Non-invasive tests for predicting liver outcomes in chronic hepatitis C patients: A systematic review and meta-analysis
2021-09-03TanatYongpisarnChanatthaThimphitthayaPassisdLaoveeravatNichaWongjarupongRoongruedeeChaiteerakij
Tanat Yongpisarn, Chanattha Thimphitthaya, Passisd Laoveeravat, Nicha Wongjarupong, Roongruedee Chaiteerakij
Tanat Yongpisarn, Chanattha Thimphitthaya, Division of Gastroenterology, Department of Medicine, Faculty of Medicine, Chulalongkorn University and King Chulalongkorn Memorial Hospital, Thai Red Cross Society, Bangkok, Bangkok 10330, Thailand
Passisd Laoveeravat, Division of Digestive Diseases and Nutrition, Department of Medicine, University of Kentucky, Lexington, KY 40536, United States
Nicha Wongjarupong, Department of Internal Medicine, University of Minnesota, Minneapolis, MN 55455, United States
Roongruedee Chaiteerakij, Division of Gastroenterology, Department of Medicine, Faculty of Medicine, Chulalongkorn University and King Chulalongkorn Memorial Hospital, Thai Red Cross Society, Bangkok 10330, Thailand
Roongruedee Chaiteerakij, Center of Excellence for Innovation and Endoscopy in Gastrointestinal Oncology, Faculty of Medicine, Chulalongkorn University, Bangkok 10330, Thailand
Abstract BACKGROUND Liver fibrosis leads to liver-related events in patients with chronic hepatitis C (CHC) infection.Although non-invasive tests (NITs) are critical to early detection of the development of liver fibrosis, the prognostic role of NITs remains unclear due to the limited types of NITs and liver outcomes explored in previous studies.AIM To determine the prognostic value of NITs for risk stratification in CHC patients.METHODS The protocol was registered in PROSPERO (International Prospective Register of Systematic Reviews; no.CRD42019128176).The systematic review was performed in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.Search was performed using MEDLINE and EMBASE databases under a timeframe from the inception of the databases through February 25, 2020.We restricted our search to CHC cohort studies reporting an association between liver fibrosis assessed by NITs and the development of hepatocellular carcinoma, decompensation, or mortality.Pooled hazard ratios (HR) and area under the receiver operating characteristic (AUROC) for each NIT were estimated using a random effects model.Subgroup analyses were performed for NITs assessed at pre-treatment or post-treatment with sustained virologic response (SVR), treatment with either pegylated interferon and ribavirin or direct acting antiviral, Eastern or Western countries, and different cutoff points.RESULTS The present meta-analysis included 29 cohort studies, enrolling 69339 CHC patients.Fibrosis-4 (FIB-4) index, aspartate aminotransferase to platelet ratio (APRI) score, and liver stiffness measurement (LSM) were found to have hepatocellular carcinoma predictive potential with pooled adjusted HRs of 2.48 [95% confidence interval (CI): 1.91-3.23, I2 = 96%], 4.24 (95%CI: 2.15-8.38, I2 = 20%) and 7.90 (95%CI: 3.98-15.68, I2 = 52%) and AUROCs of 0.81 (95%CI: 0.73-0.89, I2 = 77%), 0.81 (95%CI: 0.75-0.87, I2 = 68%), and 0.79 (95%CI: 0.63-0.96, I2 = 90%), respectively.Pooled adjusted HR with a pre-treatment FIB-4 cutoff of 3.25 was 3.22 (95%CI: 2.32-4.47, I2 = 80%).Pooled adjusted HRs for post-treatment with SVR FIB-4, APRI, and LSM were 3.01 (95%CI: 0.32-28.61, I2 = 89%), 9.88 (95%CI: 2.21-44.17, I2 = 24%), and 6.33 (95%CI: 2.57-15.59, I2 = 17%), respectively.Pooled adjusted HRs for LSM in patients with SVR following direct acting antiviral therapy was 5.55 (95%CI: 1.47-21.02, I2 = 36%).Pooled AUROCs for post-treatment with SVR FIB-4 and LSM were 0.75 (95%CI: 0.55-0.95, I2 = 88%) and 0.84 (95%CI: 0.66-1.03, I2 = 88%), respectively.Additionally, FIB-4 and LSM were associated with overall mortality, with pooled adjusted HRs of 2.07 (95%CI: 1.49-2.88, I2 = 27%) and 4.04 (95%CI: 2.40-6.80, I2 = 63%), respectively.CONCLUSION FIB-4, APRI, and LSM showed potential for risk stratification in CHC patients.Cutoff levels need further validation.
