Indeterminate liver lesions on gadoxetic acid-enhanced magnetic resonance imaging of the liver: Case-based radiologic-pathologic review
2021-10-11JurateNoreikaiteDekanAlbashaVijayChidambaramAnkurAroraAshokKatti
Jurate Noreikaite, Dekan Albasha, Vijay Chidambaram, Ankur Arora, Ashok Katti
Jurate Noreikaite, Dekan Albasha, Vijay Chidambaram, Ankur Arora, Ashok Katti, Department of Radiology, Liverpool University Hospitals NHS Foundation Trust, Liverpool L7 8XP, United Kingdom
Abstract Different histopathological manifestations of focal liver lesions show varying common and uncommon imaging findings and some pathologies may show similar appearance despite of different histopathology. It is necessary to characterise focal liver lesions accurately as not only benign and malignant lesions are managed differently, but also certain benign lesions have differing management. These lesions are increasingly being detected due to rapid growth of use of crosssectional imaging as well as improvement in image quality and new imaging techniques. Contrast enhanced magnetic resonance imaging (MRI) is considered the gold standard technique in characterising focal liver lesions. Addition of gadoxetic acid has been shown to significantly increase diagnostic accuracy in the detection and characterization of liver abnormalities. Classic imaging characteristics of common liver lesions, including their behaviour on gadoxetic acid enhanced MRI, have been described in literature over recent years. It is important to be familiar with the typical aspects of these lesions as well as know the uncommon and overlapping imaging features to reach an accurate diagnosis. In this article, we will review the well-described characteristic imaging findings of common and rare focal liver lesions and present several challenging cases encountered in the clinical setting, namely hepatocellular adenoma, focal nodular hyperplasia, hepatic angiomyolipoma, hepatocellular carcinoma, intrahepatic cholangiocarcinoma, neuroendocrine tumours as well as a pleomorphic liposarcoma of the liver.
Key Words: Indeterminate liver lesions; Magnetic resonance imaging; Gadoxetic acid; Hepatobiliary phase; Hepatocellular carcinoma
INTRODUCTION
Recent years have seen a rapid growth of the use of cross-sectional imaging as well as an increase in image quality and new imaging techniques. This has led to a rise in the detection of a variety of benign and malignant focal liver lesions. It is necessary to characterise focal liver lesions accurately as not only benign and malignant lesions are managed differently, but also certain benign lesions have differing management. The ability to accurately identify various liver lesions on imaging also saves the patient from biopsy or other invasive interventions needed to reach a diagnosis, which carry associated complications such as bleeding, abdominal pain, or even mortality[1,2].
Contrast enhanced magnetic resonance imaging (MRI) is considered the gold standard technique in characterising focal liver lesions because it provides superior tissue contrast resolution, safe contrast agent profile and is ionising radiation free. Gadoxetate disodium (Primovist, Bayer Schering Pharma), also known as gadoxetic acid, in particular, has been shown to significantly increase diagnostic accuracy in the detection and characterisation of focal liver lesions[3,4]. It provides dynamic vascular phases [arterial phase (AP), portal venous phase (PVP) and equilibrium phases] and due to its progressive distribution into functional hepatocytes and bile ducts also a hepatobiliary phase (HBP). Gadoxetic acid has been demonstrated to be invaluable in detecting hepatocellular carcinoma (HCC) in the cirrhotic liver and distinguishing between focal nodular hyperplasia (FNH) and hepatocellular adenoma (HCA)[4-6].
Different histopathological manifestations of focal liver lesions show varying common and uncommon imaging findings and some pathologies may show similar appearance despite different histopathology. Classic imaging characteristics of common liver lesions, including their behaviour on gadoxetic acid enhanced MRI, have been described in literature over recent years. It is important to be familiar with the typical aspects of these lesions as well as know the uncommon and overlapping imaging features to reach an accurate diagnosis. In this article, we will review the welldescribed characteristic imaging findings of focal liver lesions and present several challenging cases encountered in the clinical setting.
