The Brown Algae Saccharina japonica and Sargassum horneri Exhibit Species-Specific Responses to Synergistic Stress of Ocean Acidification and Eutrophication
2021-08-30LIUYuxinCAOJiazhenCHUYaoyaoLIUYanWANGQiaohanGONGQingliandLIJingyu
LIU Yuxin, CAO Jiazhen, CHU Yaoyao, LIU Yan, 2), WANG Qiaohan, 2), GONG Qingli, 2), and LI Jingyu, 2),
The Brown AlgaeandExhibit Species-Specific Responses to Synergistic Stress of Ocean Acidification and Eutrophication
LIU Yuxin1), CAO Jiazhen1), CHU Yaoyao1), LIU Yan1), 2), WANG Qiaohan1), 2), GONG Qingli1), 2), and LI Jingyu1), 2),*
1)Fisheries College,Ocean University of China, Qingdao 266003, China 2) Key Laboratory of Mariculture, Ministry of Education, Ocean University of China,Qingdao 266003, China
Ocean acidification and eutrophication are two important environmental stressors. They inevitably impact marine macroalgae, and hence the coastal ecosystem of China., as the main culture species in China, is suffering the harmful golden tide caused by. However, it remains unclear whether the detrimental effects ofoncultivation become more severe in future acidified and eutrophic scenario. In this study, we respectively investigated the effects ofCO2(400μatm and 1000μatm) and nutrients (non-enriched and enriched seawater) on the growth, photosynthesis, respiration, chlorophyll contents, and tissue nitrogen ofand. Results indicated that enrichment of nutrients contributedto utilize HCO3−. The carbon acquisition pathway shifted from HCO3−to CO2in, whileremained using HCO3−regulated by nutrient enrichment.exhibited better photosynthetic traits than, with a higher level of net photosynthetic rate and chlorophyll contents at elevatedCO2and enriched nutrients. Tissue nitrogen also accumulated richly in the thalli ofunder higherCO2and nutrients. Significant enhancement in growth was only detected inunder synergistic stress. Together,showed competitive dominance in current study. These findings suggest that increasing risk of golden tide in acidified and eutrophic ocean can most likely result in great damage tocultivation.
eutrophication; ocean acidification;;; synergistic stress
1 Introduction
The concentration of atmospheric carbon dioxide (CO2) increased approximately 130 pars per million (ppm) since the Industrial Revolution (Joos and Spahni, 2008; AOAN, 2019). Rising atmospheric CO2dissolved in seawater, causing pH reductions and alterations in chemical balances of dissolved inorganic carbon (DIC) (Feely., 2004, 2009a; Doney., 2009). These changes in pH and DIC are ineluctable consequences of rising atmospheric CO2, referred to as ocean acidification (OA) (Doney., 2009). Anthropogenic CO2emission is rising at the fastest rate after the Industrial Era (Joos and Spahni, 2008; AOAN, 2019), thus leading to a continuing decrease in seawater pH (Feely., 2004, 2009b; Doney., 2009; Feely., 2009a). OA significantly affects the physiological processes and ecological functi- ons of seaweeds and other marine organisms (Gazeau., 2007; Edmunds, 2011; Koch., 2013; Kroeker.,2013; Enochs., 2015; Gao., 2019). A body of evidence indicates that OA actively stimulates the growth of kelps, such as,andwhich were carbon limited in nearshore environment (Swanson and Fox, 2007; Xu., 2019; Hurd., 2020; Zhang., 2020). On the other hand, OA simultaneously reduces the calcification of,and other calcified algae (Reymond., 2013; Johnson and Carpenter, 2018).
Furthermore, human pollution, agricultural production and atmospheric deposition have dramatically increased since 1970s, resulting in excessive nutrients input to coastal seawater (Smith., 2003; van der Struijk and Kroeze, 2010; Strokal., 2014; Brockmann., 2018; Murray., 2019). This process leads to another environmental issue known as eutrophication (Smith., 2003). Several studies showed that water quality slightly recovered from previous eutrophic state in the Baltic Sea, Chesapeake Bay and other coastal seas (Okino and Kato, 1987; Andersen., 2017; McCrackin., 2017; Duar-te and Krause-Jensen, 2018). In contrast, severe eutrophic areas are still located at some key bays in China, including Liaodong Bay, Yangtze River Estuary and other jurisdictional seas (MEE, 2019). With exceeded nutrients supply, eutrophication can enhance the growth of phytoplankton, fast-growing filamentous and mat- forming opportunistic macroalgae (Pedersen and Borum, 1997; Wernberg., 2018). Degraded water quality from eutrophication is critical for the development, persistence and expansion of harmful algae blooms (HABs) (Heisler., 2008). Recent reports showed that microalgal blooms,-dominated green tides and-dominated golden tides have substantially increased worldwide (Glibert., 2005; Smetacek and Zingone, 2013; Kudela., 2015; Wang., 2018). HAB resulted from eutrophication affects substance circulation, primary productivity, community structure and marine ecosystem service (Norkko and Bonsdorff, 1996a,b; Glibert., 2005; Heisler., 2008; Rabouille., 2008; Smetacek and Zingone, 2013; Anderson., 2015; Kudela., 2015; Watson., 2015).
Several studies have found that coral reef systems are negatively affected by OA and nutrient enrichment (Hoegh-Guldberg., 2007; Ge., 2017; Guan., 2020). For phytoplankton, marine diazotrophs such asspp. increase their N2fixation under elevated CO2in nitrogen enriched cultures (Eichner., 2014; Hutchins and Fu, 2017). However, limited investigations aimed to reveal the ecophysiological effects of OA and eutrophication on marine macrophytes. Previous studies indicated that the growth and quality ofwere inhibited and threatened by the interactive effects of OA and eutrophication (Chu., 2019, 2020). In contrast, there was an enhanced production of amino acid and fatty acid inspecies at elevated CO2concentration and nutrient level (Gao., 2018). Thus, the responses to the synergistic stress of OA and eutrophication are species-specific in macroalgae. The rise of acidity in coastal ocean was found to be greater under eutrophication (Cai., 2011). This severe scenario potentially aggravate the disappearance of habitat-forming seaweeds worldwide (Filbee-Dexter and Wernberg, 2018; Wern- berg., 2018). It is thus important to understand how macroalgae will response to the future synergistic stress of OA and eutrophication.
The kelpis the foremost commercial harvesting alga among northwestern Pacific countries (Chung., 2017; Kim., 2017). In previous studies, the growth, photosynthesis, and nutrient uptake ofwere significantly enhanced under elevated CO2concentrations (Swanson and Fox, 2007; Zhang., 2020). Also, excess nutrient availability significantly promoted the growth and physiological performance of(Gao., 2017). On the other hand, the sheet- like macroalgaeblooms frequently occur in recent years (Liu., 2013; Xiao, 2020), whose floating thalli have caused detrimental impacts onaquaculture (Xiao, 2020). Many investigations have focused on how environmental factors affect population dynamics and distributions ofin East China Sea and Yellow Sea (Xiao, 2019; Xiao., 2020; Choi., 2020). However, it remains unclear whetheris more resilient to the synergistic stress of OA and eutrophication than.
In the present study, we investigated the synergistic stress of OA and eutrophication on growth, photosynthesis, respiration, chlorophyll contents, and tissue nitrogen of sporophytes ofandappearing in the same period. The results are expected to reveal the species-specific ecophysiological responses ofand, and determine which alga has greater resilience and interspecific competitive dominance under synergistic stress of OA and eutrophication.
2 Materials and Methods
2.1 Algal Collection and Maintenance
The sporophytes of(approximately 80cm in average length,=20) and(approximately 150cm in average length,=20) were collected in Rong- cheng, Shandong, China (36˚07´N, 120˚19´E), in December 2019. Thesamples were from cultivated populations, withtwining on, or floating between their rafts. The samples were kept in cold foam boxes filled with seawater and quickly transported to the laboratory within 8h. Healthy sporophytes were selected and rinsed several times with sterilized seawater to remove the epiphytes and detritus. More than 100 discs (1.4cm in diameter) were punched from the meristem ofwith a cork borer, and more than 100 segments (4–5cm in length) were cut from the apical part ofbranches for the subsequent experiments. The discs and segments were maintained separately in plastic tanks containing 3L filtered seawater. The seawater was renewed daily during the maintenance. These samples were maintained at an irradiance of 90μmolphotonsm−2s−1with a 12L:12D photoperiod, and 10℃, the seawater temperature of the collection area, for 3d to reduce the negative effects of excision.
