Viricidal activity of several disinfectants against African swine fever virus
2021-09-10JIANGChenggangSUNYingZHANGFanAIXinFENGXiaoningHUWeiZHANGXianfengZHAODongmingBUZhigaoHEXijun
JIANG Cheng-gang,SUN Ying,ZHANG Fan,AI Xin,FENG Xiao-ning,HU WeiZHANG Xian-feng,ZHAO Dong-mingBU Zhi-gao,HE Xi-jun
1 State Key Laboratory of Veterinary Biotechnology,Harbin Veterinary Research Institute,Chinese Academy of Agricultural Sciences,Harbin 150069,P.R.China
2 National High Containment Laboratory for Animal Diseases Control and Prevention,Harbin 150069,P.R.China
Abstract Prevention of African swine fever,a disease caused by African swine fever virus (ASFV),requires maintenance of high biosecurity standards,which principally relies on disinfection.Finding the perfect disinfectant against ASFV is difficult because of the lack of relevant data.Therefore,we aimed to find the most effective disinfectant and to optimise its concentration as well as contact time to confirm the viricidal effect against ASFV in vitro.We evaluated the viricidal activity of three concentrations each of six common disinfectants against ASFV using immersion disinfection assay (IDA) and spray disinfection assay (SDA);the concentrations of these disinfectants at which complete viral inactivation occurred were almost same as the manufacturer-recommended concentrations,but the exposure times for viral inactivation are different.The following disinfectants (assay:concentration,exposure time) showed complete inactivation:iodine and acid mixed solution(IDA/SDA:0.5%,10 min);compound potassium peroxymonosulfate (IDA:0.25%,30 min;SDA:0.25%,60 min);citric acid(IDA:0.25%,60 min;SDA:0.5%,60 min);sodium dichloroisocyanurate (IDA:0.125%,60 min;SDA:0.25%,60 min);and glutaral ang deciquam (IDA/SDA:0.2%,60 min);and deciquam (IDA/SDA:0.5%,60 min).However,in the presence of organic material contamination,disinfectants did not show a marked inactivation effect.Therefore,disinfection procedures should be performed in two steps:thorough mechanical cleaning followed by application of disinfectant.In conclusion,all the tested disinfectants can inactivate ASFV;these can be used as alternative disinfectants to enhance biosecurity.
Keywords:disinfection,African swine fever virus (ASFV),virucidal effects,biosecurity
1.Introduction
African swine fever (ASF) is an infectious disease caused byAfrican swine fever virus(ASFV),which affects domestic and wild pigs of all breeds and ages.Since its identification in Kenya in 1921,ASF has remained endemic in Africa(Cwynaret al.2019).Between the late 1950s and early 1980s,ASF emerged in Europe,Russia,and South America,but it was mostly eradicated by the mid-1990s.In 2007,a highly virulent genotype II ASFV strain emerged in the Caucasus Region and subsequently spread into the Russian Federation and Europe,where it continued to circulate and spread.In August 2018,ASF was reported for the first time in China;ASF has especially affected China,which is the world’s largest pork producer,producing about 50% of the world’s pork supply,and consumer (Zhaoet al.2019).It is currently one of the most important and serious diseases of pigs mainly because of the enormous sanitary and socioeconomic consequences (Danzettaet al.2020).
ASFV is the sole member of the family Asfarviridae,and it is the only known DNA arbovirus (Dixonet al.2013).Depending on the strain,ASFV has a large (170-193 kb)double-stranded DNA genome containing 151-167 genes,which are involved in viral replication and assembly as well as in modulating host cellular functions and immune evasion.Wild pigs such as warthogs and bush pigs,and soft ticks of theOrnithodorosspecies are the natural hosts of ASFV and they can be persistently infected with no disease signs (Costardet al.2013).First ASFV isolate,Pig/Heilongjiang/2018 (Pig/HLJ/18),was isolated from a pig sample from an ASF outbreak farm in China (Zhaoet al.2019).All virus-inoculated pigs died between 6-9 days post-inoculation (p.i.),and the contact pigs died between 13-14 days post-contact (p.c.).These results indicate that Pig/HLJ/18 is highly virulent and transmissible in domestic pigs.It emphasizes the need to control and eradicate ASF in China.