BENIGN LESIONS
HCA
HCA is a rare benign liver tumour which occurs predominantly in young and middleaged women and is associated with the use of oral contraceptives or other steroid medications. In contrast to other benign liver tumours, an HCA may be complicated by malignant transformation or bleeding[7]. As such, because of its serious clinical consequences, an HCA is often treated with surgical resection while FNH is managed conservatively in the majority of cases, without the need for surgical intervention. Therefore, accurate diagnosis is important. The use of MRI with a hepato-specific contrast agent, specifically gadoxetic acid, makes the diagnosis relatively easy to reach[5,8,9].
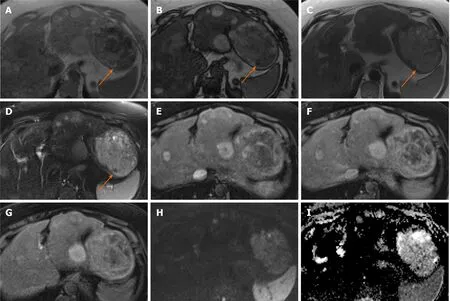
Figure 1 Hepatocellular adenoma.
Generally, typical MRI findings seen in HCA include mild to moderate high signal intensity on T2 weighted imaging (T2-WI), sometimes with small cystic areas or diffuse homogeneous steatosis of the lesion and it may show internal bleeding or atoll sign. FNH classically shows the presence of a T2-weighted (T2-W) hyperintense central scar. Both lesions show enhancement on the AP imaging and tend to be isointense in the PVP[10]. In particular, when compared with background liver parenchyma, on the HBP image an HCA is hypointense in the majority of cases whereas FNH is hyper- or isointense. FNH is composed of functional hepatocytes with abnormal biliary ductules and is therefore expected to accumulate hepatobiliary specific contrast agents, while HCA traditionally has been thought of as not having bile ductules and would often be expected to not retain such contrast[8].
The diagnostic conundrums are usually encountered when differentiating between HCA and malignant entities and characterising different molecular types of HCA (Figures 1 and 2). HCAs are classified into few major molecular subtypes: HNF1α inactivated HCA (H-HCA), inflammatory HCA (IHCA), β-catenin activated HCA (β-HCA) and β-catenin activated inflammatory HCA (β-IHCA) and sonic hedgehog HCA. The term Unclassified HCA is applied to those HCAs in which no specific mutation is identified[11]. The highest risk of malignant transformation was shown in mixed βcatenin-activated and inflammatory and β-catenin-activated forms[11]. Hepatobiliary contrast agent retention in the HBP can be seen in 83% of β-HCAs, 29% of IHCAs and not been demonstrated in H-HCA and unclassified HCAs[12]. Hyperintensity on HBP of HCAs could potentially help identify HCAs at high risk of malignancy[13]. However, this feature of high-risk HCAs makes it harder to differentiate radiologically from FNH which is hyperintense on HBP. Other MRI features may be helpful such as the presence of a central scar, the heterogeneous “periseptal” uptake of FNH on HBP, or other MR phases features. In addition, β-HCA typically demonstrates a subtle heterogenous hyperintense signal on T2-WI MRI, unlike FNH[12]. It is suggested that in patients with inflammatory HCA risk factors (such as obesity, metabolic syndrome, and alcohol use), relying on MRI features alone to differentiate FNH from inflammatory HCA may not be appropriate[8]. Histopathological analysis may be required in certain cases still, in order to achieve the final diagnosis.
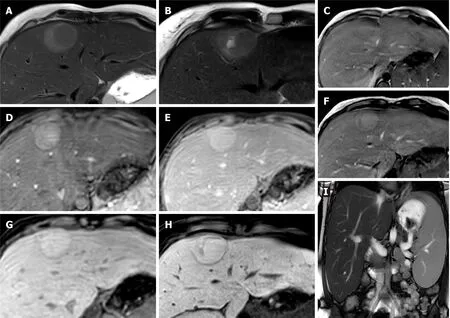
Figure 2 Hepatocellular adenoma.
