Advances in Chemical Constituents and Pharmacological Activities of Pleuropterus multiflorus
2021-08-10ShenXiaojingZhangGanjuanQiYangJuntaoWangQingJiangWeiwei
Shen Xiao-jing, Zhang Gan-juan, LÜ Qi, Yang Jun-tao, Wang Qing, Jiang Wei-wei*
Advances in Chemical Constituents and Pharmacological Activities of
Shen Xiao-jing1,2, Zhang Gan-juan1, LÜ Qi1, Yang Jun-tao1, Wang Qing1, Jiang Wei-wei1*
(1. College of Science, Yunnan Agricultural University,Kunming 65020, China;2. Yunnan Key Laboratory of Pharmacology for Natural Products, Kunming Medical University,Kunming 650500, China)
, known as one of precious perennial Chinese traditional medicine, belonging togenus and Polygonaceae family, distributed mainly in Sichuan, Yunnan, Guizhou and southern Shanxi and Gansu in ChinaThe modern studies showed thatcould be used for liver injury, cancer, diabetes, alopecia, atherosclerosis, and neurodegenerative diseases as well. In recent years, liver injuries caused by takinghave been reported worldwide. Therefore, the model of safety monitoring and risk management ofis very important. Thechemical constituents and medicinal activities of, including the toxic effects were summarized for the further studies and development, which is beneficial for the strengthening standardization of clinical applications, basic science research, quality control in manufacturing.
; Chemical constituent; Pharmacological activities; Review
Traditional Chinese Medicine (TCM) plays a significant role in Chinese civilization and is widely used in western societies, Asia, Africa and the Middle East now[1].(hereinafter as PM), belonging togenus and Polygonaceae family, is known as one of precious perennial Chinese traditional medicine, also called Heshouwu in China, which is dried root tuber of. It is distributed mainly in provinces of Sichuan, Yunnan, Guizhou and southern Shanxi and Gansu in ChinaThe PM utilization in traditional Chinese at the first time can be traced back to 973 A.D., recorded in Kaibao Bencao, an encyclopedia of medical plants. PM possesses variously pharmacological activities officially listed in the Chinese Pharmacopoeia. There are two forms of PM decoctions in the Chinese Pharmacopoeia (2015): Raw Radix(RPM) andPreparata (PMP). PM has been widely used for strengthening bones and muscles, preventing premature graying of the hair and treating seminal emission and menstrual complaints due to their multiple beneficial effects to human body in China. RPM contributes to detoxification and bowel relaxation, while PMP tonifies the liver and kidney, benefits essence of blood and black beard, and relieves hyperlipidemia, fatty liver, and osteoporosis. The modern studies indicated that PM can be used for liver injury, cancer, diabetes, alopecia, atherosclerosis, and neurodegenerative diseases as well. PM is also used in many Chinese medicinal supplements to improve general health. Along with the development of medical values of PM in recent years, it has been prepared as a tonic food and beverage and has become popular in Asia and many othercountries. However, liver injuries caused by taking PM have been reported worldwide. More than 130 compounds, including anthraquinones, stilbenes, phenolic acid, phospholipids, flavones and dianthrone derivatives, have been isolated from PM. Anthraquinones and stilbenes might relate to the toxic components.
Therefore, this paper summarized the chemical constituents and pharmacological activities of PM to provide a complete overview for the information cur- rently available, which will facilitate further research and exploitation of PM and is beneficial for the strengthening standardization of clinical applications, basic science research, quality control in manufac- turing.