2.2 Culture Experiment
The culture experiment was conducted over a period of 6d under combinations twoCO2levels (400μatm and 1000 μatm) and two nutrient levels (non-enriched natural seawater and nutrient-enriched seawater). The nutrient- enriched level was enriched 50% PESI medium (Tatewaki, 1966), which was made by sterilized seawater from coas- tal Qingdao. There was a total of 4 experimental treatments and each had 3 replicates. Four individuals were cultured in each of 12 gently aerated side-arm flasks, in which each contained 500mL non-enriched or enriched seawater at 10℃. The culture medium was renewed on the third day of the experiment.
2.3 Carbonate Chemistry Parameters
For the treatments under twoCO2levels, the samples were cultured in two CO2incubators (GXZ-380C-C02, Jiangnan Instruments Factory, Ningbo, China). The 400 μatm was achieved by bubbling ambient air. And the 1000μatm was obtained through gas cylinders of the incubator. The pH value of the medium in each flask was measured by a pH meter (Orion STAR A211; Thermo Scientific). The salinity was measured by a seawater salimeter (0–100‰, Aipli). Other indirectly measured carbonate chemistry parameters of all treatments were calculated based on the pH values, salinity,CO2levels, the equilibrium constants1and2for carbonic acid dissociation, andBfor boric acid, using CO2SYS software (Robbins and Kleypas, 2018).
2.4 Measurement of Growth
The growth ofandwas determined by weighing fresh weight (FW) of discs or thalli. The discs and thalli were gently scrubbed with tissue paper to remove water from the surface before being wei- ghed. The relative growth rate (RGR) was calculated as the following formula:
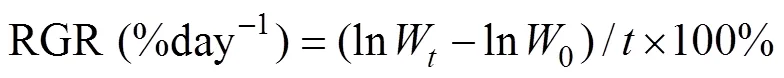
whereis the time period of culture experiment,0is the initial FW,is the FW afterdays of culture.
2.5 Measurement of Photosynthesis and Respiration
The net photosynthetic rate (Pn) and the respiration rate (d) of the samples was measured by a manual oxygen meter (FireSting O2II, Pyro Science). After measuring the FW, four discs or segments of each replicate were transferred to the oxygen electrode cuvette with 330mL culture medium from their own flasks. The medium was magnetically stirred during the measurement to ensure the even diffusion of oxygen. The irradiance and temperature conditions were set the same as the growth chambers. The samples were set to acclimate to the conditions in the cuvette for 5min before the measurements. The oxygen concentration in the medium was recorded per minute for 10min. The increase of oxygen content in the medium within 5min was defined as the Pn, and the decrease of oxygen content in darkness in the following 5min was defined as Rd. The Pnand Rdwere presented as μmolO2min−1g−1FW.
2.6 Measurement of Chlorophyll Contents
Approximately 0.2g (FW) of the samples from every replicate were used for the extraction of photosynthetic pigments. The discs or segments were dipped in 2mL dimethyl sulfoxide for 5min, and the absorbance of supernatant was determined at 665, 631, 582, and 480nm in the ultraviolet absorbance spectrophotometer (U-2900, HITACHI, Tokyo, Japan). Then the same samples were added 3mL acetone, setting for 2h. Before the measurements, 1mL methanol and 1mL distilled water was added to the supernatant. The absorbance was obtained at 664, 631, 581, and 470nm. The contents of chlorophyll (Chl)andwere calculated according to the following equation:
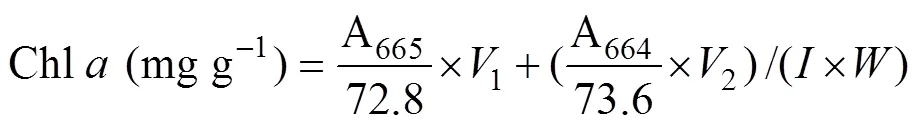
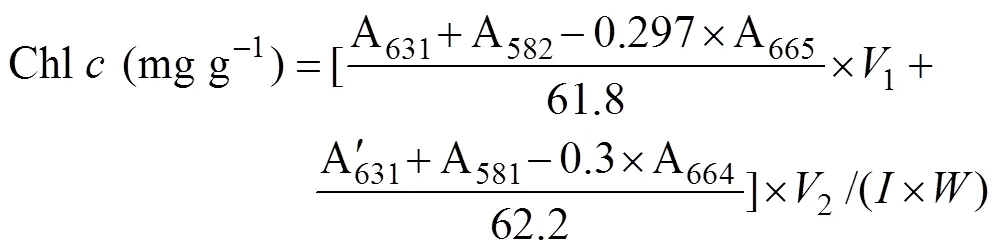

2.7 Measurement of Tissue Nitrogen
One disc or segment was randomly selected from every replicate for the measurement of tissue nitrogen (TN) contents. The samples were completely dried at 80℃, and ground into powder. About 2–3mg powder was used to measure the TN contents in the elemental analyzer (Vario EL III, Elementar, Germany). The TN contents were normalized to %DW.
2.8 Data Analysis
Results were expressed as mean ±standard deviation (=3). Prior to the analysis, the data were conformed to a normal distribution (Shapiro-Wilk test,>0.05) and homogeneity of variance (Levene’s test,>0.05). Two- way analysis of variance (ANOVA) was conducted to as- sess the combined effects ofCO2and nutrient levels on carbonate chemistry parameters, RGR,n,d, Chl, Chl, and TN. Tukey honest significance difference (HSD) was conducted to determine the significance levels of factors (<0.05). Pearson correlation coefficient (PCCs) was conducted to analyze the correlations of each experimental indicator withCO2and nutrients levels (<0.05). Data were analyzed in SPSS 22.0 software.
3 Results
3.1 Carbonate Chemistry Parameters of Culture Medium
At the sameCO2level, two-way ANOVA showed thatnutrients had no significant effects on any parameter (Table 1). In the culture medium of, elevatedCO2decreased the pH by 0.3 and CO32−by 57%, but it increased the DIC by 12%, HCO3−by 22%, and CO2by 187% in both the non-enriched and enriched nutrient treatments. In the culture medium of, elevatedCO2decreased the pH by 0.4 in both nutrient levels and CO32−by 75% (non-enriched) and 65% (enriched), but it increased the DIC by 27% (non-enriched) and 4% (enriched), HCO3−by 13% (non-enriched) and 5% (enriched), and CO2by 191% in both nutrient treatments.

Table 1 Parameters of the seawater carbonate system at different pCO2 and nutrient conditions
Notes: L-N is the lowCO2and non-enriched condition, L-E is the lowCO2and enriched condition, H-N is the highCO2and non-enriched condition, and H-E is the highCO2and enriched condition. DIC is dissolved inorganic carbon, and TA is total alkalinity. Data are reported as means ±SD (=3). Different superscript letters indicate significant differences in one parameter between treatments (<0.05).
3.2 Growth
The differences inCO2and nutrients yielded no significant effects on RGR of, but nutrients significantly promoted the growth of(Fig.1). At both 400μatm and 1000μatm, the RGR ofdecreased due to enriched nutrient. In contrast, the RGR ofsignificantly increased in excessive nutrient availability (=4.550,<0.05). PCCs showed that RGR ofpositively correlated with bothCO2and nutrients. In contrast, RGR ofpositively correlated withCO2, but negatively correlated with nutrients (Table 2). Together,showed more promotive growth under the synergistic stress.