FNH
FNH is the second most frequent benign hepatic tumour (haemangioma being the most common). It is found most typically in women in their 3rd-5thdecades of life. FNH is rarely symptomatic and usually found incidentally[14], unless very large in which case it can cause vague abdominal pain. There is some debate whether FNH is caused by or associated with use of oral contraceptives, but it may promote the growth of FNH. An FNH, contrary to HCA, has no malignant potential or life-threatening complications, and as such a surgical resection or further evaluation is not required if a diagnosis can be made confidently on imaging.
FNH is believed to represent a local hyperplastic response of hepatocytes to a congenital vascular anomaly. It is a proliferation of normal, non-neoplastic hepatocytes that are abnormally arranged. Normal portal venous structures are not present, but most lesions contain thick-walled arterial vessels that provide outstanding arterial supply; therefore haemorrhage, infarction and necrosis would be extremely rare[14]. Although the lesions have well-demarcated margins, they do not have a true capsule, which is consistent with their hyperplastic rather than neoplastic nature.
Typical MR features of FNH are iso- or mild hypointensity on T1-weighted imaging (T1-WI) and an iso- or slightly hyperintense lesion on T2-W sequences. FNH is known to have a classic central stellate fibrovascular scar, which is only seen in about 50% of cases and when present usually shows a high signal intensity on T2-WI. FNH is homogeneously and strongly enhanced on AP except for the central scar. It becomes isointense to the liver parenchyma during portal phase, with the central scar remaining relatively hypointense. The central scar typically shows enhancement in delayed phase. On the HBP FNH becomes iso- to hyperintense compared to surrounding liver without or with hypointense central scar[10]. Size of > 5 cm, presence of multiple lesions and evidence of haemorrhage and necrosis are considered atypical[15]. Rarely FNH may contain fat. Cases mimicking HCC, for example complete perfusion defect on HBP[16], and various enhancement patterns (Figure 3), such as a peripheral ring-like enhancement without a visible central scar, have also been described[16,17].
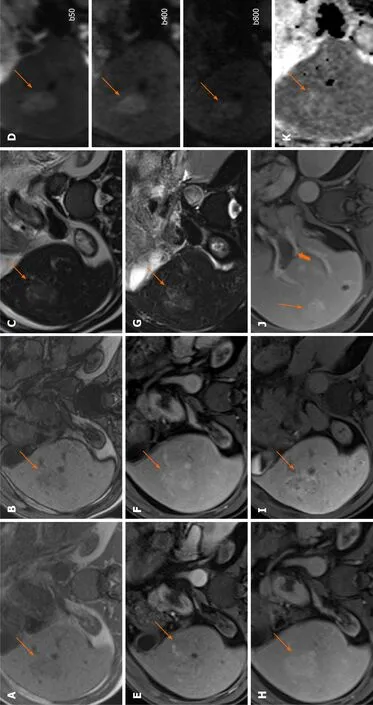
Figure 3 Focal nodular hyperplasia.

Figure 4 Hepatic angiomyolipoma.
Hepatic angiomyolipoma
Hepatic angiomyolipoma (HAML) is a rare, hepatic mesenchymal neoplasm which more frequently occurs in the kidneys, with the liver representing the second most common site of involvement[18]. It is found in both males and females, and in a majority of cases is asymptomatic. The tumour consists of 3 components: fat, vascular and smooth muscle. These components can vary significantly within each lesion and it is this heterogeneity that proves the preoperative diagnosis by imaging difficult (Figure 4).
The presence of fatty areas and solid tissue components is considered typical, however due to a significant overlap of the imaging features, most HAMLs are misdiagnosed as HCC with fatty metamorphosis. Both of these lesions show comparable dynamic enhancement patterns during the AP, followed by low signal intensity on PVP or late dynamic phases[19,20]. Generally, HAMLs are lacking hepatocytes, whereas HCCs contain hepatocytes with various degrees of malignant change, which in turn leads to a more homogeneous hypointensity on HBP compared with that of the spleen and sharper margins in HAML, compared to heterogeneous signal intensity and the ill-defined margin of HCCs at the HBP[19].
