Portable and automated analyzer for rapid and high precision in vitro dissolution of drugs
2021-09-14ZhongmeiChiSiqiZhoXiujunCuiYunxingFengLiYng
Zhongmei Chi , Siqi Zho , Xiujun Cui , Yunxing Feng , Li Yng ,*
a Key Laboratory of Nanobiosensing and Nanobioanalysis at Universities of Jilin Province, Department of Chemistry, Northeast Normal University,Changchun,130024, China
b Jingke-Oude Science and Education Instruments Co., Ltd, Changchun,130024, China
Keywords:Dissolution test analyzer Portable Automated Immediate-release drug Fixed dose combination drug
ABSTRACT We developed a novel portable and automated dissolution test analyzer for rapid and high precision in vitro dissolution testing of drugs.The analyzer consists of a flow-through-cell drug dissolution system,an automated sequential sampling system, a high-speed capillary electrophoresis (HSCE) system, and a data acquisition system. Combining the high-temporal resolution flow-gating sampling approach with HSCE,which has outstanding advantages of efficient separation and resolution,the analyzer can achieve rapid analysis and exhibits the ability in miniaturization for on-site assessment of different active pharmaceutical ingredients.To integrate the flow-through-cell dissolution system with HSCE,a specially designed flow-gating-injection (FGI) interface was employed. The performance of the analyzer was investigated by analyzing the dissolution of immediate-release drugs including single dose (amoxicillin dispersible tablets) and fixed dose combination (amoxicillin and clavulanate potassium) drug tablets with the high-temporal resolutions of 12 s and 20 s, respectively. The dissolution profiles of different active pharmaceutical ingredients could be simultaneously and automatically monitored with high repeatability and accuracy. The analyzer was successfully utilized for the pharmaceutical quality control and bio-relevant dissolution testing,as well as in vivo-in vitro correlation analysis.Our portable analyzer is miniaturized, convenient and of low-cost, and will provide a valuable tool for dissolution testing in pharmaceutical research and development.
1. Introduction
In vitro dissolution testing is an essential part in the process of drug research and development [1,2]. The standardized tests were developed back to the 1970s by United States Pharmacopeia(USP)and the dissolution guidance was adopted by the Food and Drug Administration(FDA)of the United States in 1997[3].Nowadays,it is widely realized that rapid and efficient in vitro dissolution testing is of pivotal importance, not only for evaluation and controlling of drug quality [4-7] but also for forecasting the respective in vivo performance including in vitro-in vivo correlation (IVIVC), bioequivalence (BE) and bioavailability [8-16].
During the past decades, in vitro dissolution testing has been routinely used as an analytical tool to evaluate the performance and quality of pharmaceutical dosage forms [17-20]. This boosts the development of commercial-available analytical devices [21,22].Most types of commercial devices, however, are only used as a sample preparation apparatus for dissolving drug tablets,and even are equipped with an automated sampling system. To achieve dissolution data, most of the devices are usually off-line coupled with additional analytical technique (HPLC, spectrophotometry,etc.), for the detection of active pharmaceutical ingredients at particular intervals during the dissolution process[23,24].Such an off-line procedure is difficult to integrate all the necessary parts for dissolution testing, including drug dissolving, sample injection,separation and detection of ingredients. A portable and miniaturized analyzer for complete dissolution testing can reduce both time and labor consumption to achieve accurate analytical results.
Recent efforts have been made to develop analyzers for on-line dissolution testing[25-27].Ideally,the analyzer should be capable of providing real-time information about the different active pharmaceutical ingredients during dissolution process, with integration of all the necessary steps for on-line analysis.An attractive methodology is the application of optical fiber to on-line record information every 15-30 s of the drug dissolution process. While the methodology has already progressed to the commercial stage,the optical fiber-based dissolution testing faces challenges including media turbidity due to undissolved components and baseline off-sets upon dissolution time [28,29]. Moreover, devices based on optical fiber dissolution testing are difficult for quantification of fixed dose combination pharmaceutical formulations,especially those with highly overlapping features and spectral interferences that require the assistance of complicated mathematical models [30-32].
Very recently,we have reported a novel method for dissolution testing with high-temporal resolution and efficient separation of active ingredients [33]. The method combines a programcontrolled sequential injection and high-speed capillary electrophoresis(HSCE)to a flow-through dissolution cell.In that paper,we successfully demonstrated the feasibility of the method by analyzing the dissolution profiles of drugs. Here, we extend our study to develop a portable dissolution analyzer based on the method reported. The analyzer is battery-powered and highly compact, integrating a flow-through-cell drug dissolution system,an automated sequential sampling system, an HSCE system and a data acquisition system.Taking immediate-release drugs including single dose (amoxicillin dispersible tablets) and fixed dose combination (amoxicillin and clavulanate potassium) drug tablets as the testing models, we show that the developed analyzer can perform robust and automatic in vitro drug release testing and is timesaving without tedious operations. Several tests including quality control(QC)dissolution test,bio-relevant dissolution test as well as IVIVC evaluation were performed to show the capability of the device in drug research and development. The present study pushes our newly-developed method to next important state, a commercial-available portable and automatic device for dissolution testing.
2. Experimental
2.1. Chemicals
Drug tablets, including amoxicillin dispersible tablets (each containing 0.25 g amoxicillin) and amoxicillin/clavulanate potassium tablets (each containing 0.25 g amoxicillin and 0.125 g clavulanate potassium), were purchased from local pharmacies. The details of the purchased products are given in Table S1. Standard amoxicillin trihydrate and clavulanate potassium were supplied by Henan Wanjia Shouhua Biotechnology Co., Ltd. (Xinyang, China).Sodium dihydrogen phosphate, disodium hydrogen phosphate(PB), ammonium acetate, acetic acid (HAc), and hydrochloric acid(HCl)were purchased from Beijing Chemical Works(Beijing,China).Sodium chloride,sodium hydroxide,sodium taurocholate,lecithin,maleic acid, glyceryl monooleate, sodium oleate and pepsin were obtained from Sigma Aldrich(St.Louis,MO,USA).All reagents and chemicals were of analytical grade and were used without further purification. Ultrapure water was provided by Milli-Q Water System(Millipore, Billerica, MA, USA).