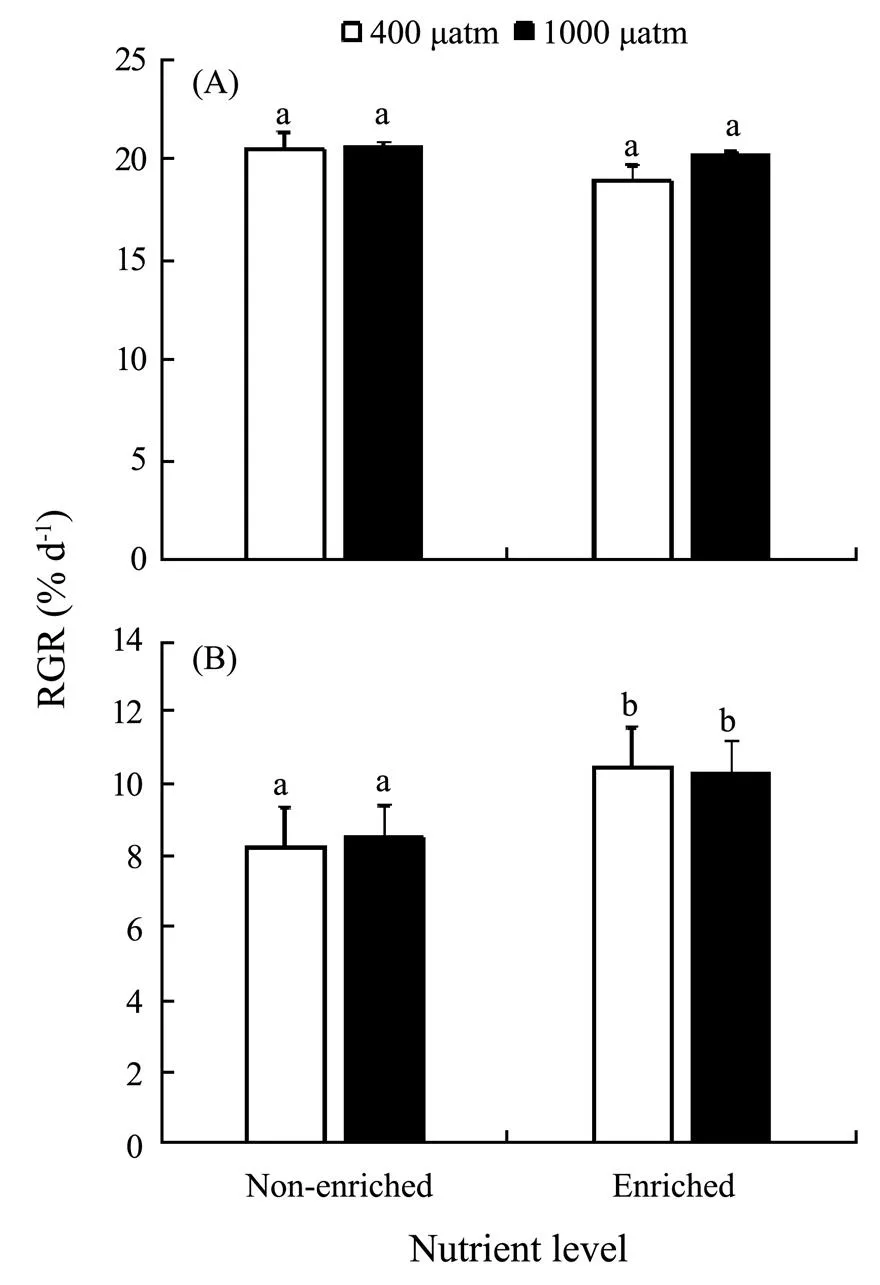
Fig.1 Relative growth rate (RGR) of S. japonica (A) and S. horneri (B) cultured at different pCO2 and nutrient conditions for 6d. Data are reported as means±SD (n=3). Different letters above the error bars indicate significant differences between treatments (P<0.05).
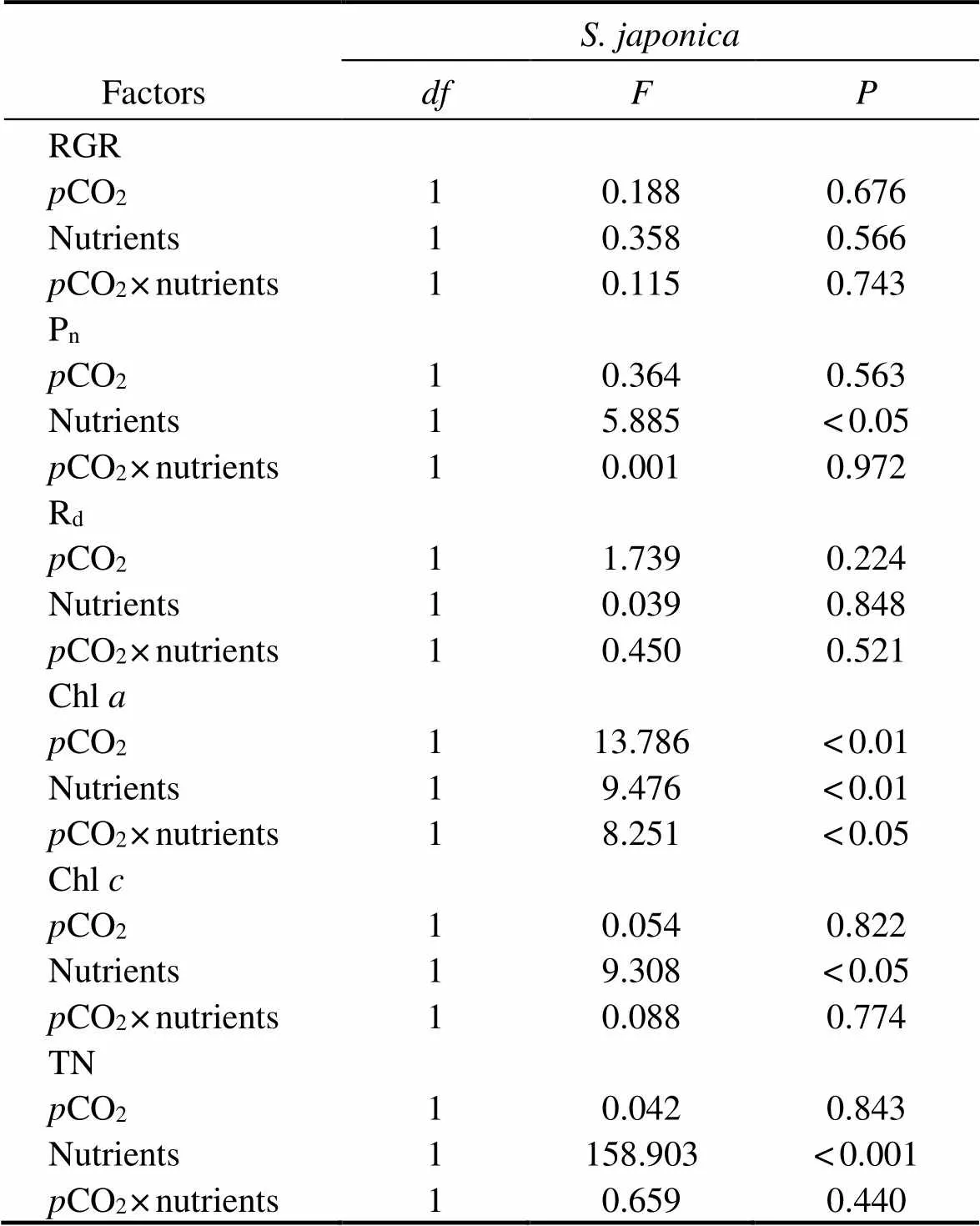
Table 2 Analysis of variance (two-way ANOVA) examining the statistical differences of experimental parameters of S. japonica among pCO2 and nutrients
3.3 Photosynthesis and Respiration
As shown in Fig.2, nutrient enrichment significantly increased thenofat both CO2concentrations (=5.885,<0.05). While no significant effect was detected in,nwas lower in nutrient-enriched condition. PCCs showed thatninhad positive correlations withCO2and nutrients. Whilepositively correlated withCO2, but negatively correlated with nutrients (Table 4). Photosynthesis ofwas greater than that ofat elevatedCO2and nutrients.
Thedinshowed a similar trend to(Fig.2). No significant effects ondof both algae were found in all treatments. At 400 μatm, Rdof both species was lower in excess nutrients. The correlation between Rdand nutrients ofwas positive, but that ofwas negative (Table 3). Respiration ofwas also greater than that ofunder synergistic stress.