Currently control measures include culling of infected pigs and maintenance of high biosecurity standards,which principally relies on disinfection.Disinfection and the proper use of disinfectants is the basic and most important aspect of biosecurity.It is based on inactivating pathogenic microorganisms in objects or surfaces,and is one of the effective ways to cut off the transmission of infectious diseases.Characteristics of the ideal disinfectant are as follows:fast action,durability,non-toxicity,and minimal risk of environmental contamination (Juszkiewiczet al.2019).Incorrect definition of activity parameters (i.e.,concentration,contact time,and range) may lead to the improper use and ineffectiveness of disinfectant products.It is difficult to find the perfect disinfectant against ASFV because of the lack of data regarding disinfectants.The aim of this study was to find the most effective disinfectant and to optimise its concentration as well as contact time to confirm the virucidal effect against ASFVin vitro.
2.Materials and methods
Primary porcine alveolar macrophages (PAMs) were collected from 30-40-day-old specific-pathogen-free pigs,and the cells were maintained in 10% FBS RPMI 1640 medium (Thermo Scientific,USA) at 37°C with 5% CO2incubator.An ASFV strain,HLJ/18-DP148R-del,was constructed and stored by our laboratory (Chenet al.2020).PAM cells were infected with diluted ASFV to determine the 50% cell culture infectious dose (CCID50) titre of residual virus using the Spearmann-Karber endpoint titration method(Hierholzer and Killington 1996).
Six commercially available disinfectants were selected such that the active substances were representative of the main groups of chemical compounds containing,as active substances,respectively:citric acid (94 g per 100 g),compound potassium peroxymonosulfate(effective chlorine≥10%),glutaral (5%) ang deciquam (5%),deciquam (10%),sodium dichloroisocyanurate (effective chlorine≥10%),iodine (1.5%),and phosphoric acid (15%).
Three concentrations of each disinfectant were used:citric acid,1,0.5 and 0.25%;compound potassium peroxymonosulfate,1,0.5 and 0.25%;glutaral ang deciquam,0.4,0.2 and 0.1%;deciquam,1,0.5 and 0.25%;sodium dichloroisocyanurate,1,0.5 and 0.25%;and iodine and acid mixed solution,2,1 and 0.5%.All disinfectants were diluted in 400 ppm calcium carbonate to simulate hard water conditions.All disinfectants were neutralised with Dey-Engley neutralising broth (Sigma-Aldrich,USA).
By immersion disinfection assay,8 µL of RPMI-1640 medium containing 100 CCID50ASFV was incubated with 72 µL disinfectant for 10,30,and 60 min at room temperature,and subsequently,neutraliser was added to this mixture.After 10 min,the mixture was diluted to 800 µL with RPMI-1640 medium+1% FBS,and 200 µL of the mixture was added to basic overgrown PAM cells in a 96-well plate at 37°C with 5% CO2.By spraying disinfection assay,the membrane pieces (1 cm×1 cm triangle) were inoculated with 15 µL of ASFV,and immediately sprayed twice with disinfectants from a distance of about 20 cm;10,30,or 60 min later,the pieces were placed in 800 µL RPMI-1640 medium+1% FBS,and infected PAM cells in 96-well plates at 37°C with 5% CO2.Cell status and fluorescence were observed every 24 h.In addition,negative controls without virus,positive controls with virus,and neutralisation controls were included in each experiment.
3.Results
In the positive control group,over 90% of cells showed green fluorescence (Fig.1);however,in the negative control group,no green fluorescence was observed (Fig.2).Therefore,absence of green fluorescence in the experimental group indicated that the disinfectant completely inactivated the virus.
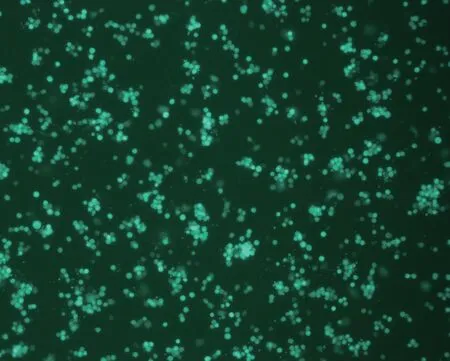
Fig.1 Green fluorescence observed in the positive control group (100×).

Fig.2 Absence of green fluorescence in the negative control group (100×).
In vitroimmersion disinfection assay showed the following results (fluorescence-based;Table 1):citric acid can completely inactivate ASFV at concentrations of 1,0.5 and 0.25% after incubation for 10,30 and 60 min,respectively;glutaral ang deciquam can completely inactivate ASFV at concentrations of 0.4 and 0.2% after incubation for 10 and 60 min,respectively,but not at a concentration of 0.1%even after incubating for 60 min;compound potassium peroxymonosulfate can completely inactivate ASFV at concentrations of 1,0.5 and 0.25% after incubation for 10,30 and 60 min,respectively;sodium dichloroisocyanurate can completely inactivate ASFV at concentrations of 0.5,0.25 and 0.125% after incubation for 10,30 and 60 min,respectively;deciquam solution can completely inactivate ASFV at concentrations of 1 and 0.5% after incubation for 30 and 60 min,respectively,but not at a concentration even after incubating for 0.125% until 60 min;iodine and acid mixed solution can completely inactivate ASFV at all concentrations,i.e.,2,1 and 0.5%,after incubation for 10 min.