Standard stock solutions of amoxicillin trihydrate and clavulanate potassium in a concentration of 1 mg/mL were prepared in ultrapure water and stored at 4°C.They were freshly diluted to the desired concentrations as the standard working solutions prior to analysis. The background electrolytes (BGE) used in the analysis were 5 mM(pH 9.0) and 10 mM(pH 8.5) of PB for the single-dose and the fixed-dose combination tablets respectively. All samples and buffers were filtered through a 0.22 μm syringe filter and degassed by ultrasound for 10 min before use.
Four widely-used in vitro dissolution media were used for QC testing, as recommended by European Pharmacopeia [34], i.e., (a)HCl (pH 1.2); (b) buffer consisting of HAc and sodium acetate (pH 4.5); (c) phosphate buffer (pH 6.8) and (d) distilled water. For biorelevant dissolution testing, four kinds of bio-relevant dissolution media,i.e.,fasted state simulated gastric fluid(FaSSGF),fasted state simulated intestine fluid (FaSSIF), fed state simulated gastric fluid(FeSSGF) and fed state simulated intestine fluid (FeSSIF), were prepared according to the Center for Drug Evaluation and Research as reported in the literature [35]. The details of the bio-relevant dissolution media are listed in Table S2. The volume of each of the dissolution media used in the present study was 240 mL.
2.2. In vitro dissolution testing with the analyzer
Figs.1A and B present the schematic design and the real photograph of the proposed analyzer,which was compacted into a devicebox to be easily transported. In the experiments presented in this study, the analyzer was usually transported between the two campuses of our university,the distance of which was about 15 km.No changes in physical structure or analytical performance of the device were observed at all during transport.The analyzer integrated all the necessary parts for in vitro dissolution testing, including a flowthrough-cell for dissolving drug tablets, a home-designed flowgating-injection(FGI)interface,an HSCE analysis system and a data acquisition system.Briefly,a drug tablet placed in the flow-throughcell,which was constructed according to the state of the compendial description [36], was dissolved in a dissolution media at constant temperature ((37 ± 0.5)°C) in a water bath. The pharmaceutical active ingredients dissolved from the tablet were continuously transferred via the FGI interface for HSCE separation and detection by UV absorption. The sequential injection was automatically performed so that the entire dissolution process could be on-line monitored every 10-20 s. The whole procedure was automatically controlled by a LabVIEW program.The data from the detector were read and visualized on the screen of the laptop via Bluetooth.
2.3. Pharmacokinetic data analysis for in vitro dissolution testing
For the validation process, peak integrations and data analysis were performed using 32 Karat™software (SCIEX Separations,Brea, CA, USA). All measurements were performed at least three times and the data were reported as means and standard deviations of these measurements. The details on the pharmacokinetic data analysis and the calculation of kinetic parameters,including in vitro cumulative dissolution percentage (Fdis%), dissolution efficiency(DE%), mean dissolution time (MDT), t50%or t80%(the time necessary to release 50%or 80%of a drug tablet)and similarity factor(f2),can be found in the Supplementary data.
3. Results and discussion
3.1. Dissolution device design
The present work aimed to develop an integrated and affordable instrument that can automatically on-line monitor active pharmaceutical ingredients dissolved from the drug with high accuracy and efficiency. The entire procedure for dissolution testing can be automatically recorded with a high-temporal resolution, which is essential for obtaining precise dissolution profiles, especially for immediate-release drug tablets. The device was compacted into a device-box with a size of 400 mm(length) × 300 mm (width) × 250 mm (height) and a weight of 10 kg (Fig.1B).
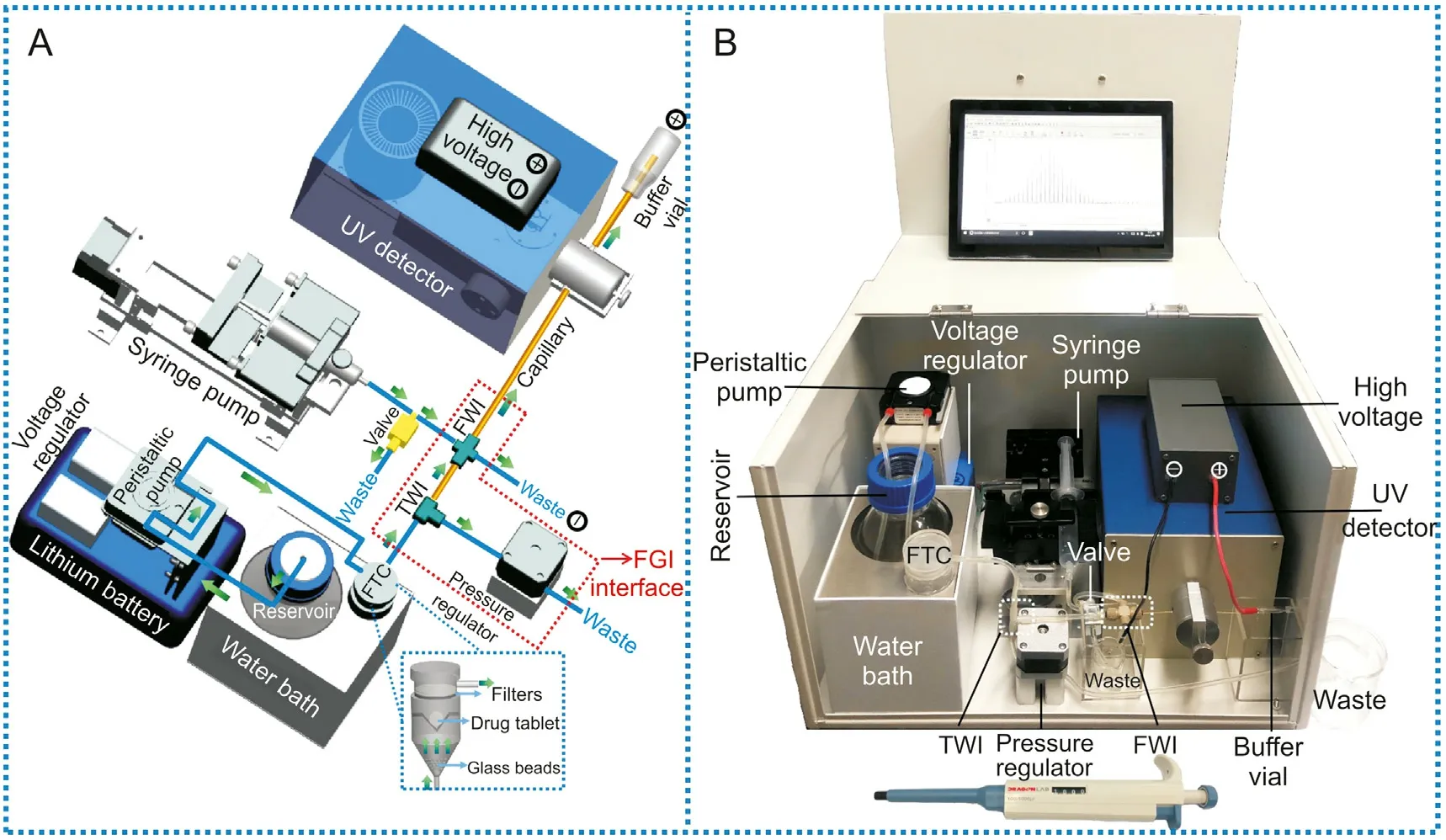