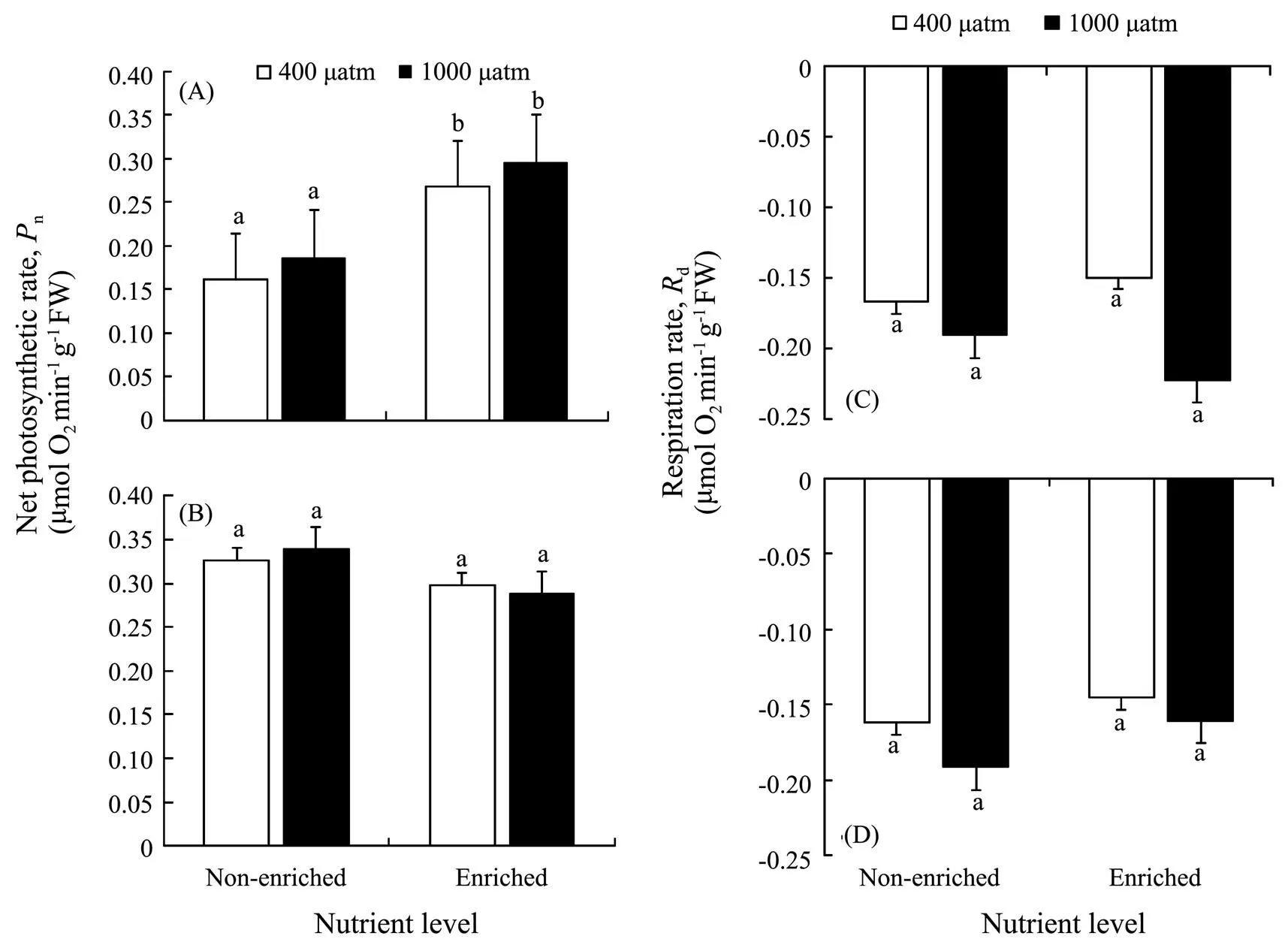
Fig.2 Net photosynthetic rate (Pn) of S. japonica (A) and S. horneri (B); Respiration rate (Rd) of S. japonica (C) and S. horneri (D) cultured at different pCO2 and nutrient conditions for 6d. Data are reported as means ±SD (n=3). Different letters above the error bars indicate significant differences between treatments (P<0.05).
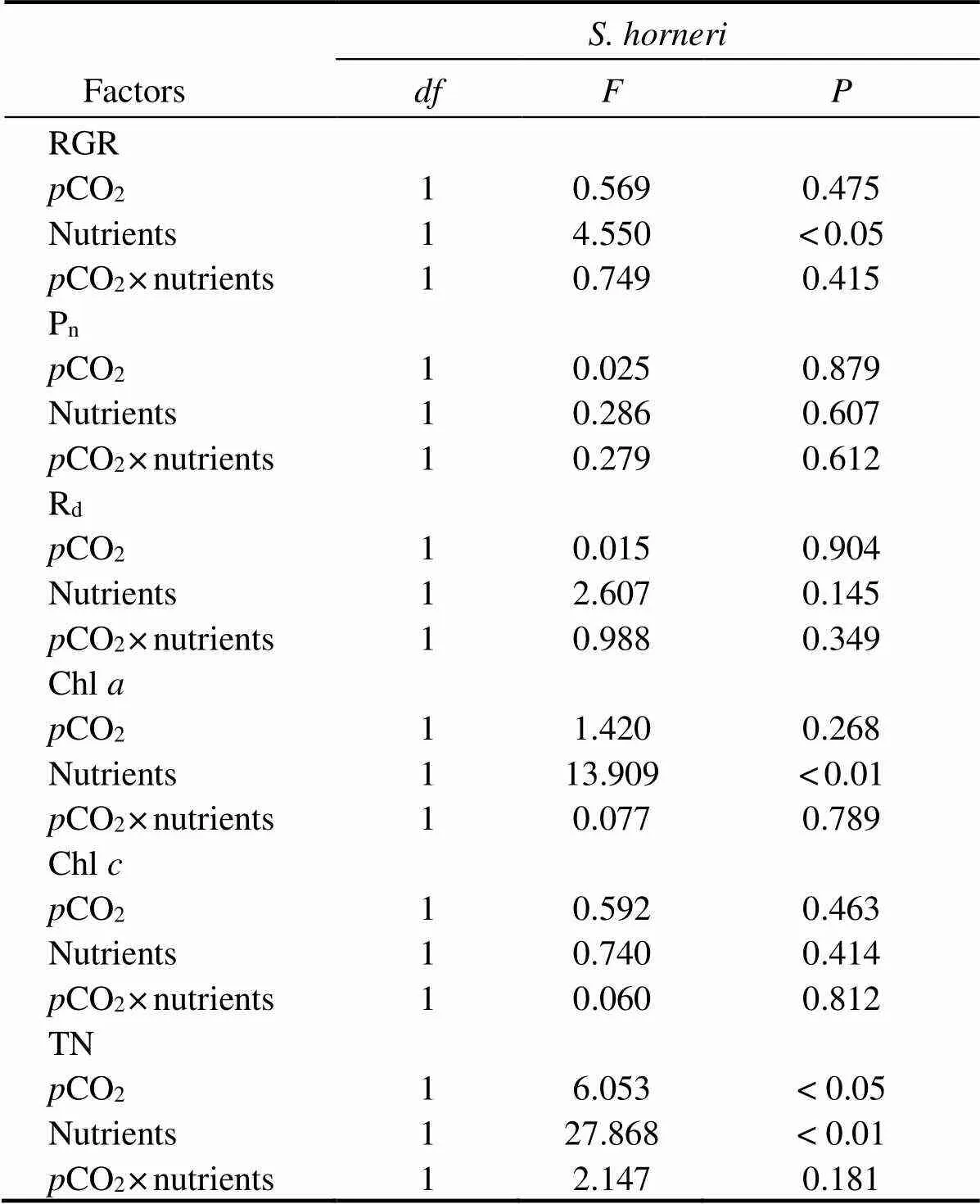
Table 3 Analysis of variance (two-way ANOVA) examining the statistical differences of experimental parameters of S. horneri among pCO2 and nutrients
3.4 Chlorophyll Contents
The Chlandcontents ofsignificantly increased under either elevatedCO2or enriched nutrient. Both chlorophyll contents reached the maximum under the synergistic stress (Fig.3). The Chlcontent ofwas significantly increased at enriched nutrients, and reached the peak in synergistic stress condition. How- ever, the Chlcontent ofincreased only withCO2elevated. NeitherCO2nor nutrients significantly affected the Chlin. PCCs showed positive correlations between ChlwithCO2and nutrients in both species. However, the correlation between Chland nutrients was significantly negative in(Table 4).
3.5 Tissue Nitrogen
The TN contents ofandsignificantly increased in nutrient-enriched condition (as seen in Fig.4). ElevatedCO2had no significant effect on the TN of, but significantly promoted the accumulation of TN in. The correlations between nutrients and TN were significantly positive in the two species. As for the correlations betweenCO2and TN, it was negative inbut positive in(Table 4).