In vitrospraying disinfection assay showed the following results (fluorescence-based;Table 2):citric acid can completely inactivate ASFV at concentrations of 1 and 0.5% after incubation for 30 and 60 min,respectively,but not at a concentration of 0.25% even after incubating for 60 min;glutaral ang deciquam can completely inactivate ASFV at concentrations of 0.4 and 0.2% after incubation for 30 and 60 min,respectively,but not at a concentration of 0.1% even after incubating 60 min;compound potassium peroxymonosulfate can completely inactivate ASFV at concentrations of 1,0.5 and 0.25% after incubation for 10,30 and 60 min,respectively;sodium dichloroisocyanurate can completely inactivate ASFV at concentrations of 0.5 and 0.25% after incubation for 10 and 60 min,respectively,but not at a concentration of 0.125% even after incubating for 60 min;deciquam can completely inactivate ASFV at concentrations of both 1 and 0.5% after incubation for 60 min but not at a concentration of 0.125% even after incubating for 60 min;and iodine and acid mixed solution can completely inactivate ASFV at concentrations of 2,1 and 0.5% after incubation for 10,10 and 30 min,respectively.
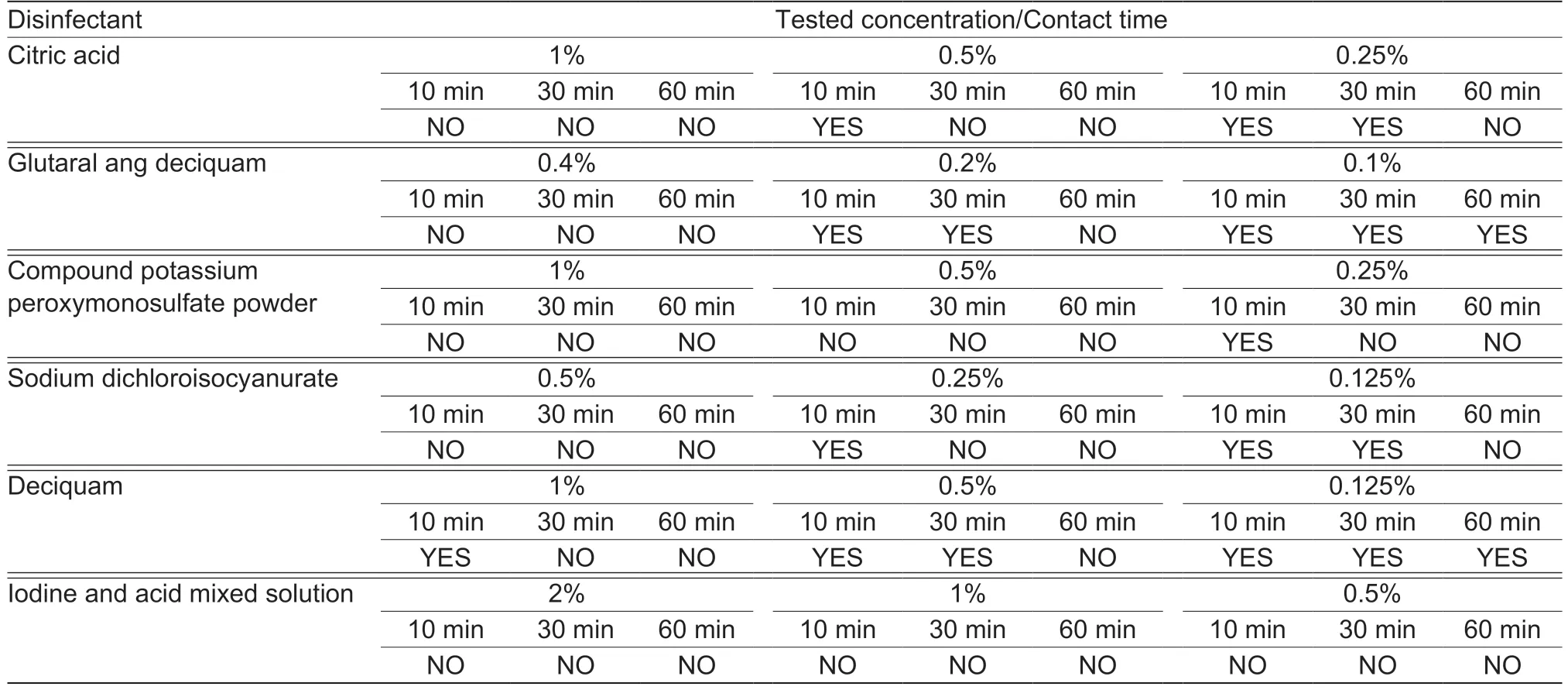
Table 1 Results of immersion disinfection assay
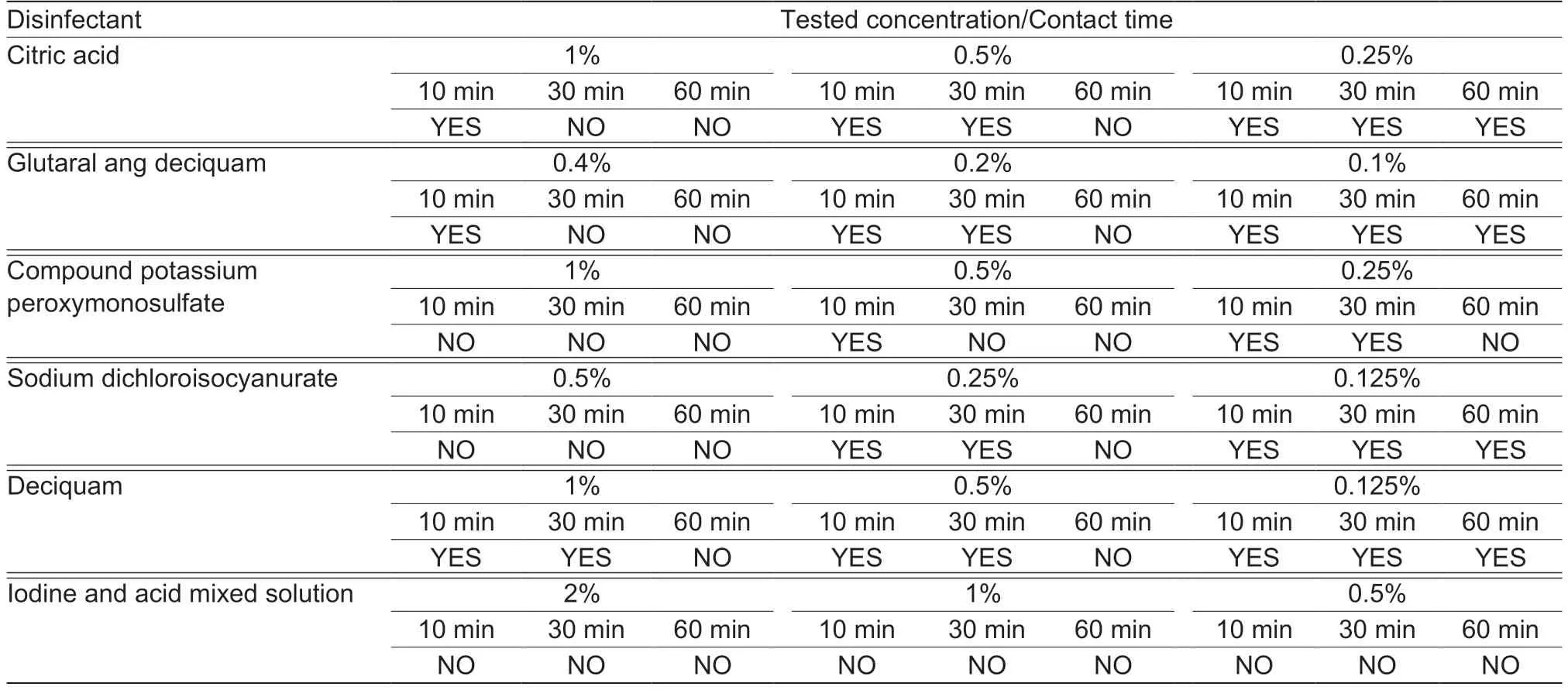
Table 2 Results of spraying disinfection assay
4.Discussion
In this study,we have demonstrated a fluorescencebasedin vitromethodology to determine the virucidal activity of disinfectants against ASFV.DP148Rgene was demonstrated to be an early transcription gene of ASFV (Reiset al.2017),and less possible to be a viral structure gene,importantly,the deletion of DP148R did not affect the virulence of ASFV in pigs (Chenet al.2020).So HLJ/18-DP148R-del may be equivalent to its parental HLJ/18 isolated from the infected pigs (Zhaoet al.2019).The replication of HLJ/18-DP148R-del can be reflected by the expression of eGFP because the genome of HLJ/18-DP148R-del contains eGFP expression box which is driven by viral p72 promoter of ASFV,which is convenient to detect ASFV infection or not.We found that iodine and acid mixed solution showed the best activity against ASFV among the six disinfectants tested;immersion and spraying disinfection