Unpacking the challenge of gastric varices: A review on indication,timing and modality of therapy
2021-09-03KarlVazMariosEfthymiouRhysVaughanAdamTestroHinBoonLewLeonardoZorronChengTaoPuSujievvanChandran
Karl Vaz, Marios Efthymiou, Rhys Vaughan, Adam G Testro, Hin-Boon Lew, Leonardo Zorron Cheng Tao Pu,Sujievvan Chandran
Karl Vaz, Marios Efthymiou, Rhys Vaughan, Adam G Testro, Leonardo Zorron Cheng Tao Pu, Sujievvan Chandran, Department of Gastroenterology and Hepatology, Austin Health, Heidelberg 3084, Victoria, Australia
Marios Efthymiou, Rhys Vaughan, Sujievvan Chandran, University of Melbourne, Parkville 3052, Victoria, Australia
Hin-Boon Lew, Department of Radiology, Austin Health, Heidelberg 3084, Victoria, Australia
Abstract Upper gastrointestinal bleeding from oesophageal or gastric varices is an important medical condition in patients with portal hypertension.Despite the emergence of a number of novel endoscopic and radiologic therapies for oesophagogastric varices, controversy exists regarding the indication, timing and modality of therapy.The aim of this review is to provide a concise and practical evidence-based overview of these issues.
Key Words: Upper gastrointestinal bleeding; Portal hypertension; Gastric varices; Variceal band ligation; Variceal obliteration; Sclerotherapy; Transjugular intrahepatic portosystemic shunt; Balloon-occlusion retrograde transvenous obliteration
INTRODUCTION
Incidence
The incidence of gastric varices (GV) is 15%-20% from endoscopic epidemiological studies of portal hypertension[1,2].Unlike oesophageal varices that tend to be present in the lamina propria mucosae and superficial submucosa, GV lie deep in the submucosa and as such can be difficult to differentiate from prominent gastric rugae with standard endoscopy.Endoscopic ultrasonography studies have demonstrated that a proportion of GV are undiagnosed on standard diagnostic endoscopy[3,4].However, this may not be clinically significant, as the size of the varix is one of the characteristics that predict risk of haemorrhage, and larger GV are less likely to remain undetected by standard endoscopy.
Both GV and oesophageal varices develop as a consequence of portal hypertension.Portal hypertension may lead to reversal of flow through the portal circulation, with two common outlets -viathe coronary (gastric) vein to the right and left gastric veins, andviathe splenic vein to the short and posterior gastric veins[5].The former supply the distal oesophagus and cardia of the stomach where transmitted pressures and increased flow lead to formation of oesophageal and cardio-oesophageal varices.The latter supply the fundus whereby increased pressures and flow through this system leads to the formation of fundal varices.In a haemodynamic study of oesophageal and and GV by Watanabeet al[5], 78% of patients with portal hypertension had the majority of collateral flow through the left and right gastric veins, likely accounting for the difference in incidence between oesophageal and GV.
In the same study, GV were demonstrated to bleed at lower portal pressures than oesophageal varices, largely due to the higher prevalence of gastro-renal shunts in those with GV.These shunts decompress the portal system.This finding has since been confirmed in further studies[6,7].
GV CLASSIFICATION
Sarin and Kumar[8] seminal paper on the anatomical classification of GV from 1989 remains the most widely accepted method for describing GV.They are divided into two groups, with further sub-classification (Figure 1): (1) Gastroesophageal varices (GOV) - are continuation of oesophageal varices that extend beyond the gastroesophageal junction.These are divided into: (a) Type 1 (GOV1) - those that extend along the lesser curve of the stomach.These account for 75% of all GV[2]; (b) Type 2 (GOV2) - those that extend along the greater curve of the stomach into the fundus; (2) Isolated GV (IGV) - occur in the absence of oesophageal varices and are sub-classified into: (a) Type 1 (IGV1) - located in the fundus and do not extend to the cardia.They are also called fundal varices; (b) Type 2 (IGV2) - can occur anywhere in the stomach (i.e., body, antrum, pylorus).These are rare, occurring in < 5% of those with GV.