Fig.1. (A)The 3D Pro Engineer design and(B)a photograph of the portable dissolution analyzer which integrates a flow-through-cell and an automated sequential injection with HSCE by a home-designed FGI interface. The green arrows in Fig. 1A indicate the flow direction. The enlarged part presented in Fig. 1A shows the flow-through-cell for drug dissolution, which is constructed according to the state of the compendial description. A pipette is placed near the analyzer in the picture in Fig.1B to indicate the size of the analyzer. HSCE: high-speed capillary electrophoresis; FTC: flow-through-cell; TWI: three-way interface; FWI: four-way interface; FGI: flow-gating-injection.
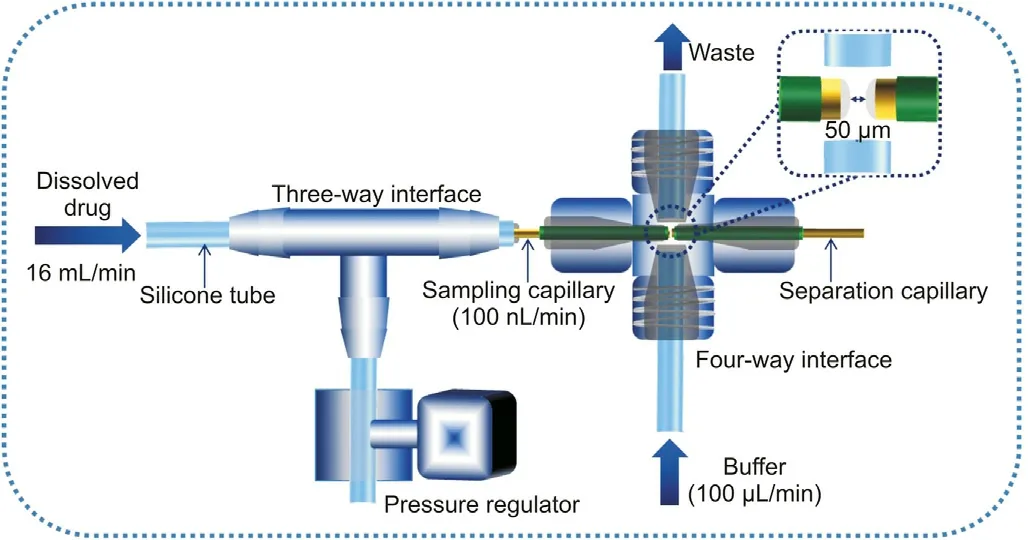
Fig.2. Schematic diagram of the FGI interface that connects the dissolution output and the inlet of the capillary for HSCE. The inset figure presents the injection space between the sampling capillary and the separation capillary.
We integrated the flow-through-cell dissolution system with short-capillary-based HSCE (Fig.1A), which allowed for rapid and efficient separation,and thus could achieve simultaneous detection of multiple pharmaceutical ingredients during the dissolution process. The short-capillary based HSCE exhibited the advantages for rapid and high-efficient separation and detection, which is important for the precise determination of multiple analytes in a sample. The integration of the system, however, has rarely been reported[37].This can be possibly due to the complex modules for sample injection. In the case of dissolution testing of drug tablets,the critical challenge lies in the fact that the rate of the medium flow from the dissolution cell is so high (e.g.,16 mL/min) that it is difficult for HSCE analysis,which requires a small amount of sample injected.To overcome this issue,we developed a specially designed FGI interface that connected the dissolution output and the inlet of the capillary for HSCE (Fig. 2). The FGI interface contained a pressure regulator, a three-way interface (TWI), a four-way interface(FWI)and a sampling capillary connecting them.The TWI was used as the flow-splitter to drive the dissolution solution to the sampling capillary. Under the back-pressure produced by a program-controlled regulator (stepping motor), the solution(16 mL/min flow rate) containing the pharmaceutical ingredients,which were dissolved from the drug in the flow-through dissolution cell, was divided to enter the 2 cm-length sampling capillary with a reduced flow-rate of only 100 nL/min. This allowed us to couple to the following HSCE analysis in which an injection of a nanoliter amount of sample was required.The FWI made by an offthe-shelf commercial micro-cross assembly (PEEK, 0.006′′ID,1/32 OD Tubing, 5/16-24 Coned, Upchurch Scientific, Oak Harbor, WA,USA) was used to connect the sampling capillary to the HSCE capillary for separation and detection. The ends of both the sampling capillary and the separation capillary (total length 12 cm)were tapered (see the inset in Fig. 2), sleeved with microtight sleeves(0.015′′ID and 0.025′′OD,Upchurch Scientific,Oak Harbor,WA,USA),and were tightly inserted through two opposite channels of the micro-cross. The injection space is defined as the distance between the capillary ends in the interface adjusted to be about 50 μm.Such interface lay in the boundary between conventionally used circular fused silica capillaries and planar microfluidic devices.With the help of microferrules and female nuts, the precise positioning and alignment of the capillaries could be easily achieved along with the off-the-shelf micro-cross assembly; therefore, the sample could be well transferred. The diameters of the other channels of the FWI were carefully expanded to tightly fit the silicone tubes (1 mm OD). These channels were connected to deliver and drain the buffer solution from the injection space via a microsolenoid valve. The FGI interface enabled a linking of dissolution system and HSCE with a sufficient low dead volume and allowed further miniaturization.