In a study by Wanget al[21], absence of a pseudo capsule, presence of an early draining vein and tumour vessels, and a higher apparent diffusion coefficient (ADC) in the hypervascular hepatic tumour on the MRI were helpful to distinguish a HAML from fat-containing HCC. The presence of an early draining vein is considered a conspicuous dilated or non-dilated vessel originating from the tumour with draining to the portal vein, hepatic vein, or inferior vena cava. A tumour pseudo capsule is defined as a thin hyperintense rind in the equilibrium phase.
Although historically HAML is considered a benign lesion, few case reports have discovered a potential for malignant transformation with evidence of recurrence[20,22,23]. As such, the potential risk of malignant changes of HAML needs to be recognised and some authors suggest that these lesions should be followed up after surgery.
MALIGNANT L ESIONS
HCC
HCC is the commonest primary hepatic malignancy, showing an increasing worldwide prevalence[24,25]. Cirrhosis constitutes a crucial risk factor for the development of HCC with the estimated prevalence of cirrhosis among patients with HCC of 80%-90%[26]. Having an underlying liver disease impacts the management and therapeutic options. Due to high rates of intrahepatic recurrence, the prognosis for patients with advanced HCC remains poor[27], however when diagnosed at an early stage, curative treatments such as surgical resection, liver transplantation, and radiofrequency ablation are possible. Hence, precise imaging diagnosis in patients with early-stage HCC is crucial.
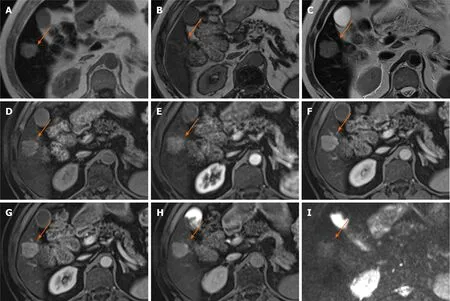
Figure 6 Hepatocellular carcinoma.
To address this, the Liver Imaging Reporting and Data System (LI-RADS) was created. It is a comprehensive system for standardising the terminology, technique, interpretation, reporting, and data collection of liver imaging. The primary blood supply of normal hepatocytes isviathe portal venous system, in contrast to HCC which is supplied by abnormal hepatic arteries. Consequent imaging features are of a lesion which enhances during the late AP (non-rim) with subsequent progressive washout of contrast relative to background liver parenchyma and a peripheral rim of enhancement (pseudocapsule) on either PVP or delayed phase imaging[28,29]. Apparent hypointensity relative to liver in the transitional phase may potentially represent hyperenhancement of liver rather than reduced enhancement of the mass, therefore it is recommended that when gadoxetate disodium is administered as contrast media, washout is evaluated only in the PVP[30]. Additional major LI-RADS features include threshold growth (increase in size of 50% or more within 6-mo time during follow-up imaging) and size.
Hypointensity on HBP is considered an ancillary feature favouring malignancy and HBP isointensity an ancillary feature suggesting benignity[28]. However, hyperintensity on HBP phase has been demonstrated in 8.8%–13.6% of HCCs[31,32]. Such HCCs are rather difficult to differentiate from FNH on gadoxetic acid enhanced MR (Figures 5-9).

Figure 7 Hepatocellular carcinoma.
A study by Kitaoet al[33] found that the washout pattern was observed in only 57% of HBP hyperintense HCCs at dynamic MRIvs95.8% on dynamic computed tomography (CT). The reason for this is thought to be that gadoxetic acid is already taken up into tumour cells in the transitional phase by hyperintense HCCs. Therefore, the addition of CT may be helpful as AP enhancement and washout pattern at dynamic CT, as well as a decrease in ADC ratio, were shown to be independent predictors of hyperintense HCC[33]. Overall, hyperintense HCCs seem to have clinical and histologic features that might be related with more favourable outcomes[31].
