly there was no significant increase in aminotransferases, which is the anticipated side effect in this subset of patients[27].
There is a single case report of the combination of lenvatinib and pembrolizumab in a patient with advanced HCC with Child-Pugh B 8 with an overall survival of 22 mo at the time of initial presentation[28]. It will be essential to see the effect of atezolizumab and bevacizumab in patients with Child-Pugh B cirrhosis. Patients with ascites will be of interest, as bevacizumab can reduced ascites in patients with various gynaecological malignancies.
ICI INDUCED HEPATITIS IN PATIENTS OF HCC
ICI induced hepatitis is a vital complication that needs particular emphasis in patients with HCC. Patients with HCC have mild hepatic dysfunction due to underlying cirrhosis, and this can make the diagnosis of ICI induced hepatitis more challenging. In the IMBrave 150 trial, 14% of patients in the atezolizumab bevacizumab arm developed a rise in ALT with 3.6% developing grade 3 or 4 increase[6]. In a large multicentre retrospective analysis of 164 patients with ICI induced hepatitis, 30.5% and 45.7% of patients developed grade 2 and grade 3 hepatitis, respectively, with a median time of onset of 61 d. The most common presentation was asymptomatic laboratory abnormalities. In patients with symptomatic presentations, flu-like symptoms like fatigue/anorexia, nausea, emesis, abdominal/back pain and arthralgia/myalgia were the most common. Steroids were used in 92.1% of patients and second-line immunosuppression was required in 22.6% of patients. On rechallenge, there was a modest risk of hepatitis recurrence. Out of 164 patients, only one had HCC and only two patients received atezolizumab as one of the ICIs[29].
ROLE OF ATEZOLIZUMAB AND BEVACIZUMAB IN MANAGEMENT OF ADVANCED HCC IN SPECIAL SUBSETS OF PATIENTS
Multifocal HCC or advanced HCC can occur in a special subgroup of patients like patients with a history of autoimmune hepatitis, pre-existing autoimmune disease, solid organ transplants, inflammatory bowel disease, significant cardiovascular disease, patients on haemodialysis, active human immunodeficiency virus (HIV) infection or patients living with HIV disease. These patients provide unique challenges during the management of advanced HCC. Pinteret al[24] and Rimassaet al[30] comprehensively review the challenges in managing these patients. Table 4 summaries the most suitable lines of therapy for these subsets of patients.
FUTURE CONSIDERATION FOR CHANGE IN THERAPEUTIC LANDSCAPE FOR SECOND LINE SETTINGS IN ADVANCED HCC FOR PATIENTS PROGRESSED ON THE ATEZOLIZUMAB AND BEVACIZUMAB COMBINATION
The choice of second-line therapies for patients developing progressive disease on atezolizumab and bevacizumab combination is uncertain. The regorafenib and cabozantinib studies included prior VEGF exposure and 3% of patient in the CELESTIAL trial received prior immunotherapy[3,4]. Sonbolet al[15] in their network meta-analysis speculate that cabozantinib and regorafenib may be more suitable second-line therapies as compared to sorafenib and lenvatinib as they were only used in VEGF naïve patients. The efficacy of the VEGF directed antibody ramucirumab and single-agent checkpoint inhibitors such as nivolumab and pembrolizumab is also questionable in second-line settings for patients treated with this combination. It will be important to consider trials with dual checkpoint blockade, such as the combination of the anti-CTLA-4 antibody line ipilimumab and PD-1 inhibitor nivolumab or PD-1 inhibitors with TKIs like cabozantinib and regorafenib in second-line settings for patients who have progressed on the atezolizumab and bevacizumab combination[15].

Table 4 Advanced hepatocellular carcinoma in special subset of population with absolute and relative contraindication for atezolizumab and bevacizumab combination
CONCLUSION
Atezolizumab and bevacizumab is the current first-line standard of care systemic therapy option for patients with advanced or unresectable HCC unsuitable for locoregional therapy with Child-Pugh A cirrhosis with no contraindication to either atezolizumab and bevacizumab. Current ESMO and NCCN guidelines support this recommendation[31,32]. The ESMO guidelines report the substantial benefit with this combination with estimated ESMO magnitude of clinical benefit score of 5 with an absolute survival gain of additional 9.6 mo as compared to sorafenib[31].
杂志排行
World Journal of Hepatology的其它文章
- Coronavirus disease 2019 and non-alcoholic fatty liver disease
- Epigenetic mechanisms of liver tumor resistance to immunotherapy
- Advances in the management of cholangiocarcinoma
- Herbal and dietary supplement induced liver injury: Highlights from the recent literature
- Challenges in the discontinuation of chronic hepatitis B antiviral agents
- Liver Kidney Crosstalk: Hepatorenal Syndrome