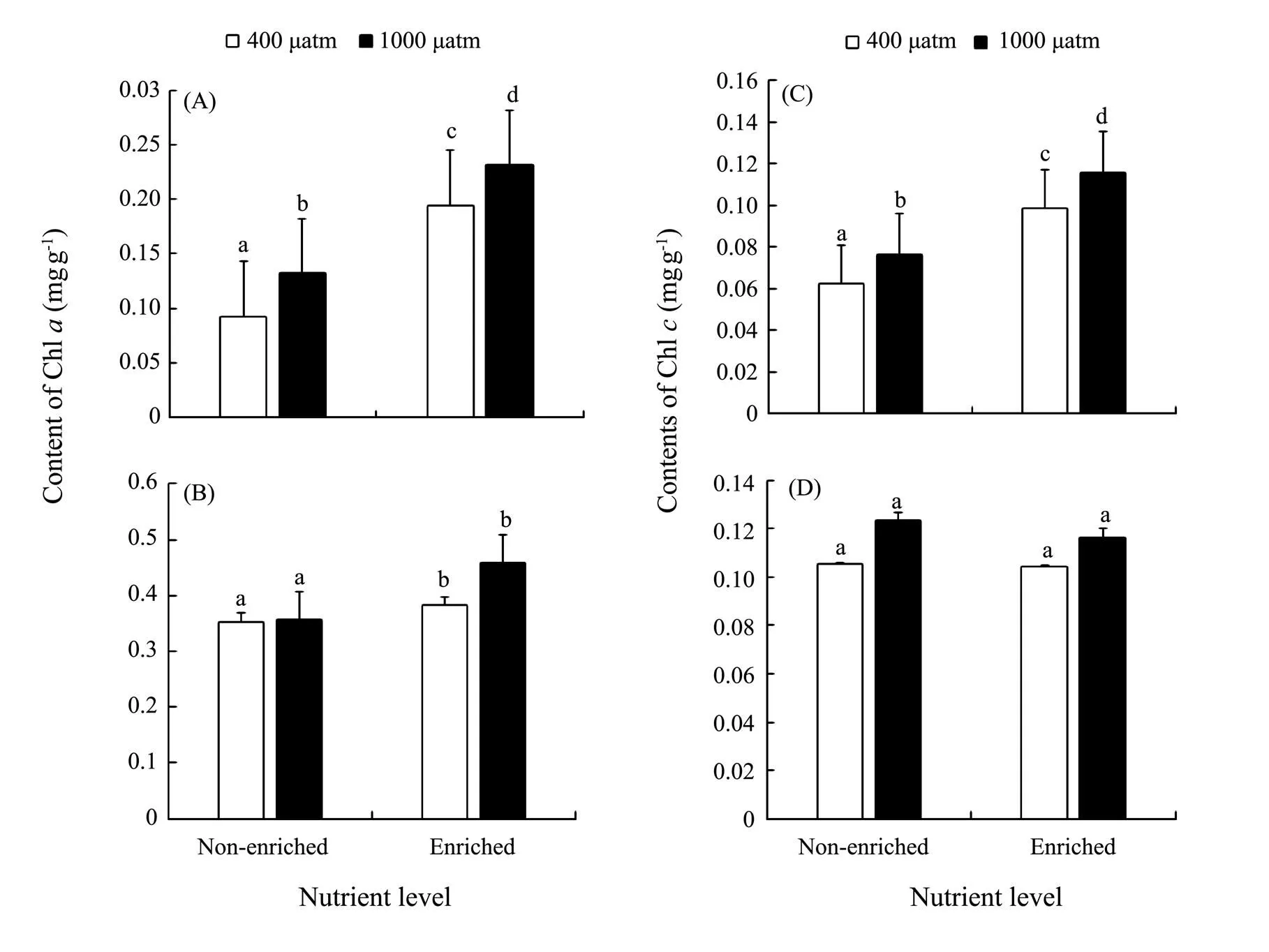
Fig.3 Chl a of S. japonica (A) and S. horneri (B); Chl c of S. japonica (C) and S. horneri (D) cultured at different pCO2 and nutrient conditions for 6d. Data are reported as means±SD (n=3). Different letters above the error bars indicate significant differences between treatments (P<0.05).
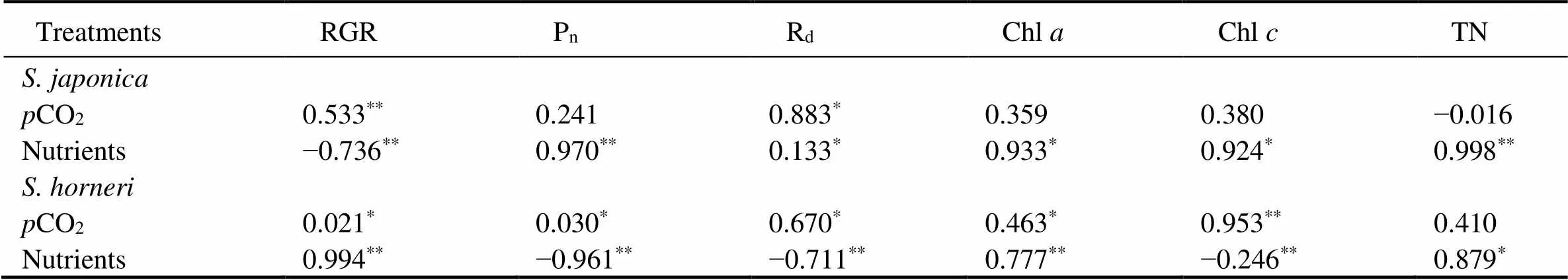
Table 4 The Pearson correlation coefficient (PCCs) of various experimental indicators of S. japonica and S. horneri with pCO2 and nutrients levels
Notes:*indicates significant correlation (<0.05),**indicates highly significant correlation (<0.01).
4 Discussion
There was a same increase pattern of DIC in the culture medium ofunder two nutrient concentrations, but different case was found in the culture medium of(Table 1). The effects of the synergistic stress of OA and eutrophication on algae may depend on their precise carbon acquisition pathways. The HCO3−inthe culture medium ofwas lower in enriched nutrient than in non-enriched treatments, indicating more HCO3−utilization paralleled with enriched nutrients. Many macroalgae use HCO3−rather than dissolved CO2under current seawaterCO2concentration (Israel and Hophy, 2002; Badger, 2003; Koch., 2013), due to their ribulose-1,5-bisphosphate carboxylase/ oxygenase (Rubisco) is not substrate-saturated at current atmospheric CO2level (Reiskind., 1988). Marine macroalgae have species-specific responses to elevated CO2because of their various capacities and strategies in CO2-concentrating mechanisms (CCMs) to utilize HCO3−in seawater (Wu., 2008; Raven and Hurd, 2012). Furthermore, DIC acquisition interacts with phosphorus and nitrogen availability (Giordano., 2005), but it remains unclear howregulates CCMs under excessive nutrient supply. The results indicate that enrichment of nutrients contributedto the utilization of HCO3−. When exposed to elevatedCO2, macroalgae may reduce the use of HCO3−by down-regulating their CCMs, and begin to rely on CO2as the primary carbon source (Bjork., 1993; Axelsson., 2000; Cornwall., 2012). This phy- siological process may have occurred in, thus leading to the DIC of culture medium remained at the same level after increasingCO2under the two nutrient conditions. In contrast, this study provides an evidence that eutrophication restrains the shift of carbon acquisition pathway into cope with higher CO2concentration.
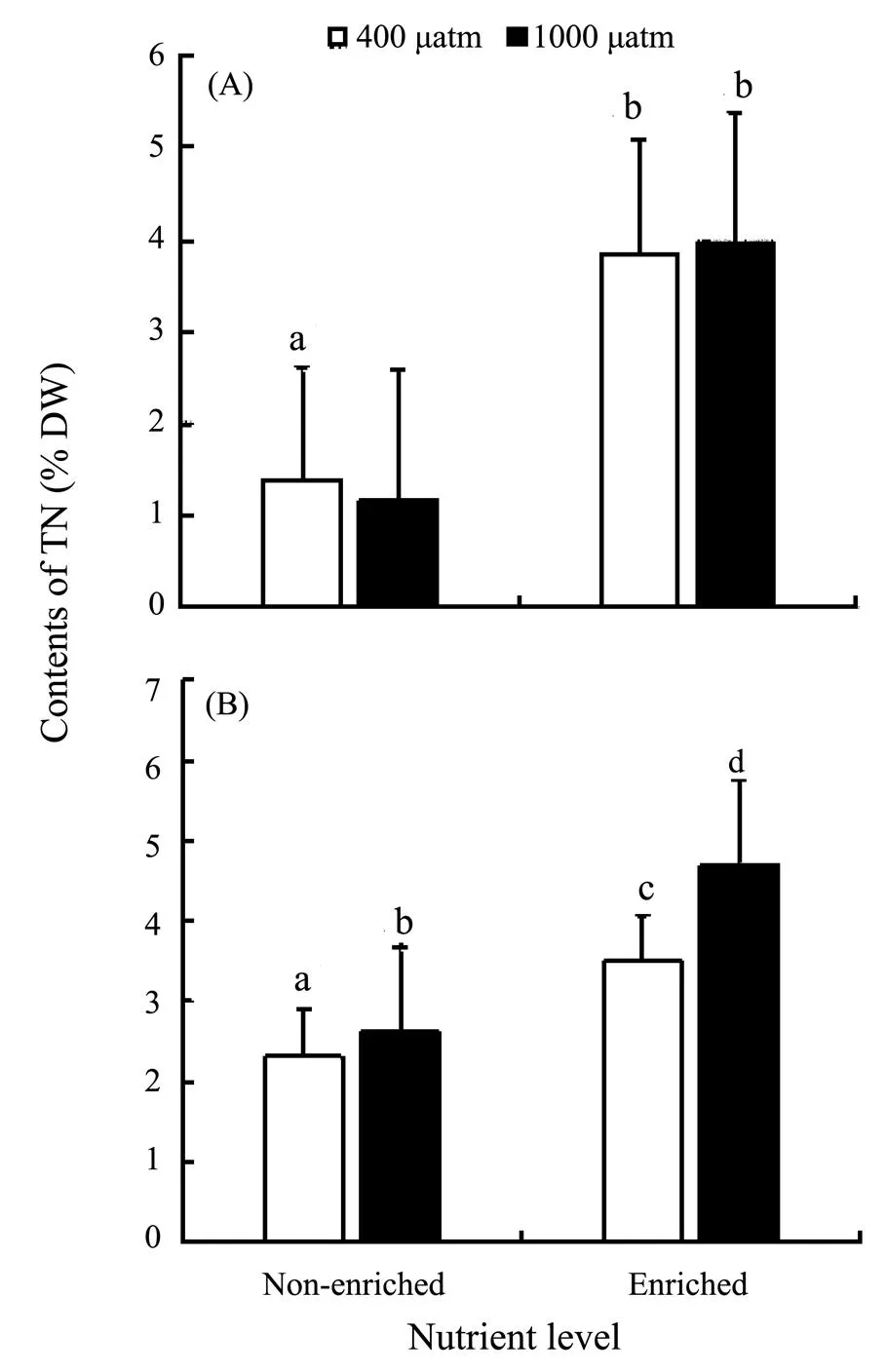
Fig.4 Tissue nitrogen (TN) of S. japonica (A) and S. horneri (B) cultured at different pCO2 and nutrient conditions for 6 days. Data are reported as means ± SD (n=3). Different letters above the error bars indicate significant differences between treatments (P<0.05).