A home-built voltage regulator was developed to electronically drive all the necessary assemblies, including a syringe pump, a peristaltic pump, a micro-solenoid valve, a pressure regulator, a high voltage power supply and a UV detector. The regulator contained the rechargeable lithium batteries to provide a stable output of 24 V and can give sufficient power for continuous running the device for more than 4 h for the drugs investigated in the present study. The regulator was a benefit for making the device compact and portable. Concerning the drugs analyzed in the present study,about 15 min was required for one run,so at least 16 analyses could be done before changing the batteries.
3.2. Automated controlling
For the dissolution testing using the developed device, the sample dissolved from the flow-through-cell was continuously injected for HSCE analysis via the FGI.The key issue for automation of the device was to control the timing sequence of the whole analytical process, including injection, separation and detection.This was accomplished by automatically controlling the microsolenoid valve for sample injection and the high voltage power supply for HSCE.The flow chart of the automated controlling using a LabVIEW program is shown in Fig.3A.The program controlled the temperature for the water bath, the pumps, the solenoid valve, as well as the high voltage for separation. The interfaces for usercontrol and parameter-setting are presented in Fig. 3B. Once the temperature in the water bath was constant,which was monitored by a DS18B20 sensor and adjusted by a thermoelectric cooler, the pumps were started up (the peristaltic pump for driving the dissolution media to the flow-through-cell and the syringe pump for delivering background electrolyte (BGE)); thus the dissolution testing began.The automated controlling of an HSCE run consisted of the following four steps:
3.2.1. Loading
The sample from the flow-through-cell was allowed to load into the sampling capillary when the high voltage for HSCE was turned off and the micro-solenoid valve was switched to direct the BGE flow to the waste vial to prevent it from entering the injection space.
3.2.2. Injection
After 2-s loading,a high voltage of-5 kV was then applied while the position of the micro-solenoid valve was maintained,injecting the sample electrokinetically into the separation capillary. In the present study, an injection time of only 1 s was sufficient for efficient separation and good sensitivity.
3.2.3. Washing
Before HSCE analysis, the sample remained in the injection space should be washed out by the BGE flow.This was achieved by turning off the power supply and switching back the position of the micro-solenoid valve to direct the BGE flow to the FWI.
3.2.4. Separation
A high voltage of-15 kV was applied across the separation capillary for HSCE separation and detection.
Once the HSCE analysis was completed,the sample was loaded again by switching both the micro-solenoid valve and the high voltage power supply, and another “loading-injection-washingseparation” sequence automatically began. Obviously, each sequence corresponded to one time-point of dissolution, and the entire dissolution process could be automatically monitored with high-temporal resolution and efficient separation.
3.3. Performance of the analyzer
To optimize the performance of the analyzer, we first investigated various parameters involved in the dissolution testing,including the flow rate of the medium in the flow-through-cell,the factors in the FGI interface (i.e., the gap distance, the flow rates of the BGE and the sample,the time of loading,injection and washing)and HSCE separation (the concentration and pH of the buffer, the separation voltage). The details of the optimization process and results are provided in Figs. S1 and S2. Under the optimal experimental conditions, the performance of the analyzer was evaluated for in vitro on-line dissolution testing of amoxicillin and amoxicillin/clavulanate potassium drugs. Fig. 4A presents the electropherogram of analysis of a mixture sample of standard amoxicillin trihydrate(50 μg/mL)and clavulanate potassium(25 μg/mL),which was continuously delivered from the flow-through-cell to the FGI,followed by twenty-sequential injections and HSCE detection. The results showed the excellent repeatability of sequential analysis with RSD(n =20)<1.63%and <1.19%for peak areas of amoxicillin trihydrate and clavulanate potassium, respectively. Baseline separation could be achieved in each sequence with a resolution of 1.8(RSD <1.45% (n =20)) (see the inset in Fig. 4A).
In Fig. 4B, we present a typical electropherogram showing the entire dissolution process of a fixed dose combination drug tablet.Each sequence corresponded to one time-point of the dissolution process. As the dissolution time evolved, the absorption peak of each active pharmaceutical ingredient (amoxicillin trihydrate and clavulanate potassium) first increased to the maximum and then decreased until it eventually disappeared after 800 s,indicating the dissolution was completed.In this case,one sequence required 20 s for efficient separation of the two active ingredients (see the inset in Fig. 4B), indicating the temporal resolution of the dissolution testing was 20-s.The results showed the feasibility of the analyzer for the simultaneous and quantitative determination of multiactive pharmaceutical ingredients in dissolution testing of the drug.For the fixed dose combination drug investigated in the study,the dissolution profiles of both ingredients were obtained via the electropherogram from only one HSCE run(Fig.4C).For single dose drug tablets, which contain only one active pharmaceutical ingredient, the dissolution profile could be obtained with a highertemporal resolution of only 12-s (Fig. S3).