assays showed that it can completely inactivate ASFV in 10 min at 0.5% concentration.Adding the surfactants,phosphoric acid and sulphuric acid to iodine facilitate its dispersion in water,decrease its volatility,increase its solubility and permeability,thereby greatly enhancing the active groups in iodine-oxidised microorganism protein,and combined with the amino group of the protein to denature it,making the inactivated effect stronger (McDonnell and Russell 1999).Compound potassium peroxymonosulfate was also found to effectively inactive ASFV as shown by immersion disinfection assay (0.25%,30 min) and spraying disinfection assay (0.25%,60 min).Compound potassium peroxymonosulfate continuously produces hypochlorous acid,a new ecological oxygen that oxidises and chlorinates pathogens in water through a chain reaction,interferes with the DNA and RNA synthesis of pathogens,coagulates and denatures proteins of pathogens,interferes with the activity of the enzyme system,increases the permeability of the cell membrane,and causing the loss of cell integrity,which leads to the breakdown of pathogens (Dunowskaet al.2005)(http://www.antecint.co.uk/main/virkons.htm).Furthermore,citric acid,sodium dichloroisocyanurate,and glutaral ang deciquam have similar effects against ASFV.Furthermore,deciquam can inactivate ASFV at 0.5% concentration in 60 min as shown by immersion or spraying disinfection assay.
ASFV is an enveloped virus that is more easily inactivated than is a non-enveloped virus by chemical disinfectants.In addition,enveloped viruses are less stable outside their hosts (Boone and Gerba 2007).In this study,ASFV was inactivated by all six commonly used chemical disinfectants,but at different concentrations and contact times.This test was conducted under ideal conditions.If ASFV was accompanied by materials,such as manure,bedding,straw,and feedstuffs,it will be resistant to chemical disinfectants.Spraying or soaking contaminated materials rich in protein with disinfectant is ineffective because ASFV can withstand fairly extensive pH changes (Penrithet al.2009).Dailydisinfection procedures should be performed in two steps:thorough mechanical cleaning followed by application of disinfectant.Concentrations of different disinfectants,which were effectively inactive ASFV in this test,must extend the exposure time for ASFV inactivation during an actual disinfection programme.