In this study, promotions in RGR were detected in bothandat elevatedCO2although the increases were statistically non-significant. This indicates that bothandare capable of OA resistance with atmospheric CO2increased to 1000μatm. To show which algae is competitively dominant under OA condition, we analyzed the Pn, Rd, Chl, Chland TN in both species. The results showed that enhancements to Pn, Rd, and chlorophyll contents ofwere parallel withCO2elevation. These results are in line with previous investigations on(Swanson and Fox, 2007; Zhang., 2020). The enhancement of Pnand chlorophyll contents were also found in other marine macrophytes, including,and(Kang., 2017; Li., 2018; Bao., 2019). However, the Pnand chlorophyll contents ofare twice as high as those of.increased the utilization of HCO3-to maintain its photosynthesis at a higher level. Since Pnand Chlofalso increased at 1000 μatm (Figs.2B, 3D), photosynthesis ofwas further improved on the basis of the original high level. These results indicate that higher photosynthetic level insuredpotentially greater resilience to OA in comparison to.
The significant enhancement in growth was observed inin nutrient-enriched condition, while no promotion of growth was found in(Fig.1). In this study, the concentrations of dissolved inorganic nitrate and ammonium were simultaneously increased in nutrient-enriched treatments (Tatewaki, 1966).Increase in nitrogen availability can enhance macroalgae in N uptake rates, tissue N contents, and photosynthetic rates (Valiela., 1997). These enhancements accelerate the growth of macroalgae. The significant increase in Chland TN contents were detected in both species in nutrient-en- riched treatments (Figs.3, 4). Previous studies have also determined the same positive physiological responses in,,and other macroalgae (Valiela., 1997; Kawamitsu and Boyer, 1999; Wu., 2008; Raven and Hurd, 2012; Ohlsson., 2020). The kinetics of nutrients uptake in macroalgae is affected by the physiological status and the form of nutrients (Raven and Hurd, 2012; Gao., 2017). It has been reported thatutilize ammonium first when ammonium and nitrate both exist (Wang., 2013), whilefirstly takes advantage of nitrate (Yu., 2019). We estimated according to the measured ecophysiological traits, because the exact concentrations and formations of nitrogen in culture medium were unclear.performed higher Pn, more chlorophyll and TN accumulations under nutrient-enriched condition. Thus, the eutrophic treatment in this study more significantly benefited, indicating the increased risk of-dominated golden tide in eutrophic condition.
The current study argued the responses of bothandunder synergistic stress of OA and eutrophication. Significant enhancement in chlorophyll and TN contents was observed in both species (Fig.3, Fig.4). These results indicated that bothandimproved carbon and nitrogen assimilation. The exceeding nutrient availability in eutrophic scenario regulates these physiological responses in macroalgae to hence the negative effects resulting from declining pH in OA (Young and Gobler, 2016; Chu., 2020). However, significant increase in growth was only observed on(Fig.1). Increased carbon and nitrogen assimilation inenhanced its growth more than. These advantages in ecophyisiological traits may allowremain dominant and cause damage tocultivation in future acidified and eutrophic ocean. Furthermore, the damage resulting from golden tide tocultivation is likely to be more severe.has vesicles in structure, which can keep the thalli floating and increase carbon acquisition (Smetacek and Zingone, 2013; Choi., 2020). Floatingwrap the rafts, shading the cultivatedbelow (Wu., 2019; Xiao, 2020). Thus, we suppose that increasingbiomass shaded cultivatedin a more severe environment with lower light intensity and less carbon availability (Xiao, 2020). Thedominated golden tide may cause greater damage tocultivation in acidified and eutrophic ocean. In addition, we need meso-scale experiments to estimate the increasing risk of the golden tide incultivation.
5 Conclusions
It is important to estimate the damage tocultivation by golden tide resulting fromunder the synergistic stress of OA and eutrophication. In this study, we determined that nutrient enrichment contributedto utilize HCO3−.exhibited better photosynthetic traits than, and tissue nitrogen also accumulated more in thalli ofin elevatedCO2and nutrient-enriched treatments. Furthermore, increased carbon and nitrogen assimilation enhanced the growth ofin acidified and eutrophic scenario. Together,may cause greater damage tocultivation in acidified and eutrophic ocean.
Acknowledgements
We sincerely thank Dr. Zhu Dasheng, from Shandong Lidao Oceanic Technology Company Limited, for his help in providing algal materials for the experiment. This work is funded by the Major Scientific and Technological Innovation Project of Shandong Provincial Key Research and Development Program (No. 2019JZZY020708).
Andersen, J. H., Carstensen, J., Conley, D. J., Dromph, K., Fle- ming-Lehtinen, V., Gustafsson, B. G.,., 2017. Long- term temporal and spatial trends in eutrophication status of the Baltic Sea., 92: 135-149, DOI: 10. 1111/brv.12221.
Anderson, C. R., Moore, S. K., Tomlinson, M. C., Silke, J., and Cusack, C. K., 2015. Living with harmful algal blooms in a changing world: Strategies for modeling and mitigating their effects in coastal marine ecosystems. In:. Elsevier Inc., 495-561, DOI: 10.1016/B978-0-12-396483-0.00017-0.
AOAN, 2019.–. US Department of Commerce, NOAA, Global Monitoring Laboratory.
Axelsson, L., Mercado, J., and Figueroa, F., 2000. Utilization of HCO3−at high pH by the brown macroalga., 35: 53-59, DOI: 10. 1080/09670260010001735621.
Badger, M., 2003. The roles of carbonic anhydrases in photo- synthetic CO2concentrating mechanisms., 77: 83-94, DOI: 10.1023/A:1025821717773.
Bao, M., Wang, J., Xu, T., Wu, H., Li, X., and Xu, J., 2019. Rising CO2levels alter the responses of the red macroalgaunder light stress., 501: 325- 330, DOI: 10.1016/j.aquaculture.2018.11.011.
Bjork, M., Haglund, K., Ramazanov, Z., and Pedersen, M., 1993. Inducible mechanisms for HCO3−utilization and repression of photorespiration in protoplasts and thalli of three species of(Chlorophyta)., 29: 166-173, DOI: 10.1111/j.0022-3646.1993.00166.x.
Brockmann, U., Topcu, D., Schütt, M., and Leujak, W., 2018. Eutrophication assessment in the transit area German Bight (North Sea) 2006–2014–Stagnation and limitations., 136: 68-78, DOI: 10.1016/j.marpolbul. 2018.08.060.
Cai, W. J., Hu, X., Huang, W. J., Murrell, M. C., Lehrter, J. C., Lohrenz, S. E.,., 2011. Acidification of subsurface coastal waters enhanced by eutrophication., 4: 766-770, DOI: 10.1038/ngeo1297.
Choi, S. K., Oh, H. J., Yun, S. H., Lee, H. J., Lee, K., Han, Y. S.,., 2020. Population dynamics of the ‘golden tides’ sea- weed,, on the southwestern coast of Korea: The extent and formation of golden tides., 12, DOI: 10.3390/su12072903.
Chu, Y., Liu, Y., Li, J., and Gong, Q., 2019. Effects of elevatedCO2and nutrient enrichment on the growth, photosynthesis, and biochemical compositions of the brown alga(Laminariaceae, Phaeophyta)., 2019: e8040, DOI: 10.7717/peerj.8040.