The use of this classification system has been shown to predict risk of bleeding and guides management.GOV1 varices behave similarly to oesophageal varices, and so the treatment paradigm for prophylaxis and acute variceal haemorrhage is the same for oesophageal varices.IGV1 and GOV2 varices are more difficult to control when they bleed compared with GOV1 varices and portend a poorer prognosis[2].
An alternate classification, published by Hashizumeet al[9], is a more detailed examination of GV describing the form (tortuous, nodular or tumorous), location (anterior, posterior, lesser curve or greater curve of cardia, or fundic), and colour (red or white) of the varix (Figure 2).Similar to classification systems for oesophageal varices, this classification is aimed at stratifying patients at highest risk of bleeding.In a stepwise logistic regression analysis, those with varices in the anterior or greater curve of the cardia, nodular appearance (i.e., larger size), or red colour spot had the highest predicted risk for bleeding[9].In another study, which focused on patients with fundic varices, increased size and presence of a red spot increased risk of haemorrhage[10].Advanced liver disease, as determined by the Child-Turcotte-Pugh classification, was an additional risk factor for bleeding[10].Other classification systems exist[11], however are used less frequently than those described by Sarin and Kumar[8], and Hashizumeet al[9].
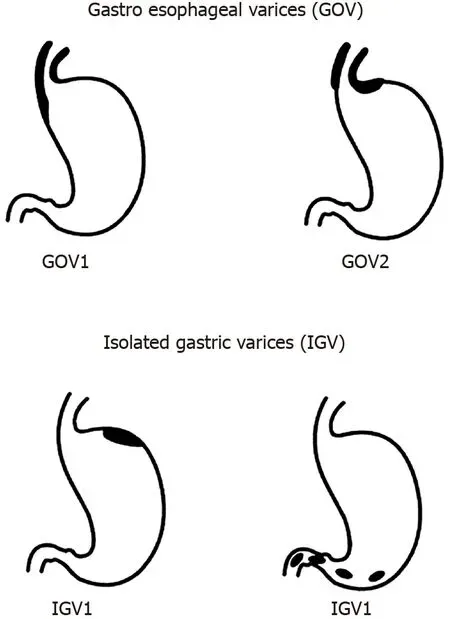
Figure 1 Classification of gastric varices according to Sarin et al[2].
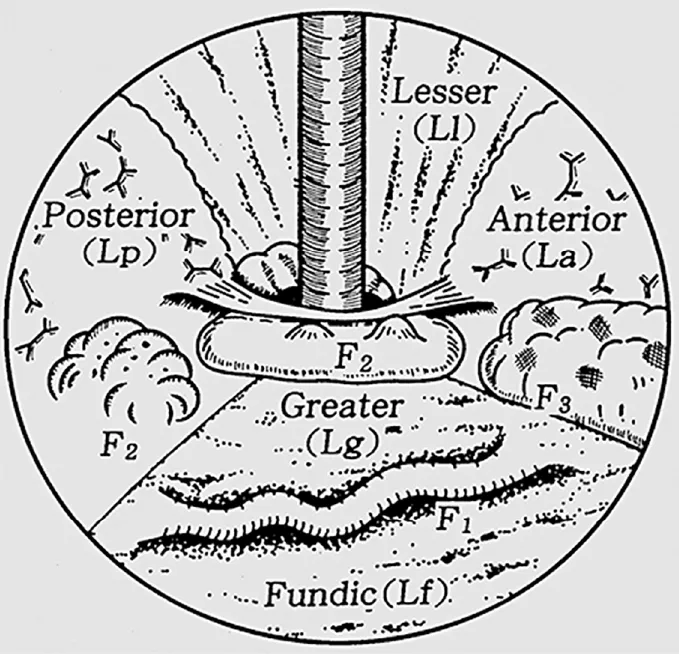
Figure 2 Classification of gastric varices according to Hashizume et al[9].
ACUTE GASTRIC VARICEAL HAEMORRHAGE
Bleeding from GV is considered definite if there is active spurting or oozing from the varix or an adherent clot or fibrin plug on the varix.GV bleeding should be considered the cause of upper gastrointestinal bleeding when a GV without high-risk stigmata is present in the absence of oesophageal varices or an alternate source of bleeding[12].