Key Words: Non-invasive tests; Prognosis; Hepatitis C virus; Hepatocellular carcinoma; Mortality; Liver-related outcomes
INTRODUCTION
Chronic hepatitis C (CHC) infection can lead to the development of liver fibrosis and cirrhosis that are commonly associated with hepatocellular carcinoma (HCC), other liver-related events (LREs), and mortality.Liver biopsy is considered the gold standard for evaluating liver fibrosis in patients with chronic liver disease.Since the introduction of non-invasive tests (NITs), biopsy use has substantially declined.Currently available NITs for liver fibrosis assessment include direct and indirect serum markers and radiologic examination such as liver stiffness measurement (LSM).According to the 2018 European Association for the Study of the Liver guidelines, the degree of liver fibrosis should be assessed by NITs in CHC patients prior to any treatment[1].The degree of liver fibrosis determines optimal treatment regimen and whether the patient requires post-treatment monitoring of HCC development.NITs are also recommended for monitoring untreated CHC patients every 1 to 2 years[2].
Although serum markers and LSM have been shown to identify accurately patients with cirrhosis (F4) and patients without fibrosis (F0), their ability to stage intermediate degrees of fibrosis and post-treatment residual fibrosis is suboptimal[2,3].The difficulties in the prediction of significant or advanced fibrosis without histologic confirmation has made risk stratification problematic for some CHC patients.For instance, the decision to pursue HCC surveillance following successful treatment of hepatitis C virus (HCV) infection [i.e.sustained virologic response (SVR)] is controversial for patients with advanced fibrosis (F3)[2,4].
Previous meta-analyses have evidenced the potential of NITs in determining prognosis.However, these syntheses included studies on chronic liver diseases from various etiologies and did not comprehensively explore all liver-related outcomes[5,6].Types of NITs investigated in these meta-analyses were also limited.In this present review, we provided an updated systematic review and meta-analysis to assess the importance of validated NITs in risk stratification specific to CHC patients.
MATERIALS AND METHODS
Literature search
The protocol was registered in PROSPERO (International Prospective Register of Systematic Reviews; no.CRD42019128176).The systematic review was performed in accordance with Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines[7].Search was performed using MEDLINE and EMBASE databases from the inception of databases to February 25, 2020.The NITs for hepatic fibrosis included in our review were retrieved from the European Association for the Study of the Liver, Asociación Latinoamericana para el Estudio del Hígado Clinical Practice Guidelines[1].The list of serum biomarkers and respective formulae are provided in Supplemental Table 1.In addition to the list of NITs, the terms prognosis, decompensation, hepatocellular cancer, chronic hepatitis C, and their related terms were selected as keywords.The details of the search strategy are provided in Supplemental Table 2.We restricted our search to cohort studies.Publications in the reference list of our included studies, publications that cited the included studies, and publications that were included in recent meta-analyses[8,9] of NITs and chronic liver diseases were also reviewed.