Table 1 summarizes several parameters showing the performance of the analyzer, including repeatability, accuracy, linearity range, and sensitivity, for dissolution testing of the fixed dose combination drug containing amoxicillin trihydrate and potassium clavulanate. A wide linear range of the active pharmaceutical ingredient was achieved with limit-of-detection(LOD)down to the level of μg/mL. The recovery testing was applied to evaluate the accuracy and precision of the analyzer, which was performed by spiking amoxicillin trihydrate and clavulanate potassium dosage at three different levels (10 μg/mL, 80 μg/mL and 150 μg/mL). The recoveries of amoxicillin trihydrate and clavulanate potassium were in the range of 98.4%-101.5%and 97.9%-100.8%,respectively.The precision of the recovery was 1.63%(RSD,n =3)for amoxicillin trihydrate and 1.19% (RSD, n =3) for clavulanate potassium. The results demonstrated good accuracy and precision of the proposed analyzer.

Fig. 3. (A) LabVIEW program flow chart for automated controlling. (B) The interfaces on the computer for user-control and parameter-setting.
The on-line dissolution testing using the proposed device was compared with the results using the traditional off-line approach.The experimental details for the traditional off-line in vitro dissolution testing are described in the Supplementary data. The resulting dissolution profiles are presented in Fig.5.The similarity factor f2between the on-line analyzer and off-line approaches was calculated to be 73.9 for amoxicillin trihydrate in the single dose drug tablets, and 58.3/62.8 for amoxicillin trihydrate/clavulanate potassium in the fixed dose combination drug tablets, indicating the good agreement between two approaches. It should be mentioned that the statistical analysis would be more accurate with larger data numbers.In the above similarity analysis,however,only three (or five) data points could be used before the complete dissolution of the single dose drug (or the fixed dose combination drug), because of the limitation of low-temporal resolution of the traditional off-line dissolution testing.On the other hand,75 or 45 data points during the entire dissolution in 15 min were obtained using the analyzer with 12-s or 20-s time-resolution. No tedious and time-consuming operations were necessary for sample collection and analysis.It is expected that the analyzer is of valuable application in precise analysis of dissolution profiles of immediaterelease drugs.
3.4. Applicability of the analyzer
Taking single dose (amoxicillin dispersible tablets) and fixed dose combination (amoxicillin and clavulanate potassium) drug tablets as testing models, we evaluated the applicability of the proposed analyzer by performing dissolution testing for QC, biorelevant and IVIVC evaluation of drugs.
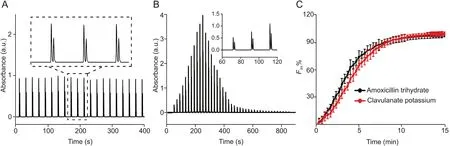
Fig. 4. (A) Electropherogram of 20 sequential analyses using the analyzer for a mixture of standard amoxicillin trihydrate (50 μg/mL) and clavulanate potassium (25 μg/mL). (B)Typical electropherogram showing the entire dissolution process of a fixed dose combination drug tablet (amoxicillin trihydrate and clavulanate potassium). The inset is the enlarged figure showing the temporal resolution of 20-s with baseline separation of both active ingredients in each sequence.(C)Dissolution profiles of both ingredients obtained from only one HSCE run using the analyzer. The experimental conditions: gap distance 50 μm, loading time 2 s, injection time 1 s, washout time 2 s, sampling rate 100 nL/min,background electrolyte (BGE) gating flow rate 100 μL/min.
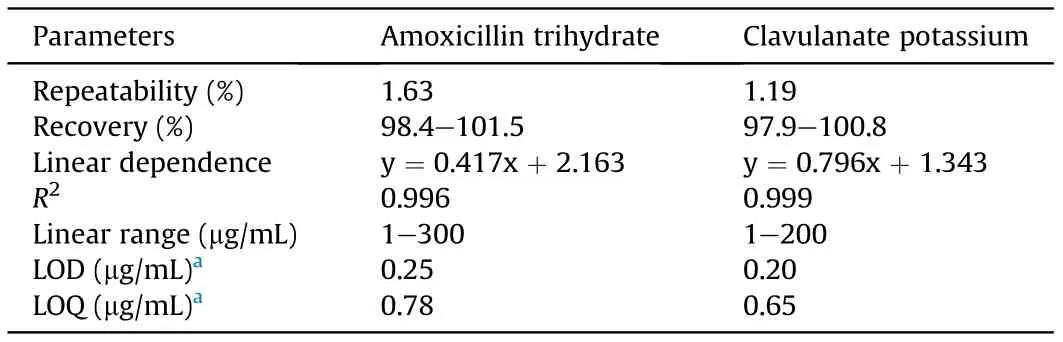
Table 1 Several performance characteristics for evaluation of the analyzer for dissolution testing for the fixed dose combination drug containing amoxicillin trihydrate and clavulanate potassium.
3.4.1. QC dissolution testing
The most commonly used dissolution media in laboratories for QC of drugs are water,HCl at pH 1.2,HAc at pH 4.5,and PB at pH 6.8 to simulate gastric fluid and intestinal fluid. In Fig. S4, we present the dissolution profiles recorded in four standards QC media by the proposed analyzer.Three batches of amoxicillin dispersible tablets or amoxicillin and clavulanate potassium tablets, which were produced from the same pharmaceutical company, were investigated. For different batches and in different QC dissolution media,the resulting t50%, t80%, MDT and DE% of each drug are shown in Fig.6.It can be seen that in either of the QC media,the dissolution time (t50%, t80%, or MDT) of batch 3 was shorter than that of the other two batches,and the corresponding DE%was larger.For each batch,there was only a slight difference in the dissolution time and DE% in different QC dissolution media. The f2factors between two batches in each QC medium were in the range of 51-88,indicating the good similarity of dissolution for the drugs of different batches.
Similar evaluations were also performed for drugs obtained from different pharmaceutical companies. Dissolution investigation of two drug tablets produced by different companies conformed to the requirements of USP [36], which should be given attention to in clinical application. The dissolution profiles are presented in Fig. S5 and the corresponding kinetic constants obtained from the profiles are shown in Fig. 7. The dissolution time was the shortest and the DE% was the largest for the tablet produced by company 2. For each drug produced by different companies investigated in the study, the good similarity in the dissolution was observed as f2factors were larger than 50.