Chu, Y., Liu, Y., Li, J., Wang, Q., and Gong, Q., 2020. Nutrient enrichment regulates the growth and physiological responses ofto ocean acidification., 19: 895-901, DOI: 10.1007/s11 802-020-4359-7.
Chung, I. K., Sondak, C. F. A., and Beardall, J., 2017. The future of seaweed aquaculture in a rapidly changing world., 52: 495-505, DOI: 10.1080/ 09670262.2017.1359678.
Cornwall, C. E., Hepburn, C. D., Pritchard, D., Currie, K. I., Mcgraw, C. M., Hunter, K. A., and Hurd, C. L., 2012. Car- bon-use strategies in macroalgae: Differential responses to lowered pH and implications for ocean acidification., 48: 137-144, DOI: 10.1111/j.1529-8817.2011. 01085.x.
Doney, S. C., Fabry, V. J., Feely, R. A., and Kleypas, J. A., 2009. Ocean acidification: The other CO2problem., 1: 169-192, DOI: 10.1146/annurev. marine.010908.163834.
Duarte, C. M., and Krause-Jensen, D., 2018. Intervention op- tions to accelerate ecosystem recovery from coastal eutrophi- cation., 5: 470, DOI: 10. 3389/ fmars.2018.00470.
Edmunds, P. J., 2011. Zooplanktivory ameliorates the effects of ocean acidification on the reef coralspp., 56: 2402-2410, DOI: 10.4319/lo.2011.56. 6.2402.
Eichner, M., Rost, B., and Kranz, S. A., 2014. Diversity of ocean acidification effects on marine N2fixers., 457: 199-207, DOI: 10.1016/j.jembe.2014.04.015.
Enochs, I. C., Manzello, D. P., Donham, E. M., Kolodziej, G., Okano, R., Johnston, L.,., 2015. Shift from coral to macroalgae dominance on a volcanically acidified reef., 5: 1083-1088, DOI: 10.1038/nclimate 2758.
Feely, R., Doney, S., and Cooley, S., 2009a. Ocean acidification: Present conditions and future changes in a high-CO2world., 22: 36-47, DOI: 10.5670/oceanog.2009.95.
Feely, R. A., Orr, J., Fabry, V. J., Kleypas, J. A., Sabine, C. L., Langdon, C., 2009b. Present and future changes in seawater chemistry due to ocean acidification. In:. American Geophysical Union, 173-188, DOI: 10.1029/2005GM000337.
Feely, R. A., Sabine, C. L., Lee, K., Berelson, W., Kleypas, J., Fabry, V. J., and Millero, F. J., 2004. Impact of anthropo- genic CO2on the CaCO3system in the oceans., 305: 362-366, DOI: 10.1126/science.1097329.
Filbee-Dexter, K., and Wernberg, T., 2018. Rise of turfs: A new battlefront for globally declining kelp forests., 68: 64-76, DOI: 10.1093/biosci/bix147.
Gao, K., Beardall, J., Häder, D. P., Hall-Spencer, J. M., Gao, G., and Hutchins, D. A., 2019. Effects of ocean acidification on marine photosynthetic organisms under the concurrent in- fluences of warming, UV radiation, and deoxygenation., 6: 322, DOI: 10.3389/fmars.2019. 00322.
Gao, G., Clare, A. S., Chatzidimitriou, E., Rose, C., and Cald- well, G., 2018. Effects of ocean warming and acidification, combined with nutrient enrichment, on chemical composition and functional properties of., 258: 71-78, DOI: 10.1016/j.foodchem.2018.03.040.
Gao, X., Endo, H., Nagaki, M., and Agatsuma, Y., 2017. Interactive effects of nutrient availability and temperature on growth and survival of different size classes of(Laminariales, Phaeophyceae)., 56: 253- 260, DOI: 10.2216/16-91.1.
Gazeau, F., Quiblier, C., Jansen, J. M., Gattuso, J. P., Middel- burg, J. J., and Heip, C. H. R., 2007. Impact of elevated CO2on shellfish calcification., 34: L07603, DOI: 10.1029/2006GL028554.
Ge, C., Chai, Y., Wang, H., and Kan, M., 2017. Ocean acidifi- cation: One potential driver of phosphorus eutrophication., 115: 149-153, DOI: 10.1016/j.mar polbul.2016.12.016.
Giordano, M., Beardall, J., and Raven, J. A., 2005. CO2con- centrating mechanisms in algae: Mechanisms, environmen- tal modulation, and evolution., 56: 99-131, DOI: 10.1146/annurev.arplant.56.032 604.144052.
Glibert, P., Anderson, D., Gentien, P., Granéli, E., and Sellner, K., 2005. The global, complex phenomena of harmful algal blooms., 18: 136-147, DOI: 10.5670/oceanog. 2005.49.
Guan, Y., Hohn, S., Wild, C., and Merico, A., 2020. Vulnerabi- lity of global coral reef habitat suitability to ocean warming, acidification and eutrophication., 26: 5646-5660, DOI: 10.1111/gcb.15293.
Heisler, J., Glibert, P. M., Burkholder, J. M., Anderson, D. M., Cochlan, W., Dennison, W. C.,., 2008. Eutrophication and harmful algal blooms: A scientific consensus., 8: 3-13, DOI: 10.1016/j.hal.2008.08.006.
Hoegh-Guldberg, O., Mumby, P. J., Hooten, A. J., Steneck, R. S., Greenfield, P., Gomez, E.,., 2007. Coral reefs under rapid climate change and ocean acidification., 318: 1737-1742, DOI: 10.1126/science.1152509.
Hurd, C. L., Beardall, J., Comeau, S., Cornwall, C. E., Havenhand, J. N., Munday, P. L.,., 2020. Ocean acidifi- cation as a multiple driver: How interactions between changing seawater carbonate parameters affect marine life., 71: 263-274, DOI: 10. 1071/MF19267.
Hutchins, D. A., and Fu, F., 2017. Microorganisms and ocean global change., 2: 17058, DOI: 10. 1038/nmicrobiol.2017.58.
Israel, A., and Hophy, M., 2002. Growth, photosynthetic proper- ties and Rubisco activities and amounts of marine macroalgae grown under current and elevated seawater CO2concentra- tions., 8: 831-840, DOI: 10.1046/j. 1365-2486.2002.00518.x.
Johnson, M. D., and Carpenter, R. C., 2018. Nitrogen enrich- ment offsets direct negative effects of ocean acidification on a reef-building crustose coralline alga., 14 (7): 20180371, DOI: 10.1098/rsbl.2018.0371.
Joos, F., and Spahni, R., 2008. Rates of change in natural and anthropogenic radiative forcing over the past 20000 years., 105: 1425-1430, DOI: 10.1073/ pnas.0707386105.
Kang, J. W., Kambey, C., Shen, Z., Yang, Y., and Chung, I. K., 2017. The short-term effects of elevated CO2and ammonium concentrations on physiological responses in(Rhodophyta)., 20: 18, DOI: 10.1186/s41240-017-0063-y.
Kawamitsu, Y., and Boyer, J. S., 1999. Photosynthesis and carbon storage between tides in a brown alga,., 133: 361-369, DOI: 10.1007/s002270 050475.
Kim, J. K., Yarish, C., Hwang, E. K., Park, M., and Kim, Y., 2017. Seaweed aquaculture: Cultivation technologies, cha- llenges and its ecosystem services., 32: 1-13, DOI: 10. 4490/algae.2017.32.3.3.
Koch, M., Bowes, G., Ross, C., and Zhang, X. H., 2013. Clima- te change and ocean acidification effects on seagrasses and marine macroalgae., 19: 103-132, DOI: 10.1111/j.1365-2486.2012.02791.x.
Kroeker, K. J., Kordas, R. L., Crim, R., Hendriks, I. E., Ramajo, L., Singh, G. S.,., 2013. Impacts of ocean acidification on marine organisms: Quantifying sensitivities and interac- tion with warming., 19: 1884-1896, DOI: 10.1111/gcb.12179.
Kudela, R. M., Bickel, A., Carter, M. L., Howard, M. D. A., and Rosenfeld, L., 2015. The monitoring of harmful algal blooms through ocean observing: The development of the California harmful algal bloom monitoring and alert program. In:. Elsevier Inc., 58-75, DOI: 10.1016/B978-0-12-802022-7.00005-5.
Li, Y., Zhong, J., Zheng, M., Zhuo, P., Xu, N., 2018. Photope- riod mediates the effects of elevated CO2on the growth and physiological performance in the green tide alga., 141: 24-29, DOI: 10.1016/j.marenvres.2018.07.015.