5.Conclusion
Six common disinfectants can inactivate ASFV in the absence of organic materials and can be used as different alternative disinfectants,especially to control ASFV,which contaminates animal farms.Results showed the recommended disinfectants (assay:optimized concentration,exposure time) for complete inactivating ASFV:iodine and acid mixed solution (IDA/SDA:0.5%,10 min);compound potassium peroxymonosulfate (IDA:0.25%,30 min;SDA:0.25%,60 min);citric acid (IDA:0.25%,60 min;SDA:0.5%,60 min);sodium dichloroisocyanurate (IDA:0.125%,60 min;SDA:0.25%,60 min);and glutaral ang deciquam (IDA/SDA:0.2%,60 min);and deciquam (IDA/SDA:0.5%,60 min).
Acknowledgements
This work was supported by the National Key R&D Program of China (2018YFC1200600),the grant from the State Key Laboratory of Veterinary Biotechnology Program(SKLVBP201801),and the National Science and Technology Major Project of China (2018ZX10734401-018-002).
Declaration of competing interest
The authors declare that they have no conflict of interest.
Ethical approval
All experiments with live ASFV were conducted within the enhanced biosafety level 3 (P3+) facilities in the Harbin Veterinary Research Institute (HVRI) of the Chinese Academy of Agricultural Sciences approved by the Ministry of Agriculture and Rural Affairs and China National Accreditation Service for Conformity Assessment.
杂志排行
Journal of Integrative Agriculture的其它文章
- Errata regarding previously published articles
- Application of methyl jasmonate postharvest maintains the quality of Nanguo pears by regulating mitochondrial energy metabolism
- Melatonin treatment induces chilling tolerance by regulating the contents of polyamine,γ-aminobutyric acid,and proline in cucumber fruit
- Linking changes in the soil microbial community to C and N dynamics during crop residue decomposition
- Modification of total and phosphorus mineralizing bacterial communities associated with Zea mays L.through plant development and fertilization regimes
- Yield performance and optimal nitrogen and phosphorus application rates in wheat and faba bean intercropping