3.4.2. Bio-relevant dissolution testing
The in vitro bio-relevant dissolution testing was investigated for each drug using four different bio-relevant media (FaSSGF, FaSSIF,FeSSGF,and FeSSIF),which could better represent the physiological conditions. The dissolution profiles of both drugs in four biorelevant media are shown in Fig. S6. The kinetic parameters obtained from the dissolution profiles are presented in Table 2.80%of the dissolution of an amoxicillin dispersible drug was completed in 10 min in either of the bio-relevant media(Fig.S6)and completed dissolution was achieved within 15 min. For fixed dose combination drugs, the dissolution rate of amoxicillin and clavulanate potassium was faster in FaSSGF than in other bio-relevant media.Overall, the solubility of both drugs in FaSSGF and FeSSGF was much higher than that in FeSSIF and FaSSIF. The results demonstrate that the in vitro dissolution testing of the drug in the biorelevant medium which simulated the human gastrointestinal tract medium can be easily detected by the proposed analyzer,which can better predict the in vivo role and the behavior.
3.4.3. IVIVC dissolution testing
The in vivo dissolution testing was performed on male rats aged 8 weeks (26-36 g), purchased from Liaoning Living Organism(Dalian,China).See the Supplementary data for more details of the in vivo experiments. The individual plasma concentration versus time profiles of amoxicillin dispersible drugs and amoxicillin and clavulanate potassium drugs in rats are shown in Fig. S7 and Table S3. The pharmacokinetic behaviors of amoxicillin trihydrate and clavulanate potassium were consistent with the onecompartment model. The in vivo absorption fraction (Fabs%) of the drug was calculated using the appropriate IVIVC model of Wagner-Nelson,described as the following equation [38]:

where Fabs%is in vivo absorption fraction,Ctis drug concentration of plasma in t time, AUC0-tis the area under plasma concentrationtime curves from 0 to t, AUC0-∞is the area under plasma concentration-time curves from 0 to∞, and keis elimination rate constant.
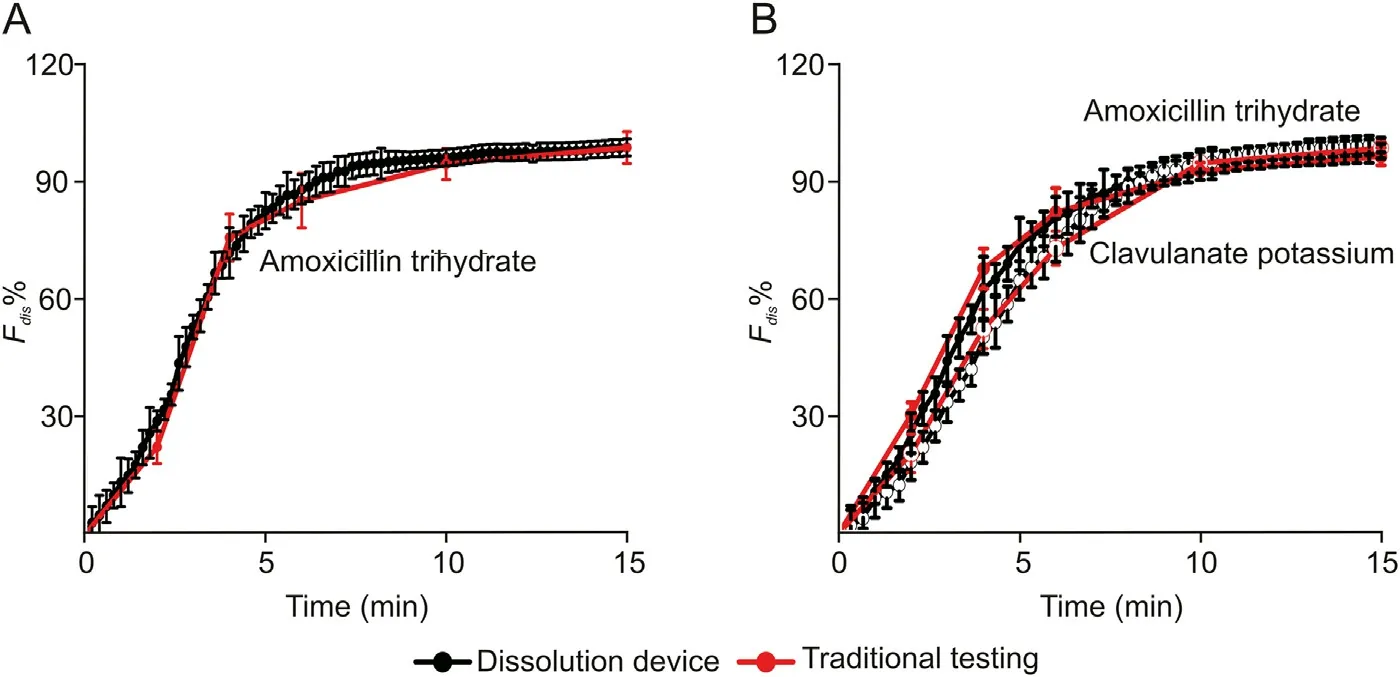
Fig. 5. Comparison of dissolution profiles using the proposed analyzer with those using traditional off-line dissolution testing for (A) amoxicillin dispersible drug tablets and (B)amoxicillin and clavulanate potassium drug tablets. Other conditions were the same as those in Fig. 4.