Liu, D., Keesing, J. K., He, P., Wang, Z., Shi, Y., and Wang, Y., 2013. The world’s largest macroalgal bloom in the Yellow Sea, China: Formation and implications., 129: 2-10, DOI: 10.1016/j.ecss.2013.05. 021.
McCrackin, M. L., Jones, H. P., Jones, P. C., and Moreno-Ma- teos, D., 2017. Recovery of lakes and coastal marine eco- systems from eutrophication: A global meta-analysis.,62: 507-518, DOI: 10.1002/lno.10441.
MEE, 2019.. Beijing, 1-22.
Murray, C. J., Müller-Karulis, B., Carstensen, J., Conley, D. J., Gustafsson, B. G., and Andersen, J. H., 2019. Past, present and future eutrophication status of the Baltic Sea., 6: 2, DOI: 10.3389/fmars.2019.00002.
Norkko, A., and Bonsdorff, E., 1996a. Rapid zoobenthic com- munity responses to accumulations of drifting algae., 131: 143-157, DOI: 10.3354/meps 131143.
Norkko, A., and Bonsdorff, E., 1996b. Population responses of coastal zoobenthos to stress induced by drifting algal mats., 140: 141-151, DOI: 10. 3354/meps140141.
Ohlsson, L. O., Karlsson, S., Rupar-Gadd, K., Albers, E., and Welander, U., 2020. Evaluation ofandfor biogas production and nutrient recycling., 140: 105670, DOI: 10. 1016/j.biombioe.2020.105670.
Okino, T., and Kato, K., 1987. Lake Suwa–Eutrophication and its partial recent recovery., 14: 373-375, DOI: 10. 1007/BF00208212.
Pedersen, M., and Borum, J., 1997. Nutrient control of estuarine macroalgae: Growth strategy and the balance between nitro- gen requirements and uptake., 161: 155-163, DOI: 10.3354/meps161155.
Rabouille, C., Conley, D. J., Dai, M. H., Cai, W. J., Chen, C. T. A.,., 2008. Comparison of hypoxia among four river- dominated ocean margins: The Changjiang (Yangtze), Miss- issippi, Pearl, and Rhône Rivers.,28: 527-1537, DOI: 10.1016/j.csr.2008.01.020.
Raven, J. A., and Hurd, C. L., 2012. Ecophysiology of photo- synthesis in macroalgae. In:. Spring- er, 105-125, DOI: 10.1007/s11120-012-9768-z.
Reiskind, J. B., Seamon, P. T., and Bowes, G., 1988. Alternative methods of photosynthetic carbon assimilation in marine macroalgae., 87: 686-692, DOI: 10.1104/ pp.87.3.686.
Reymond, C. E., Lloyd, A., Kline, D. I., Dove, S. G., and Pan- dolfi, J. M., 2013. Decline in growth of foraminiferunder eutrophication and ocean acidification scenarios., 19: 291-302, DOI: 10. 1111/gcb.12035.
Smetacek, V., and Zingone, A., 2013. Green and golden seaw- eed tides on the rise., 504: 84-88, DOI: 10.1038/na ture12860.
Smith, S. V., Swaney, D. P., Talaue-McManus, L., Bartley, J. D., Sandhei, P. T., McLaughlin, C. J.,., 2003. Humans, hy- drology, and the distribution of inorganic nutrient loading to the ocean., 53: 235-245, DOI: 10.1641/0006- 3568(2003)053[0235:HHATDO]2.0.CO;2.
Strokal, M., Yang, H., Zhang, Y., Kroeze, C., Li, L., Luan, S.,., 2014. Increasing eutrophication in the coastal seas of China from 1970 to 2050., 85: 123- 140, DOI: 10.1016/j.marpolbul.2014.06.011.
Swanson, A. K., and Fox, C. H., 2007. Altered kelp (Lamina- riales) phlorotannins and growth under elevated carbon dioxide and ultraviolet-B treatments can influence associated intertidal food webs., 13: 1696-1709. DOI: 10.1111/j.1365-2486.2007.01384.x.
Tatewaki, M., 1966. Formation of a crustaceous sporophyte with unilocular sporangia in., 6: 62-66, DOI: 10.2216/i0031-8884-6-1-62.1.
Valiela, I., McClelland, J., Hauxwell, J., Behr, P. J., Hersh, D., and Foreman, K., 1997. Macroalgal blooms in shallow estua- ries: Controls and ecophysiological and ecosystem conse- quences., 42: 1105-1118, DOI: 10.4319/lo.1997.42.5_part_2.1105.
van der Struijk, L. F., and Kroeze, C., 2010. Future trends in nutrient export to the coastal waters of South America: Impli- cations for occurrence of eutrophication., 24: 1-14, DOI: 10.1029/2009GB003572.
Wang, B., Xin, M., Wei, Q., and Xie, L., 2018. A historical overview of coastal eutrophication in the China Seas., 136: 394-400, DOI: 10.1016/j.marpolbul. 2018.09.044.
Wang, Y., Xu, D., Fan, X., Zhang, X., Ye, N., Wang, W.,., 2013. Variation of photosynthetic performance, nutrient up- take, and elemental composition of different generations and different thallus parts of., 25: 631-637, DOI: 10.1007/s10811-012- 9897-y.
Watson, S. B., Whitton, B. A., Higgins, S. N., Paerl, H. W., Brooks, B. W., and Wehr, J. D., 2015. Harmful algal blooms. In:. Elsevier Inc., 873-920, DOI: 10.1016/B978-0- 12-385876-4.00020-7.
Wernberg, T., Krumhansl, K., Filbee-Dexter, K., and Pedersen, M. F., 2018. Status and trends for the world’s kelp forests. In:. Elsevier, 57-78, DOI: 10.1016/B978-0-12-805052-1.00003-6.
Wu, H., Feng, J., Li, X., Zhao, C., Liu, Y., Yu, J., amd Xu, J., 2019. Effects of increased CO2and temperature on the physiological characteristics of the golden tide blooming ma- croalgaein the Yellow Sea, China., 146: 639-644, DOI: 10.1016/j.mar polbul.2019.07.025.
Wu, H. Y., Zou, D. H., and Gao, K. S., 2008. Impacts of in- creased atmospheric CO2concentration on photosynthesis and growth of micro- and macro-algae., 51: 1144-1150, DOI: 10.1007/s11 427-008-0142-5.
Xiao, J., Wang, Z., Song, H., Fan, S., Yuan, C., Fu, M.,., 2020. An anomalous bi-macroalgal bloom caused byandseaweeds during spring to summer of 2017 in the western Yellow Sea, China., 93: 101760, DOI: 10.1016/j.hal.2020.101760.
Xu, D., Brennan, G., Xu, L., Zhang, X. W., Fan, X., Han, W. T.,., 2019. Ocean acidification increases iodine accumula- tion in kelp-based coastal food webs., 25: 629-639, DOI: 10.1111/gcb.14467.
Young, C. S., and Gobler, C. J., 2016. Ocean acidification acce- lerates the growth of two bloom-forming macroalgae., 5: e0155152, DOI: 10.1371/journal.pone.0155 152.
Yu, J., Li, J., Wang, Q., Liu, Y., and Gong, Q., 2019. Growth and resource accumulation of drifting(Fucales, Phaeophyta) in response to temperature and nitro- gen supply., 18: 1216- 1226, DOI: 10.1007/s11802-019-3835-4.
Zhang, X., Xu, D., Guan, Z., Wang, S., Zhang, Y., Wang, W.,., 2020. Elevated CO2concentrations promote growth and photosynthesis of the brown alga., 32: 1949-1959, DOI: 10.1007/s 10811-020-02108-1.
. Tel: 0086-532-82032377 E-mail: qdlijingyu@ouc.edu.cn
November 25, 2020;
March 2, 2021;
March 30, 2021
© Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2021
(Edited by Ji Dechun)
杂志排行
Journal of Ocean University of China的其它文章
- Numerical Modelling for Dynamic Instability Process of Submarine Soft Clay Slopes Under Seismic Loading
- DcNet: Dilated Convolutional Neural Networks for Side-Scan Sonar Image Semantic Segmentation
- Bleaching with the Mixed Adsorbents of Activated Earth and Activated Alumina to Reduce Color and Oxidation Products of Anchovy Oil
- Effects of Dietary Protein and Lipid Levels on Growth Performance, Muscle Composition, Immunity Index and Biochemical Index of the Greenfin Horse-Faced Filefish (Thamnaconus septentrionalis) Juvenile
- Transcriptome Analysis Provides New Insights into Host Response to Hepatopancreatic Necrosis Disease in the Black Tiger Shrimp Penaeus monodon
- Genome-Wide Patterns of Codon Usage in the Pacific Oyster Genome