Study selection
Two reviewers (TY and CT) independently searched for studies on the prognosis of CHC patients based on non-invasive staging of liver fibrosis.Title and abstract of the studies were initially screened.The full-text of these studies were then independently assessed for eligibility by the two reviewers.Cohort studies that met the following criteria were included: (1) NITs documented and used to identify CHC patients who had a risk of developing LREs including hepatic decompensation, HCC, and/or mortality.Hepatic decompensation (HD) was defined as the development of variceal bleeding, hepatic encephalopathy, ascites, spontaneous bacterial peritonitis, jaundice, and/or hepatorenal syndrome; (2) Patients were free of HCC and HD at enrollment; (3) Development of HD, HCC and mortality were assessed; and (4) Outcomes of interest were reported by hazard ratio (HR), relative risk, or area under the receiver operating characteristic (AUROC).Whereas studies of any size or language were included, the following studies were excluded: (1) Case-control studies, cross-sectional studies, case series, and conference abstracts; and (2) Trials enrolling patients with no evidence of HCV infection or when more than 10% of the patients were co-infected with HBV.Publications detailing the same patient cohorts but reporting different outcomes of interest were selected for separate analysis.When publications from the same cohort described the same outcomes, the study with the most comprehensive data or with the longest follow-up was selected for each outcome[10].Any disagreement over study eligibility between reviewers was resolved through discussion with a third reviewer (PL).
Data extraction
A standardized form was used to extract data from the selected papers.Data included study characteristics (primary author, country, publication year, patient enrollment period, duration of follow-up), patient characteristics (age, sex, co-infection, baseline levels of NITs, fibrosis stages, HCV treatment regimen, response), method of NITs, endpoint (HD, HCC, overall and liver-related mortality), HR and AUROCs with 95% confidence intervals (95%CI), and control variables used for the adjusted analysis.Two reviewers (TY and CT) extracted the data independently, discrepancies were identified and discussed with a third reviewer (PL).Any missing data from the publications were requested from the study authors.
Risk of bias
A quality assessment of prognostic studies was performed independently by TY and CT using the Quality In Prognosis Studies tool[10].Any disagreements between the reviewers over the risk of bias in particular studies were resolvedviadiscussion with a third reviewer (PL).
Statistical analysis
Primary analysis assessed the performance of NITs in the prediction of LRE development in CHC patients.The analysis of each outcome was computed using a random-effects model.Since relative risk was provided by only one study[11], it was not included in our meta-analysis.Inverse variance method was used to pool the results.Unadjusted and adjusted HRs were pooled separately.Additionally, the significance of each NIT’s prognostic value was assessedvsthe random value (mean AUROC of each NIT was compared with 0.50 or the “random” value representing the absence of prognostic value).We then pooled the results, and 0.50 was added back to illustrate the overall prognostic value of each NIT.The AUROCs of different NITs were then compared usingt-tests to identify any statistical difference in terms of prognostic ability.Subgroup analyses based on timing of liver fibrosis assessment (before or after HCV treatment) were performed when possible.Heterogeneity between studies was considered whenI2value was greater than 50%.Publication bias was first evaluated by constructing funnel plots.Egger's linear regression test was also performed due to possible bias ascertained from funnel plots.All analyses were conducted using Review Manager (RevMan) [Computer program], Version 5.3.Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014, and ProMeta (Version 3) [Computer software] (Internovi, Cesena, Italy).
RESULTS
Study selection
After removing duplicate publications, 17248 papers were identified and screened by title and abstract.Of these, 104 full articles met our predefined selection criteria and were further examined.We further excluded 65 publications due to the following reasons: Non-relevant outcomes (n= 32), outcomes not reported as risk ratio (n= 13), patients meeting our exclusion criteria,e.g., prior history of HCC (n= 10), studies of the same patient cohorts (n= 5), and NITs being used as diagnostic tests for HCC or HD (n= 5) (Figure 1).
Among the 39 cohort studies matching our selection criteria, 29 studies (69339 HCVinfected patients) were selected for quantitative analysis, with the 10 remaining studies slated only for qualitative analysis.
These 39 included studies enrolled a total of 77920 participants between 1990 and 2015.Seventeen and 22 studies were conducted in Western[12-28] and Asian countries[11,29-49], respectively (Table 1, Supplemental Table 3).