An appearance of smooth hypointense rim in the HBP could also improve the detection of tumour capsule and the diagnosis of HCC[34].
Intrahepatic cholangiocarcinoma
Intrahepatic cholangiocarcinoma (ICC) is the second most common primary hepatic tumour. Although it accounts for only 3% of gastrointestinal malignancies, the incidence of ICC has been rising worldwide[35]. Risk factors include chemical exposure, liver flukes, biliary tract disease (primary sclerosing cholangitis, hepatolithiasis, Caroli’s disease), viral hepatitis, metabolic syndrome, cirrhosis, smoking and alcohol[35,36]. Of note, a large proportion of ICC patients (38.9%) have no identifiable risk factors[36] and further studies are required to explore this.
ICC can be classified into three types according to the Liver Cancer Study Group of Japan classification based on morphologic features with each type demonstrating its characteristic imaging features: Mass-forming (the most common, definite mass in the liver parenchyma), periductal-infiltrating (extends longitudinally along the bile duct, often resulting in dilatation of the peripheral bile duct), and intraductal growth (proliferating towards the lumen of the bile duct like a papilla or tumour thrombus)[37]. As part of the focal liver lesions review, we will discuss the appearances of the massforming ICC on gadoxetic acid enhanced MRI.
The mass-forming ICC shows an irregular, but well-defined margin with hyperintensity at T2-WI and low signal at T1-WI. Capsular retraction, encasement of vessels without the formation of a grossly perceivable tumour thrombus, and presence of satellite nodules are often seen[38]. The usual enhancement pattern demonstrated by ICC is peripheral irregular enhancement in the AP and gradual centripetal enhancement on subsequent phases. Similarly to HCC, due to the pseudo-washout effect on gadoxetic acid-enhanced MRI, it is recommended that washout is assessed on PVP[39,40]. Histologically the viable tumour cells are often seen at the periphery of the tumour, while the central portion is composed of a variable degree of fibrosis. The majority of the tumours with severe fibrosis show delayed enhancement[38]. Intrahepatic mass-forming cholangiocarcinomas lack hepatocytes and in turn are often hypointense on HBP which helps to delineate the lesion itself, the satellite nodules and intrahepatic metastases due to strong enhancement of normal liver parenchyma on HBP[41]. Tumours with intermediate signal intensity on HBP tend to correlate with poor prognosis and histologically are shown to have more abundant fibrous stroma[42]. Therefore, imaging with gadoxetic acid could be used for prognostication. In a study by Choiet al[40] peritumoral bile duct dilatation and HBP target appearance (peripheral hypointense rim compared with the central area of the lesion) were independent factors suggestive of ICC (Figure 10).

Figure 8 Hepatocellular carcinoma.
Neuroendocrine tumours
Neuroendocrine tumours (NETs) consist of a vast heterogeneous group of malignancies which are derived from embryonic neural crest tissue found in various organs. The gastrointestinal tract accounts for 54.5%-73.7% of the tumours[43,44]. Within the gastrointestinal tract, the small intestine is the most common site, followed by the rectum, appendix, colon, and stomach. NETs comprise approximately 1%–2% of all gastrointestinal tumours. In the liver, NETs usually represent metastases from other sites, therefore other primary sites should be examined when a NET is suspected in the liver. Tumours with no identifiable primary site typically originate from unrecognised, small or “burned-out” gastroenteropancreatic NETs[45], however a primary hepatic location, while extremely rare, has been reported in the literature[46-48].
NET liver metastases generally are hyperintense on T2-WI. Hypervascular metastases regularly show heterogeneous intense enhancement in the AP and ring enhancement is also a frequent finding[49]. Hypovascular metastases are best appreciated on PVP, similar to CT, and appear as low-signal intensity lesions relative to the liver parenchyma (Figures 11 and 12). Perilesional enhancement is frequent in the venous phase. A peripheral low-signal intensity area may be observed on the delayed phase[49]. Because of high signal intensity on T2-WI, NET liver metastases may be difficult to distinguish from cavernous haemangioma, however, unlike NET metastases, haemangiomas do not typically washout and less commonly restrict diffusion. While variable lesion enhancement is seen with dynamic postcontrast images, NET liver metastases generally demonstrate hypoenhancement relative to liver parenchyma on HBP images[50] and HBP imaging is shown to improve detection of NET liver metastases[51,52].