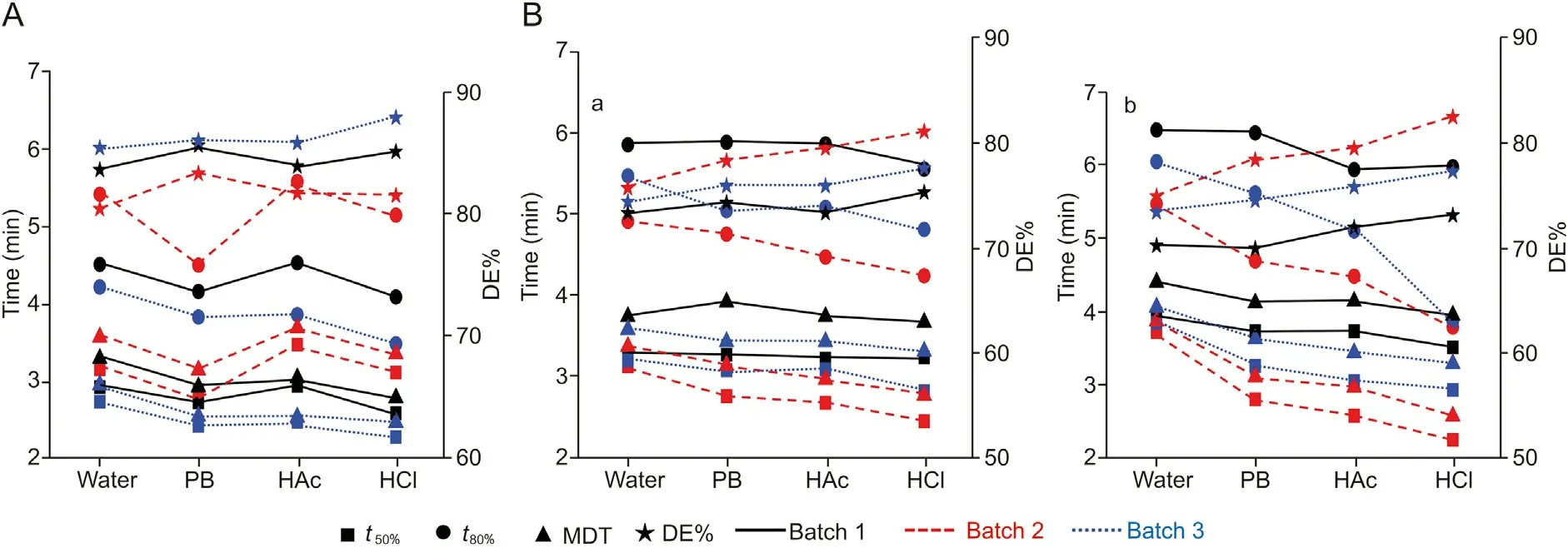
Fig.6. Drug release kinetics parameter derived from the dissolution profiles for different batches of(A)amoxicillin dispersible tablets and(B)amoxicillin and clavulanate potassium tablets (a and b:results for active ingredient amoxicillin and clavulanate potassium respectively)dissolved in four different quality control(QC) dissolution media(water, PB,HAc and HCl). MDT: mean dissolution time; DE%: dissolution efficiency.
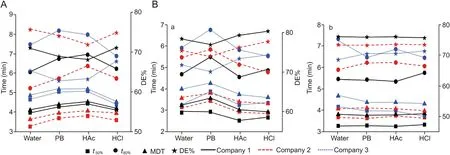
Fig. 7. Drug release kinetics parameter derived from the dissolution profiles in four different QC dissolution media (water, PB, HAc and HCl) for (A) amoxicillin dispersible tablets and (B) amoxicillin and clavulanate potassium tablets (a and b: results for active ingredient amoxicillin and clavulanate potassium respectively) produced by different companies.
Level A correlation(e.g.,a point-to-point relationship),which is the most informative and recommended by the U.S.Food and Drug Administration [39], was employed to establish the relation between Fdis% and Fabs% of active pharmaceutical ingredient of drugs obtained by deconvolution of pharmacokinetic data.A time-scaling factor(Tscale),which was used to normalize the dissolution time for in vitro and in vivo dissolution testing [40,41], was obtained by linear regression of in vitro-in vivo dissolution time relationship at three typical dissolution time points,i.e.,at the release percentages of 30%, 50% and 90%, respectively. The value of the Tscalewas calculated to be 0.054.Thus the dissolution time at a certain in vivo dissolution percentage was normalized by multiplying by 0.054.The calculated Fabs%and Fdis%for amoxicillin dispersible drugs and amoxicillin and clavulanate potassium drugs at different dissolution times were compared. The results are presented in Fig. 8. The linear regression equations between Fabs%and Fdis%are y =0.976x-2.479 (amoxicillin dispersible drugs), y =0.992x-5.756 and y =0.949x +0.734(amoxicillin and clavulanate potassium drugs),with the R2values higher than 0.979. The high R2values of each regression equation indicate that the in vivo and in vitro dissolution are highly correlated in a positive and linear fashion [42]. The results demonstrate the feasibility to predict the drug dissolution in vivo through the dissolution test in vitro by using the proposed analyzer.
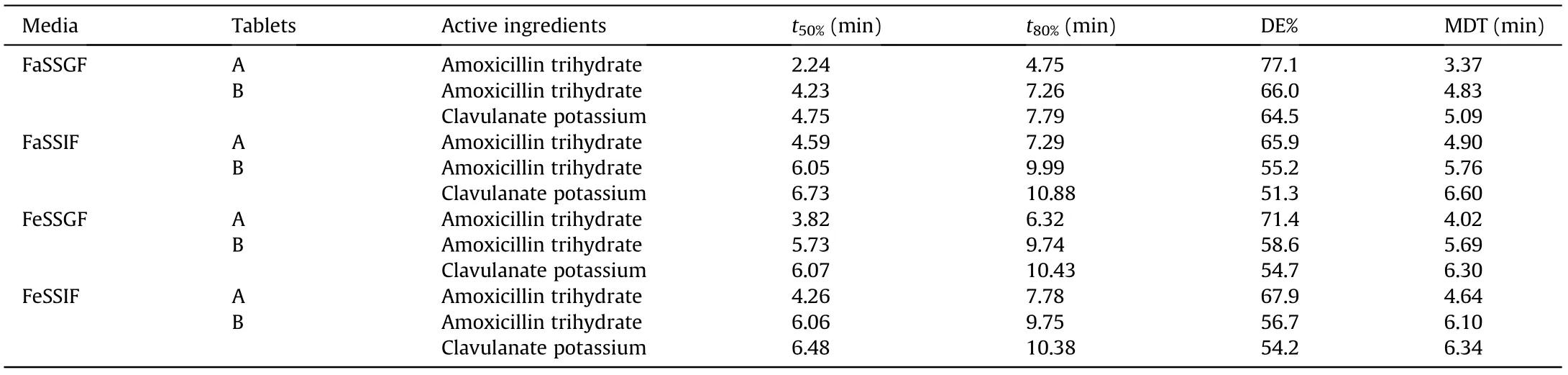
Table 2 Drug release kinetics parameters for amoxicillin immediate-release tablets(A)and amoxicillin and clavulanate potassium tablets(B)using four bio-relevant dissolution media.