Table 1 Characteristics of the cohort studies included in the systematic review
The performance of the Fibrosis-4 (FIB-4) index, aspartate aminotransferase to platelet ratio (APRI) score, and LSM tests for the prediction of LREs and mortality were characterized in 20, 11, and 19 studies, respectively.LSM was mainly performed by ultrasound-based transient elastography (TE), except in two studies that used either magnetic resonance elastography (MRE)[30], or 2D-shear wave elastography (2DSWE)[29].
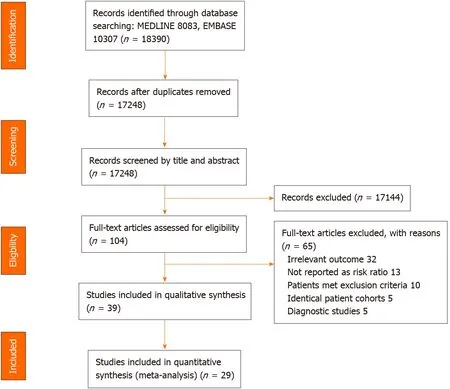
Figure 1 Flow diagram of search methodology and selection process.
The primary outcomes of interest were HCC, overall mortality, and liver-related mortality in 21[11,13-15,29-44,49], 12[3,12,17-24,38,46], and 10[16,18,20,21,25,27,28,45,47,48] studies, respectively.Twelve studies selected HD or a compound of LREs as relevant outcome(s)[12,17-21,23,24,26,38,43,46].Characteristics of all the studies are summarized in Table 1 and Supplemental Table 3.
Eleven studies enrolled patient cohorts with HCV and human immunodeficiency virus co-infection[12,16,18,21,22,24-28,45].Fifteen reports included only patients who were successfully treated,i.e.having SVR[13,14,23,29-31,33,36,38-40,44,46,47,49], while two studies enrolled only patients with cirrhosis[13,15].All studies had a mean or median follow-up time of at least 1 year.
FIB-4, APRI, and LSM were among the most extensively explored NITs (Table 2).We did not conduct quantitative analysis using other NITs due to their very limited usage (n= 1 for Forns index[37] and Fibrotest[22],n= 0 for other NITs).
The included studies were mostly rated as low risk of bias (n= 27)[11-14,16,19-23,25,26,28-30,32,33,35-39,42-44,46,48] (Supplementary Table 4, Supplementary Figure 1).However, five studies were rated as high risk of bias because of concerns about selective reporting of multivariate analysis and other biases[34,40,41,45,49].Only 13 studies provided the number of patients lost to follow-up[13,14,17,20-22,24,28,32,36,37,44,45].The agreement between the two reviewers’ assessment was excellent (93%).

Table 2 Pooled unadjusted and adjusted hazard ratios of pre- and post-treatment fibrosis-4 index, aspartate aminotransferase to platelet ratio index, liver stiffness measurement for the prediction of hepatocellular carcinoma development
Association between NITs and HCC risk
Among NITs included in the present analysis, FIB-4 score was the most studied NIT for its role in HCC prediction.Eleven studies including 1891 HCC cases examined the relationship between FIB-4 values and HCC development[13,29,30,33,38-42,44,49].The FIB-4 cutoffs selected in these studies ranged from 2.5 to 4.5.All these studies reported a significant positive association between high FIB-4 values and risk of HCC development, with pooled unadjusted and adjusted HRs of 5.17 (95%CI: 4.03-6.63,I2= 76%) and 2.48 (95%CI: 1.91-3.23,I2= 96%), respectively (Figure 2A).
Five studies totaling 169 HCC cases evaluated the prognostic value of APRI and found a statistically significant positive association between high APRI values and HCC occurrence[31,33,36,40,41].The APRI cutoffs used in these studies ranged from 0.5 to 2.0.The overall pooled unadjusted and adjusted HRs were 5.27 (95%CI: 2.34-11.83,I2= 91%) and 4.24 (95%CI: 2.15-8.38,I2= 20%), respectively (Figure 2B).