Figure 9 Hepatocellular carcinoma.
Primary hepatic NETs (PHNETs) generally grow slowly and only become clinically evident at an advanced stage. They most often appear as an endocrinologically silent hepatic mass and are less frequently associated with typical carcinoid syndrome, unlike extrahepatic NETs[47]. In preoperative imaging, PHNETs are often misdiagnosed as HCC or cholangiocarcinoma. Radiological findings are similar for both primary and metastatic NETs[53]. Similarly to NET liver metastases, PHNETs tend to be hypervascular and markedly enhance, and while they are usually solid, cystic PHNETs have been described. Fluid-fluid levels have also been described in some cases[46,54] (Figure 13). Most lesions demonstrate delayed contrast wash-out due to hypervascularity and central necrosis, but progressive enhancement has also been reported[55]. ADC values typically show restricted diffusion.
Liposarcoma
Liposarcoma is a rare malignant mesenchymal tumour usually located in the retroperitoneal space and the deep soft tissues of the extremities, particularly those of the thigh. Hepatic location is extremely rare, few cases have been reported in the literature[56]. Early diagnosis of primary liposarcoma of liver is difficult. In liver, they are often misdiagnosed as adenomas (Figure 14).

Figure 10 Intrahepatic cholangiocarcinoma.
Generally minimal enhancement is seen in liposarcomas that are well-differentiated, and more so with round cell, pleomorphic, and dedifferentiated subtypes[56]. Associated non-adipose masses, thickened or nodular septa, prominent foci of high T2 signal, and areas of enhancement are all features suspicious for liposarcoma[57]. Higher grade liposarcomas commonly contain little to no macroscopic fat and may not confound the MRI diagnosis of predominantly fatty lesions. Areas of haemorrhage and necrosis can be seen.
CONCLUSION
The various types of liver lesions demonstrate diverse imaging appearances due to common and uncommon features as well as overlapping imaging findings. Familiarising with these entities and their characteristic appearances can help in making an accurate diagnosis.
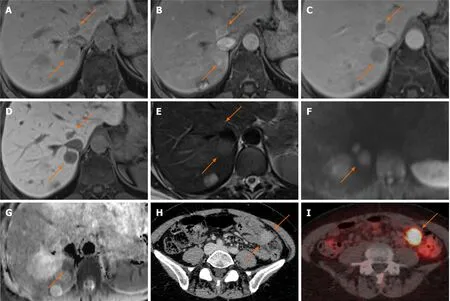
Figure 11 Neuroendocrine carcinoma metastases.

Figure 12 Neuroendocrine carcinoma metastases.

Figure 13 Neuroendocrine carcinoma.
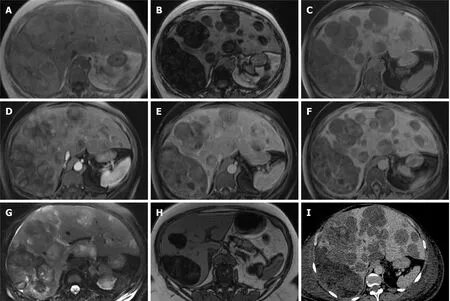
Figure 14 Pleomorphic liposarcoma.
杂志排行
World Journal of Hepatology的其它文章
- Coronavirus disease 2019 and non-alcoholic fatty liver disease
- Epigenetic mechanisms of liver tumor resistance to immunotherapy
- Advances in the management of cholangiocarcinoma
- Herbal and dietary supplement induced liver injury: Highlights from the recent literature
- Challenges in the discontinuation of chronic hepatitis B antiviral agents
- Liver Kidney Crosstalk: Hepatorenal Syndrome