Pre-endoscopic management
Pre-endoscopic management follows that for oesophageal variceal bleeding, namely use of splanchnic vasoconstrictors (i.e., terlipressin or octreotide) to reduce portal pressures, prophylactic antibiotics (e.g., ceftriaxone), and a restrictive transfusion protocol[13,14].The same medical treatment is instituted in patients with a presumed diagnosis of variceal haemorrhage with known portal hypertension who present with symptoms of upper gastrointestinal bleeding.
Endoscopic management
As outlined above, GOV1 varices are considered an extension of oesophageal varices and so at the time of haemorrhage should be treated in the same manner as bleeding oesophageal varices [i.e., endoscopic variceal band ligation (EVBL), Figure 3][2,12-14].One small prospective randomized controlled trial (RCT) reported numerically higher haemostasis and lower re-bleeding rates in the subset of patients with GOV1 haemorrhage treated with endoscopic variceal obturation (EVO) rather than EVBL[15], although this did not reach statistical significance.Both treatments may be considered equally efficacious for GOV1 varices.
Current guidelines support the use of cyanoacrylate injection - either asN-butyl-2-cyanoacrylate (e.g., Histoacryl®) or 2-octyl-cyanoacrylate (e.g., Dermabond) - for acutely bleeding fundic varices (GOV2 and IGV1), in a procedure termed EVO[13,14].Cyanoacrylate is a tissue adhesive that rapidly polymerizes upon contact with water/blood, leading to a change in the liquid composition to one of a hard brittle acrylic plastic.Majority evidence for its use stems from uncontrolled retrospective and prospective studies[16], with one RCT demonstrating a statistically non-significant increased haemostasis rate when compared to alcohol-based sclerotherapy (89%vs62%)[17].Haemostasis rates for cyanoacrylate glue injection are 80%-100%, with rebleeding rates of 10%-60%[16].Complications are rare, with fever and pain being the most common, while the most feared is embolization of the glue into systemic beds that can lead to ischaemia in those tissues (e.g., stroke, myocardial infarction, splenic infarction, pulmonary embolus).
Thrombin injection has been utilized as an alternative to cyanoacrylate for EVO for almost three decades; however, like cyanoacrylate, evidence for its use is taken from small, uncontrolled studies[16].It appears safe, with few adverse procedure-related outcomes, and haemostasis and re-bleeding rates similar to cyanoacrylate.
Sclerotherapy, the injection of a sclerosant agent into the varix, has gone out of favour for the treatment of gastric variceal haemorrhage due to unacceptably high rebleeding rates of up to 90%-100%[16].This is often due to ulceration at the point of injection resulting from the high volume of sclerosant required to obliterate GV, with a large amount of sclerosant flowing away from the variceal bedviaco-existent gastrorenal shunts that occur with high prevalence in patients with GV[16].Adverse events include fever, and retrosternal and abdominal pain.
IGV-2 varices are rare; hence little evidence exists as to the optimal endoscopic management.In general, it is accepted that they should be treated according to GOV2/IGV1 varices with EVO.
Salvage therapy
Balloon tamponade with Sengstaken-Blakemore, Minnesota or Linton-Nachlas tubes can be utilized in patients with bleeding from GOV1, GOV2 and IGV1 varices.They will not be effective for IGV2 varices, given the ectopic location of the culprit lesion.The Linton-Nachlas tube may be preferred in gastric variceal haemorrhage if available, as the gastric balloon has greater volume capacity[12].
Transjugular intrahepatic portosystemic shunt (TIPS) (Figure 4) is an effective salvage therapy for patients with endoscopically-uncontrollable bleeding oesophageal varices, however its utility in refractory gastric variceal haemorrhage is less clear[12].Although haemostasis rates exceed 90%, re-bleeding is reported to occur in 15%-30%[18-20] and concerns remain over post-TIPS encephalopathy, which can be recalcitrant to standard medical therapy and necessitate revision of the TIPS.Several hypotheses have been proposed to explain the risk of re-bleeding[21,22]; ‘Proximity’ theory - feeding vessels to GV lie further away from a TIPS shunt than feeding vessels to oesophageal varices, hence the shunt is less effective in decompressing GV; ‘Throughput’ theory - gastro-renal shunts that occur in high frequency in association with bleeding GV compete with the TIPS for portal flow and can continue to feed the gastric variceal bed; ‘Recruitment’ theory - development of new feeder vessels after proximal embolization of a GV.