Eight studies with 387 HCC cases investigated the association between LSM and HCC risk[14,15,29,30,32,34,35,38].The LSM cutoffs chosen for each study were all unique and ranged from 3.75 to 30.Consistent with FIB-4 score and APRI results, the overall pooled unadjusted and adjusted HRs were 9.45 (95%CI: 4.49-19.92,I2= 70%) and 7.90 (95%CI: 3.98-15.68,I2= 52%), respectively (Figure 2C).
Subgroup analyses were performed for NITs assessed at pre-treatment and posttreatment with SVR.Pooled adjusted HRs for pre-treatment FIB-4 and LSM were 3.20 (95%CI: 1.77-5.80,I2= 97%) and 3.76 (95%CI: 1.77-8.02,I2= 7%), respectively.Pooled adjusted HRs for post-treatment with SVR FIB-4, APRI, and LSM were 3.01 (95%CI: 0.32-28.61,I2= 89%), 9.88 (95%CI: 2.21-44.16,I2= 24%), and 6.33 (95%CI: 2.57-15.59,I2= 17%), respectively (Figure 2).The prognostic ability of these NITs remains valid even after the introduction of direct-acting antiviral (DAA) therapy.Pooled unadjusted and adjusted HRs for LSM in patients with SVR following DAA therapy were 6.80 (95%CI: 3.54-13.05,I2= 0%) and 5.55 (95%CI: 1.47-21.02,I2= 36%), respectively (Supplementary Figure 2).
To determine the optimal cutoff for HCC prediction, we pooled the results using a pre-treatment FIB-4 cutoff of 3.25 as this cutoff was applied in four studies, accounting for over 51360 CHC patients (Supplementary Figure 3).We found that the pooled, unadjusted and adjusted HRs were 4.79 (95%CI: 3.58-6.42,I2= 85%) and 3.22 (95%CI: 2.32-4.47,I2= 80%), respectively, for predicting HCC development.
Given the high heterogeneity of the analysis of pre-treatment FIB-4, we performed subgroup analyses by location of study.We found that, in the subgroup of Asian countries, pooled unadjusted and adjusted HRs of 4.91 (95%CI: 3.60-6.70,I2= 18%) and 3.12 (95%CI: 1.31-7.42,I2= 87%) for the pre-treatment FIB-4 and HCC development (Supplementary Figure 4).TheI2of pooled unadjusted HR decreased from 76% to 18%, while theI2of pooled adjusted HR slightly decreased from 97% to 87%.We hypothesized that the remaining high heterogeneity stemmed from the variety of FIB-4 cutoff used in the different studies.
Figure 3 shows the performance of NITs for HCC prediction.FIB-4 score, APRI, and LSM was significantly greater than random (AUROC = 0.5), with pooled AUROCs of 0.81 (95%CI: 0.73-0.89,I2= 77%), 0.81 (95%CI: 0.75-0.87,I2= 68%), and 0.79 (95%CI: 0.63-0.96,I2= 90%), respectively.The pooled AUROCs of FIB-4 and APRI were both statistically higher than that of the LSM,P< 0.0001 for both, respectively.


We further analyzed the prognostic values of NITs before and after HCV treatment.For the pre-treatment period, the pooled AUROC of FIB-4 score was significantly greater compared to APRI (0.88, (95%CI: 0.83-0.92,I2= 0%)vs0.77, (95%CI: 0.70-0.84,I2= 36%),P< 0.0001).For NITs assessed at post-treatment among patients with SVR, the pooled AUROC of LSM was 0.84 (95%CI: 0.66-1.03,I2= 88%), which was statistically higher than that of FIB-4 (pooled AUROC 0.75, 95%CI: 0.55-0.95,I2= 88%),P< 0.0001.The pooled AUROC of pre-treatment LSM and post-treatment APRI score was not estimated due to the limited number of studies (n= 1 each).