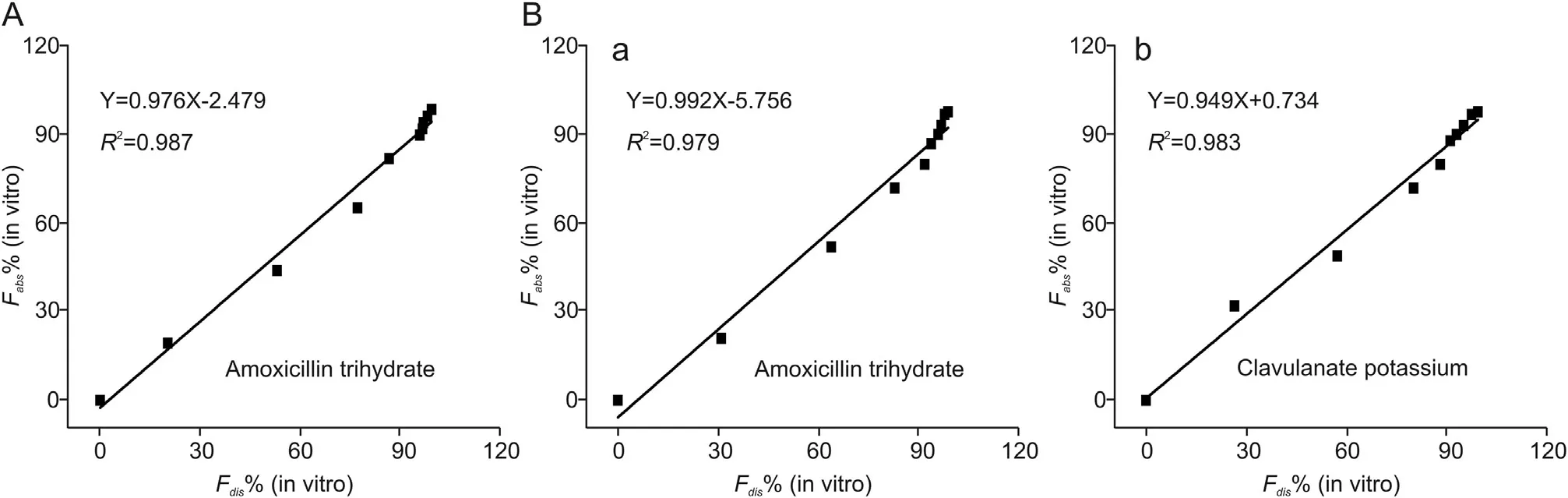
Fig.8. Level A correlation showing the point-to-point relationship of(A)amoxicillin dispersible tablets and(B)amoxicillin and clavulanate potassium tablets(B)(a and b:results for active ingredient amoxicillin and clavulanate potassium respectively)between the fractions of the drug in vivo absorbed and the fraction of drug in vitro dissolution.(Tscale =0.054).
4. Conclusion
In summary, we developed a robust analyzer that could achieve automated on-line dissolution testing of drugs. The device was highly integrated and compacted into a portable device-box with the weight of 10 kg and the size of 400 mm × 300 mm × 250 mm(length×width×height).This is attributed to combining sequential high-temporal sample injection and efficient HSCE separation with a flow-through-cell for drug dissolution via a home-built FGI system,as well as automated controlling the whole procedure of on-line dissolution testing. On-line in vitro dissolutions of both amoxicillin dispersible drugs and fixed dose combination (amoxicillin and clavulanate potassium)drugs were investigated using the proposed analyzer. The performance of the analyzer allowed us to simultaneously and automatically monitor the active pharmaceutical ingredients dissolved from a tablet in the flow-through-cell with high repeatability and accuracy. The analyzer was successfully used for quality control and bio-relevant dissolution testing, as well as the establishment of IVIVC, showing the valuable application in the evaluation and controlling of drug quality.Limited in the capacity of the batteries, the analyzer would be more suitable for dissolution testing of immediate-released drugs. Another shortcoming of the analyzer is that it is not capable of running replicate tests simultaneously.Nevertheless,the proposed portable analyzer is convenient and low-cost, and exhibits some essential merits of high-temporal resolution and high-efficient separation for on-line dissolution testing, and thus will potentially be of applications in the pharmaceutical research and development.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China(Grant No.21775017)and the Natural Science Foundation of Jilin Province, China (Grant No. 20180101174JC). L.Y.would like to thank the support from Jilin Provincial Department of Education and Jilin Provincial Key Laboratory of Micronano Functional Materials (Northeast Normal University, Changchun, China).
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpha.2020.06.001.
杂志排行
Journal of Pharmaceutical Analysis的其它文章
- A simplified LC-MS/MS method for the quantification of the cardiovascular disease biomarker trimethylamine-N-oxide and its precursors
- UHPLC-MS/MS analysis of cAMP and cGMP in rat plasma as potential biomarkers of Yin-Yang disharmony in traditional Chinese medicine
- Liquid chromatography-mass spectrometry method for the quantification of an anti-sclerostin monoclonal antibody in cynomolgus monkey serum
- Plasma-metabolite-based machine learning is a promising diagnostic approach for esophageal squamous cell carcinoma investigation
- Reducing SARS-CoV-2 pathological protein activity with small molecules
- Development of the general chapters of the Chinese Pharmacopoeia 2020 edition: A review