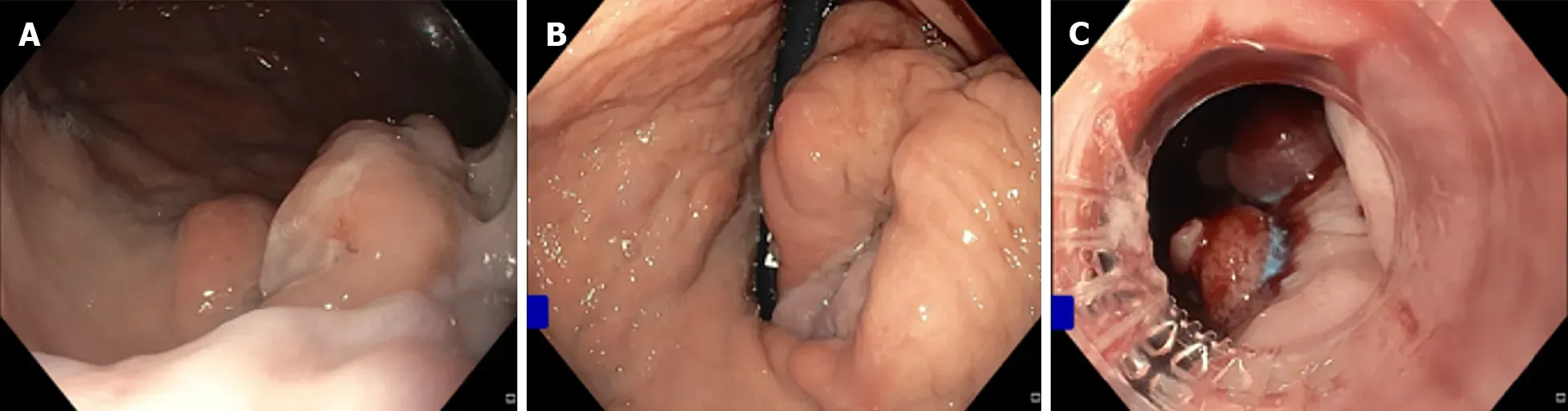
Figure 3 Endoscopic variceal banding of gastroesophageal varices 1.
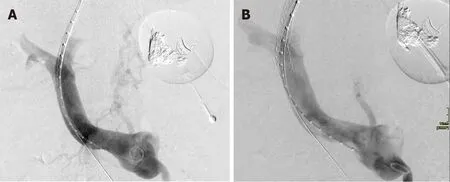
Figure 4 Transjugular intrahepatic portosystemic shunt decompressing gastric varix.
In retrospective comparison studies, Mahadevaet al[23] found TIPS to be more effective in preventing re-bleeding when compared with EVO in acute gastric variceal haemorrhage, whilst Procacciniet al[24] found no difference between the two modalities.
Balloon-occlusion retrograde transvenous obliteration (BRTO) and its modifications (coil-assisted or plug-assisted retrograde transvenous obliteration) aim to sclerose a varix without treating portal hypertension.BRTO is often reserved for use in patients with anatomy not amenable for TIPS or where TIPS is contraindicated (i.e., past history of hepatic encephalopathy or advanced synthetic liver dysfunction), and is reliant on the presence of a gastro-renal shunt for technical feasibility.Similar to TIPS, it is highly effective in achieving haemostasis with success rates > 90%[21,25].A meta-analysis of uncontrolled studies reported a clinical success rate of 97%, defined as no GV recurrence or re-bleed of acutely bleeding GV or no bleed in the case of at-risk GV that have never-bled[26].The main sclerosant used for BRTO in reported studies was ethalonamine oleate, in 94% of cases, and the most common side effect was haematuria, occurring in 70%[26].Given ethalonamine oleate is a known cause of haemolysis, the common occurrence of haematuria is somewhat expected from consequent haemoglobinuria.The antidote for this is parenteral administration of haptoglobin.Although this review by Parket al[26] included patients who underwent BRTO for acute treatment of variceal haemorrhage, primary prophylaxis and secondary prophylaxis, a breakdown of indication was not provided and subgroup analysis for this purpose not available.Another, more recently published metaanalysis[27] found that there was no significant difference in immediate haemostasis rates between the two procedures, but a higher re-bleeding rate post-TIPS [relative risk (RR) 2.61, 95% confidence interval (CI) 1.75-3.90,P< 0.01], higher post-procedural hepatic encephalopathy rate post-TIPS (RR 16.11, 95%CI: 7.13-36.37,P< 0.01), statistically non-significant higher rate of ascites in the BRTO group and statistically nonsignificant worsening in Child-Pugh status in those who received TIPS.Apart from a small pilot study out of Seoul, Korea[28] that randomly assigned 14 patients with acutely bleeding GV to up-front TIPS or BRTO, there have not been any head-to-head RCTs to ascertain the difference in safety and efficacy between these procedures in patients with acutely bleeding GV.A disadvantage of BRTO is that it can lead to the development or worsening of non-GV (oesophageal or ectopic) as portal blood flow is diverted through alternate pathways, or exacerbation or new development of ascites due to raised portal pressures.This is not an issue post-TIPS, which effectively decompresses the portal system.