Association between NITs and overall mortality
Four studies identifying 823 deaths among 3321 patients reported a significant positive association between FIB-4 score and overall mortality with pooled unadjusted and adjusted HRs of 3.06 (95%CI: 1.38-6.67,I2= 90%) and 2.07 (95%CI: 1.49-2.88,I2= 27%), respectively (Supplementary Figure 5)[16,45,47,48].Likewise, a significant positive association between LSM and overall mortality was reported from four studies containing 3663 patients with 368 deaths[20,21,25,28], with pooled unadjusted and adjusted HRs of 5.52 (95%CI: 2.81-10.85,I2= 74%) and 4.04 (95%CI: 2.40-6.80,I2= 63%), respectively (Supplementary Figure 6).

Figure 2 Unadjusted and adjusted hazard ratios of fibrosis-4 index (A), aspartate aminotransferase to platelet ratio score (B), liver stiffness measurement (C), and hepatocellular carcinoma risk.

Figure 3 Forest plots showing hepatocellular carcinoma predictive performance vs random of fibrosis-4 (A), random of aspartate aminotransferase to platelet ratio (B), and random of liver stiffness measurement (C).
The pooled HR and AUROC of APRI performance for the prediction of mortality was not estimated because only one study was included in this meta-analysis.The AUROCs for predicting overall mortality reported in individual studies are shown in Table 3.
Liver-related mortality, decompensation of cirrhosis, and composite outcomes
Due to the broad definitions of HD and LRE outcomes, we did not perform a metaanalysis on these outcomes.However, taken individually, any NIT showed statistically significant positive associations and predictive values for their respective outcomes.The HRs and AUROCs of NITs and liver-related outcomes are summarized in Tables 4 and 5[12,16-21,23-28,38,43,45-48].
Publication bias
Publication bias was assessed through Deeks funnel plots for unadjusted and adjusted HRs of NITs and LREs.The distribution of studies was symmetrical for all analyses, except for adjusted HRs of FIB-4, APRI, LSM, and HCC development, which showed asymmetry (Figure 4).Egger’s regression asymmetry test detected publication bias in adjusted HRs of FIB-4 (P< 0.001) but not in HRs of APRI or LSM (P= 0.081 and 0.097, respectively).We found that five out of eight studies that reported an adjusted HR for FIB-4 score each had more than 1000 participants[33,39-41,49].When only studies with > 1000 participants were selected for the subgroup analysis of adjusted HRs of FIB-4 and HCC development, publication bias was no longer detected (P= 0.12), suggesting that bias resulted from the inclusion of small studies.
DISCUSSION
NITs for liver fibrosis assessment play an important role in the management of HCV infection.Liver fibrosis staging is determinant for treatment prioritization and regimen in low- and middle-income countries as well as HCC surveillance.In addition to fibrosis staging, NITs are increasingly evaluated for their prognostic value.Our systematic review highlighted the potential use of FIB-4, APRI, and LSM to guide riskstratified strategies in HCV-infected patients.
We found that LSM had a higher pooled HR for HCC development than APRI and FIB-4.TE is the most validated method for LSM as judged by its clinical implementation since 2003[3].Other techniques such as MRE and 2D-SWE were also shown to have a better performance than TE in differentiating stages of fibrosis[50,51], but they are not as widely available.All of the studies included in our review performed LSM by TE, with the exception of those from Tamakiet al[30] and Hamadaet al[29], which used MRE and real-time SWE, respectively.Although both studies[29,30] evidenced higher HRs for HCC development, the difference in prognostic ability compared to TE was not explored in our meta-analysis due to the limited number of studies using MRE and 2D-SWE.
Although LSM is the most commonly used and validated NIT for liver fibrosis staging, several drawbacks can limit its use in practice such as costly equipment and maintenance, need for frequent calibration and skilled operators, and limited performance in obese patients.Therefore, the use of serologic markers such as APRI or FIB-4 score were recommended by the World Health Organization (WHO)[52] to assess hepatic fibrosis in resource-limited settings.Indeed, these scores can be easily calculated using only patient age and common laboratory data (aspartate aminotransferase, alanine aminotransferase, platelets).Considering the current recommendation to measure the degree of liver fibrosis prior to HCV treatment[2], we found that in a pre-treatment setting APRI and FIB-4 score performed well in terms of HCC prediction, with AUROCs of 0.77 and 0.88, respectively.They could provide similar, if not higher, prognostic value in comparison to LSM.
WHO has committed to eradicate viral hepatitis by 2030.Since the introduction of direct acting antiviral (DAA) therapy, the number of treated CHC patients achievingSVR has greatly increased.SVR is independently associated with improved hepatic function and prognosis[35,36].Despite achieving SVR, some patients can develop HCC or LREs suggesting that regular follow-up remains necessary[13,30,31,33,39,41,49].Non-invasive assessment of residual fibrotic burden in post-therapy patients who achieved SVR is currently unreliable[2].This issue could explain at least partly the decision of international guidelines not to recommend NITs for monitoring of posttreatment residual fibrosis[1,2].Despite its questionable diagnostic potential, we found that among patients with SVR, APRI and LSM can predict HCC development with AUROC values of 0.75 and 0.84, respectively.This was shown to be helpful even in the DAA era, as shown in our study that the adjusted HR of LSM and HCC risk in patients achieving SVR after DAA era was 5.55.

Table 3 Area under the receiver operating characteristic curves of non-invasive tests for overall mortality, liver-related mortality, and composite outcomes
Large variations in NIT cutoffs were observed in the studies included in our metaanalysis.For example, the cutoff of FIB-4 score recommended by WHO for predicting significant fibrosis (METAVIR ≥F2) is 1.45 for high sensitivity and 3.25 for high specificity[52].We found that five out of 11 studies included in this meta-analysis chose the cutoff of 3.25[13,33,41,42,49], while no studies used the cutoff of 1.45.Accordingly, we pooled the results for unadjusted and adjusted HRs of pretreatment FIB-4 using the 3.25 cutoff and found that this cutoff had a statistically significant potential to be used clinically for HCC risk stratification, with a pooled adjusted HR of 3.22 (no subgroup analysis of post-treatment SVR population was done due to the lackof studies).Notably, this does not justify excluding patients with FIB-4 below this cutoff from HCC screening, as it is still debatable whether this cutoff adequately identifies the at-risk population.Decisions regarding HCC screening in patients with low FIB-4 should be individualized based on patient risk profile.

Table 4 Unadjusted and adjusted hazard ratios of non-invasive test for the prediction of liver-related mortality
The strength of this meta-analysis resides in the inclusion of all recently validated noninvasive fibrosis tests, including both radiological and serological tests, as we aimed to make this review as comprehensive as possible.There are some limitations.Although the present meta-analysis extensively assessed several clinically relevant outcomes including HCC, HD, and overall and liver-related mortality, our analysis was nevertheless narrowed by several unavailable data such as the timing in which NITs were assessed after receiving treatment or achieving SVR.Statistical heterogeneity was found in some of our analyses.However, this could be explained by subgroup-analyses of the following factors: NITs assessed at pre-treatment or posttreatment with SVR, treatment with either pegylated interferon and ribavirin or DAA, Eastern or Western countries, and different cutoff points.For instance, statistical heterogeneity found in the analyses of pre-treatment FIB-4 and HCC development is partially explained by country of study.In the subgroup analysis on Eastern countries, there was a reduction ofI2from 76% to 18% for the unadjusted HR.Since the majority of studies are from Eastern countries with Asian participants, further studies conducted in other ethnicities are needed.Residual statistical heterogeneity seen in some of the analyses could also be explained by factors such as the presence of cirrhotic patients in the study and the type of HCV treatment regimen.Due to the limited number of studies and lack of information provided in some studies, we were unable to perform subgroup analysis on these factors.Instead, we provided this information in the figures, wherever subgroup analysis was not possible.More studies are needed to make it possible for us to explore the remaining statistical heterogeneity,by either subgroup analysis or meta-regression.