SECONDARY PROPHYLAXIS
Non-selective beta-blockers
No controlled study has demonstrated ef ficacy of non-selective beta-blockers (NSBB) for secondary prophylaxis following gastric variceal bleeding.In a RCT by Mishraet al[29], EVO was far more effective in preventing re-bleeding than propranolol, with a relative risk reduction of 80% and absolute risk reduction of 35%.Hunget al[30] and Chenet al[31] explored the adjunct use of propranolol or carvedilol, respectively, to 3-4 weekly EVO alone following gastric variceal haemorrhage in an RCT setting, albeit with no placebo arm.Neither found a difference in gastric variceal re-bleeding rates between the two groups.Of note is that both studies were conducted in the same institution with similar inclusion and exclusion criteria, except the more recent study by Chenet al[31] included all patients with any form of gastric variceal bleeding, whilst Hunget al[30] only included patients with fundic variceal haemorrhage (GOV2 or IGV1).The study by Chenet al[31] did demonstrate a significant reduction in allcause upper gastrointestinal re-bleeding in the group assigned to carvedilol (28%vs48%,P= 0.03), driven by a reduction in bleeding from portal hypertensive gastropathy, and oesophageal and gastric ulcers.However, the authors also reported on a higher incidence of adverse events in the carvedilol group (53%vs15%,P< 0.001), due to more frequent dizziness and exertional dyspnoea.Despite this, no patient in the carvedilol group discontinued therapy.The lack of efficacy with NSBB following gastric variceal haemorrhage is postulated to be due to the fact that GVs bleed at lower portal pressures, with NSBB having little effect on preventing flow of blood to the culprit variceal bedviaco-existent gastro-renal shunt[30,31].
Endoscopic therapy
Although endoscopic ultrasound (EUS) guided injection of cyanoacrylate reduces embolic complication rates, as a result of reduced volume of cyanoacrylate injected, its use during acute gastric variceal haemorrhage is limited in most centres due to access to endoscopists with expertise in EUS.However, it is an emerging therapy for secondary prophylaxis.A two-part observational comparative study by Leeet al[32] compared fortnightly EUS-guided injection of cyanoacrylate in patients presenting with acute bleeding from any type of GV with “on demand” therapy, whereby standard endoscopy and injection of cyanoacrylate was only undertaken at the time of re-bleeding.A significant reduction in re-bleeding was demonstrated in the active endoscopic treatment group (35%vs70%,P= 0.0006).There was no impact on mortality, likely due to the small number of patients in the study.In a similar cohort trial design, Bicket al[33] found that there was a lower gastric variceal and all-cause upper gastrointestinal re-bleeding rate (9%vs24%,P= 0.045 and 19%vs50%,P< 0.001, respectively) in those managed with EUS-guided cyanoacrylate injection compared with standard endoscopy guided injection.It is important to note that the standard endoscopy cohort had a higher mean MELD (17vs13,P= 0.004) and lower incidence of IGV1 varices (8%vs47%), with the latter likely accounted for by the greater sensitive of EUS for the detection of GV.