Table 5 Unadjusted and adjusted hazard ratios of non-invasive tests for the prediction of hepatic decompensation and other composite outcomes
The publication bias in adjusted HR for FIB-4 index could be explained by biased selection of outcomes in four studies.Notably, only adjusted HRs for significant variables were reported, while non-significant variables were either omitted or considered as non-significant without providing a numerical adjusted HR[39-41,49].However, through subgroup analysis, we have concluded that the publication bias detected was due to the inclusion of small studies.
CONCLUSION
FIB-4, APRI, and LSM showed predictive value in stratifying risk for CHC patients, particularly for pre-cirrhotic patients with significant fibrosis.Patients with a higher degree of fibrosis based on NITs were found to be at increased risk of complications, regardless of treatment regimen and response.Therefore, liver fibrosis measurement by NITs could benefit any HCV patient as it can determine the priority to monitor for the development of HCC and other LREs.The clinical implementation of these NITs does require future studies that can validate their respective cutoff levels.

Figure 4 Funnel plots for adjusted hazard ratios of Fibrosis-4 (A), aspartate aminotransferase to platelet ratio (B), and liver stiffness measurement (C) for the evaluation of hepatocellular carcinoma development.
ARTICLE HIGHLIGHTS
Research background
Non-invasive tests (NITs) have reduced the need for liver biopsy in chronic hepatitis C(CHC) patients.Despite its limited diagnostic performance in patients with an intermediate degree of fibrosis or in post-treatment setting, previous meta-analyses have evidenced the potential of NITs in determining prognosis.However, these studies focused on chronic liver diseases from various etiologies and did not comprehensively explore all liver outcomes.
Research motivation
The authors aimed to explore all validated NITs for liver fibrosis, specifically their ability to predict liver-related outcomes in CHC patients.
Research objectives
The main goal was to determine the prognostic value of NITs for risk stratification in CHC patients.
Research methods
A literature search was performed to identify CHC cohort studies that reported an association between liver fibrosis assessment by NITs and outcomes such as hepatocellular carcinoma.Hazard ratios (HR) and area under the receiver operating characteristic from those studies were then pooled using the random effects model.Subgroup analyses were performed based on treatment status, treatment regimen, countries, and different cutoff points.
Research results
Fibrosis-4 (FIB-4) index, aspartate aminotransferase to platelet ratio (APRI) score, and liver stiffness measurement (LSM) were found to have hepatocellular carcinoma predictive potential with pooled adjusted HR of 2.48 (95%CI: 1.91-3.23,I2= 96%), 4.24 (95%CI: 2.15-8.38,I2= 20%) and 7.90 (95%CI: 3.98-15.68,I2= 52%) and area under the receiver operating characteristic of 0.81 (95%CI: 0.73-0.89,I2= 77%), 0.81 (95%CI: 0.75-0.87,I2= 68%) and 0.79 (95%CI: 0.63-0.96,I2= 90%), respectively.
Research conclusions
FIB-4, APRI, and LSM were found to have prognostic value, and can potentially be used to stratify risk for CHC patients, regardless of their treatment status or regimen.
Research perspectives
To facilitate clinical implementation, validation of FIB-4, APRI and LSM cutoff levels are needed.
杂志排行
World Journal of Hepatology的其它文章
- Evolution of liver transplant organ allocation policy: Current limitations and future directions
- Antibiotic prophylaxis in patients with cirrhosis: Current evidence for clinical practice
- Kidney transplant from donors with hepatitis B: A challenging treatment option
- Unpacking the challenge of gastric varices: A review on indication,timing and modality of therapy
- Pathogenesis of autoimmune hepatitis
- Current state of endohepatology: Diagnosis and treatment of portal hypertension and its complications with endoscopic ultrasound