A novel endoscopic method that is gaining popularity globally is EUS-guided coiling of GV, which involves injection of metal embolization micro-coils coated with synthetic stainless steel-fibres, leading to turbulent blood flow and intravariceal clot formation to obliterate the varix[34].This can be combined with injection of cyanoacrylate glue (Figure 5), in a procedure that aims to prevent systemic embolization of the cyanoacrylate, as the coils may provide a scaffold for polymerization, as well as requiring less volume of glue injection as a result of precise delivery into the target tributary[35].The additional benefit of EUS over standard endoscopy is the ability for immediate post-treatment Doppler evaluation of the variceal bed and its afferent tributaries, to ensure complete obliteration[34,35].In a retrospective, multicentre study by Romero-Castroet al[36] comparing outcomes of 30 patients who underwent EUSguided coil (n= 11) with those who underwent EUS-guided cyanoacrylate injection (n= 19) into GVs that had previously bled (n= 23) or never bled (n= 7), both methods were highly effective in obliterating the varices (96.7% cumulatively) without a difference in re-bleeding rate.The cyanoacrylate group required more sessions to achieve obliteration (29 sessionsvs14 sessions,P= 0.29) and had lesser proportion of patients achieving variceal obliteration after a single endoscopic session (18%vs82%), whilst also having a higher reported adverse event rate (58%vs9%,P< 0.01).However, the majority of adverse events were asymptomatic pulmonary emboli detected on routine computed tomography (CT) of the chest of patients postprocedure, with no difference noted in the symptomatic adverse event rate between groups.It is also noteworthy that a statistically significant higher proportion of patients with bleeding varices and Child-Pugh C status cirrhosis were represented within the cyanoacrylate group in this study.
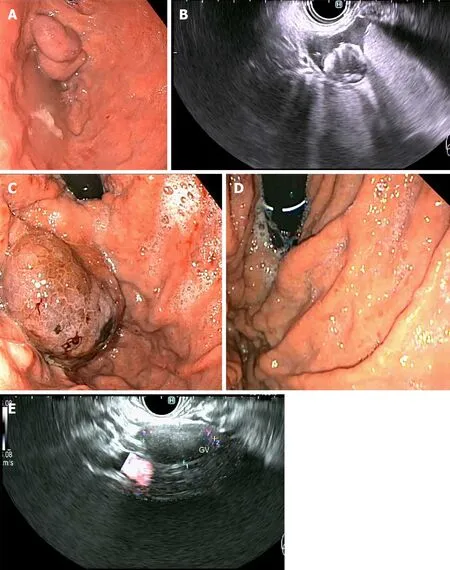
Figure 5 Primary prophylaxis of isolated gastric varices 1 with five 0.18 10 mm micronester coils in combination 1 mL cyanoacrylate glue.
Binmoelleret al[37] were the first to publish on the efficacy and safety of combined EUS-guided coil and cyanoacrylate injection for GV, predominantly in patients who had recovered from an acute gastric variceal haemorrhage (n= 28/30).The same group reported on a more extensive patient cohort (n= 152) some years later, with high obliteration rate (93%) at follow-up endoscopy, low re-bleeding rate (16%, with 50% re-bleeding events non-variceal in origin), and few procedure-related adverse events (7%; 4/9 patients with abdominal pain, 1/9 patients with pulmonary embolus)[38].Of note, 26% of patients in this study underwent treatment as primary prophylaxis, somewhat unique in the GV treatment evidence base.A single randomized trial has been performed evaluating combination coiling and cyanoacrylate injection with coiling alone, reporting a higher variceal disappearance rate on immediate postprocedure endoscopy (87%vs13%,P< 0.001), and lower re-bleeding rate (3.3%vs20%,P= 0.04), variceal reappearance rate on follow-up endoscopy at 3-mo (13%vs47%,P< 0.001), and re-intervention rate (17%vs40%,P= 0.045) in the arm allocated to combination therapy[39].The cumulative mortality rate of 28% from this study despite relatively preserved liver function in participants (90% Child Pugh A, median MELD 9.5 at enrolment) is of concern and somewhat unexplained, particularly given 10/17 patients died from uncontrolled haemorrhage and 9/10 of these were variceal in nature.
Interventional radiology
Only a single RCT has evaluated EVO with up-front TIPS for secondary prophylaxis of gastric variceal haemorrhage[40], and revealed a 71% relative risk reduction over 3 years in gastric variceal re-bleeding rate in the TIPS arm, albeit with 26% of TIPS patients suffering from hepatic encephalopathy.This study pre-dates the era of EUSguided coils and very few patients in this study had IGV1 varices.TIPS may be an attractive option in patients with concurrent ascites and/or presence of other non-GV, but less so in those with a history of prior encephalopathy or advanced synthetic liver dysfunction.
A single prospective, non-randomized study[41] and two retrospective, observational studies[42,43] have demonstrated a lower re-bleeding rate in patients treated with BRTO rather than EVO for secondary prophylaxis of variceal haemorrhage (3%-15%vs22%-71%).They each had differing inclusion criteria, with the prospective study including patients with GOV1, GOV2 and IGV1 varices, whilst the two retrospective studies both excluded patients with GOV1 varices, and one only included patients with IGV1 varices[42].
Contemporary case series have begun exploring the feasibility and safety of combined interventional radiological procedures, namely TIPS with balloon-occluded transvenous obliteration (whether in an antegrade or retrograde fashion)[44-46].Purported benefits from retrospective audits of combined procedures are reduced rebleeding and post-procedure encephalopathy rates, stable or improved liver function, and prevention or improvement of ascites.Finally, percutaneous transhepatic obliteration is an alternate route to obliteration of a gastric varix in those without a gastro-renal shunt and who may have contraindication to TIPS[47].
Given the wide array of therapeutic options available are reliant on specific anatomical features, such as feeding vessels into the variceal bed or presence of a gastro-renal shunt, appropriate imaging of the portomesenteric circulation with CT should be attained in patients with GV to allow the most anatomically suitable intervention to be chosen.
PRIMARY PROPHYLAXIS
Whilst there is a modest evidence-base for secondary prophylactic measures for bleeding GV, there is a paucity of data examining the role of primary prophylaxis.Few trials have recruited patients with the intention to treat GV prior to bleeding, and those that have[36,38,39] have done so in low numbers which prevents any meaningful subgroup analysis.
One RCT by Mishraet al[48] randomized patients with never-bled GOV2 or IGV1 ≥ 10 mm in size to cyanoacrylate injection, propranolol or no therapy in a 1:1:1 ratio.This demonstrated a significant reduction in GV bleeding in those treated with cyanoacrylate compared to those with propranolol or no therapy (10%vs38%vs53%,P= 0.003), as well as a significant reduction in bleed-related mortality between those receiving endoscopic therapy and those who received no specific therapy (0%vs20%,P= 0.025).There was no statistical difference in overall mortality between the groups (7%vs17%vs26%,P= 0.113), nor therapy-related complications (3%vs3%vs7%,P= 1.0).This suggests endoscopic cyanoacrylate therapy could be recommended in patients with GV larger than 10mm in size, and NSBB therapy considered in those with contraindication to, or declining, cyanoacrylate injection.
In subgroup analysis of another study by Bhatet al[38], 93% of patients undergoing combined EUS-guided coiling with cyanoacrylate for primary prophylaxis had no GV bleeding over a mean follow-up time of 449 d.
To date, there are no head-to-head trials comparing endoscopic therapy with radiologic interventions for primary prophylaxis, nor any specific trials to compare various endoscopic therapies (coilvscyanoacrylate or combination therapyvsmonotherapy).
CONCLUSION
Future directions
The majority of evidence for the treatment of GV stems from retrospective studies, and so there is a need for further prospective and randomized trials to better guide management.In particular, there is a paucity of data on primary prophylaxis of GV, the risk of treating small (< 10 mm) or low-risk GV, and on the optimal approach to secondary prophylaxis (endoscopic, radiologic or combined) since the advent of EUSbased combination therapy.Furthermore, little is known regarding the ideal timeframe for surveillance of GV, whether treated or untreated.
杂志排行
World Journal of Hepatology的其它文章
- Evolution of liver transplant organ allocation policy: Current limitations and future directions
- Antibiotic prophylaxis in patients with cirrhosis: Current evidence for clinical practice
- Kidney transplant from donors with hepatitis B: A challenging treatment option
- Pathogenesis of autoimmune hepatitis
- Current state of endohepatology: Diagnosis and treatment of portal hypertension and its complications with endoscopic ultrasound
- Therapeutic plasma exchange in liver failure