1 Chemical constituents
1.1 Anthraquinones
At present, more than 20 anthraquinones had been found from PM, including anthraquinone aglycones, anthraquinone glycosides and anthraquinone methyl ethers (Table 1).
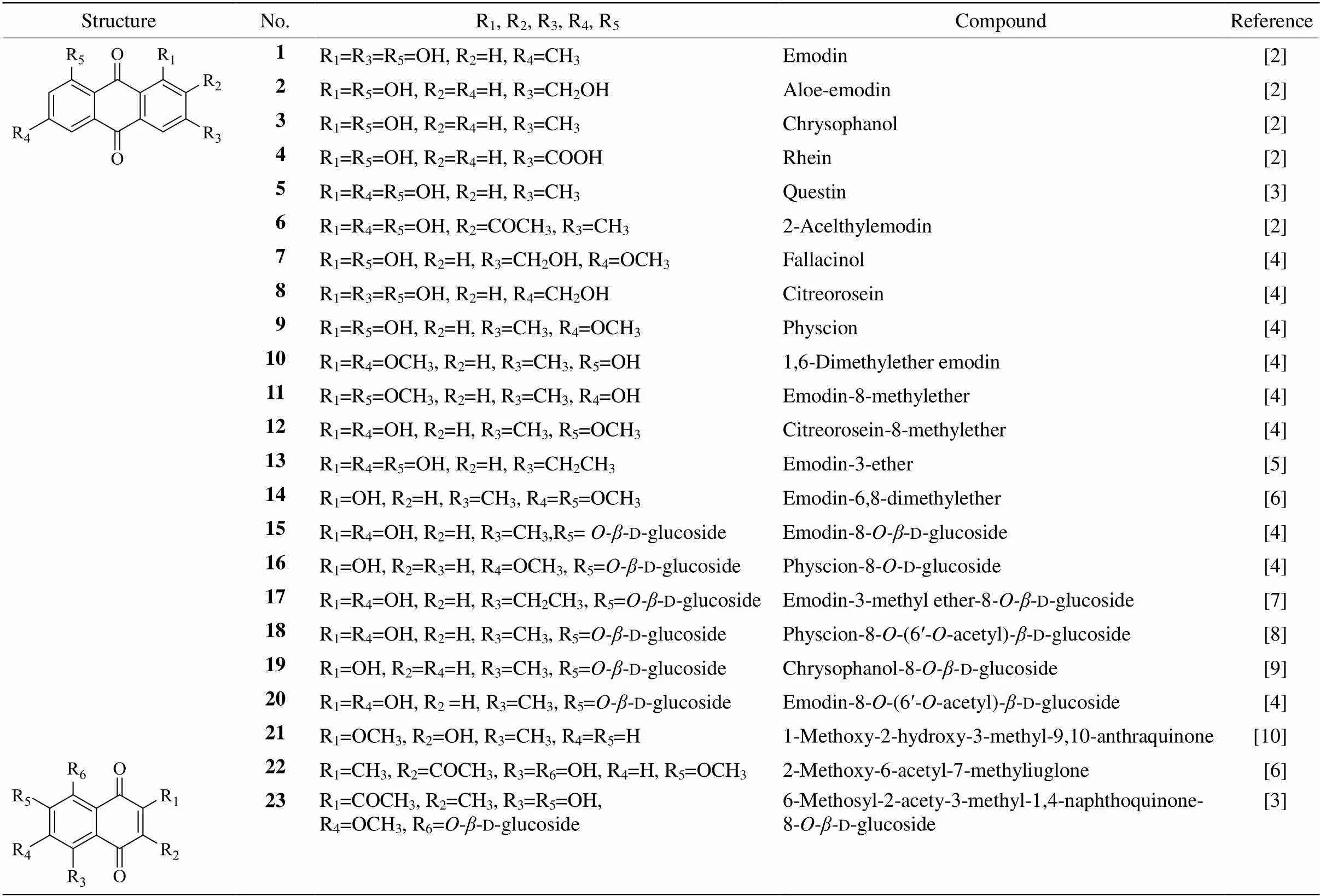
Table 1 Anthraquinones from Pleuropterus multiflorum
Compound 1 is a possessing anti-cancer activity anthraquinone derived fromPM and can dose-depen- dently inhibit the growth of HepG2 cells, perturb cell cycle progression, down-regulate the expression of genes and proteins related to glycolysis, and trigger intracellular ROS generation[11]. Compound 2 is an active ingredient of Chinese herbs, such as,,, andPM, which exhibits many pharmacological effects, including anticancer, antivirus, anti-inflammatory, antibacterial, antiparasitic, neuroprotective, and hepatoprotective activities. Therefore, it can be used to treat various diseases such as influenza virus, inflammation, sepsis, Alzheimer’s disease, glaucoma, malaria, liver fibrosis, psoriasis, Type 2 diabetes, growth disorders, and several types of cancers. However, some adverse effects of compound 2 have been reported, especially hepato- toxicity and nephrotoxicity. A poor intestinal absorption, short elimination half-life and low bioavailability of compound 2 have been demonstrated by pharmaco- kinetic studies[12]. Compound 4 is a major medicinal ingredient isolated from,,and PM, whichhas various pharmacological activities including anti-inflamema- tory, antitumor, antioxidant, antifibrosis, hepatopro- tective and nephroprotective activities[13].
1.2 Dianthrone derivatives
There are 14 dianthrone derivatives had been found from PM[14–16](Table 2). And 32 new dian- throne derivatives were tentatively characterized in PM using HPLC-UV/LTQ-FT-ICR-MS by Yang[17]. Among of them, compounds 24-27 showed moderate cytotoxic effects against KB tumor cell lines[14]. Compound 33 exhibited potential inhibitory effect on UGT1A1 activity[18].

Table 2 Dianthrone derivatives from Pleuropterus multiflorum
1.3 Stilbenes
Forty-two stilbenes (Table 3, 4, Fig. 1) have been isolated from PM[3–4,6,19–33]. Compound 38, 2,3,5,4ʹ- tetrahydroxystilbene-2--d-glucoside (TSG), as the main component ofPM was used as a standard compound for appraising PM in theChinese Pharma- copoeia[34]. Compound 38 is a bioactive natural pro- duction with anti-inflammatory and antitumor origin- nating. Itmight possess potent anti-breast cancer effect with adriamycin, which may exert a synergistic reduction of cell injury via the inhibition of vascular endothelial growth factor/phosphatidylinositol 3-kinase/ Akt pathway[35]. The antioxidant and free radical- scavenging activities of compound 38 even are much stronger than resveratrol[36].Compound 38also shown neuroprotective effect in various neurodegenerative diseases and cerebral ischemia such as Alzheimer’s disease, Parkinson’s disease and cerebral ischemia/reperfusion injury.It can inhibit apoptosis and protect neuronal cells against injury through multifunctional cytoprotective pathways[37]. Compound 38 also has the antiatherosclerosis, anti-inflammatory and anti- cardiac fibrotic effects[38–39]. Compound 38 could delay senescence and treat aging-related diseases, and even more effective than resveratrol in delaying sene- scence[40]. The moderate inhibitory activities against NO production of compound 69 and 70 had been con- firmed by Liin LPS-stimulated RAW264.7 cells[31].
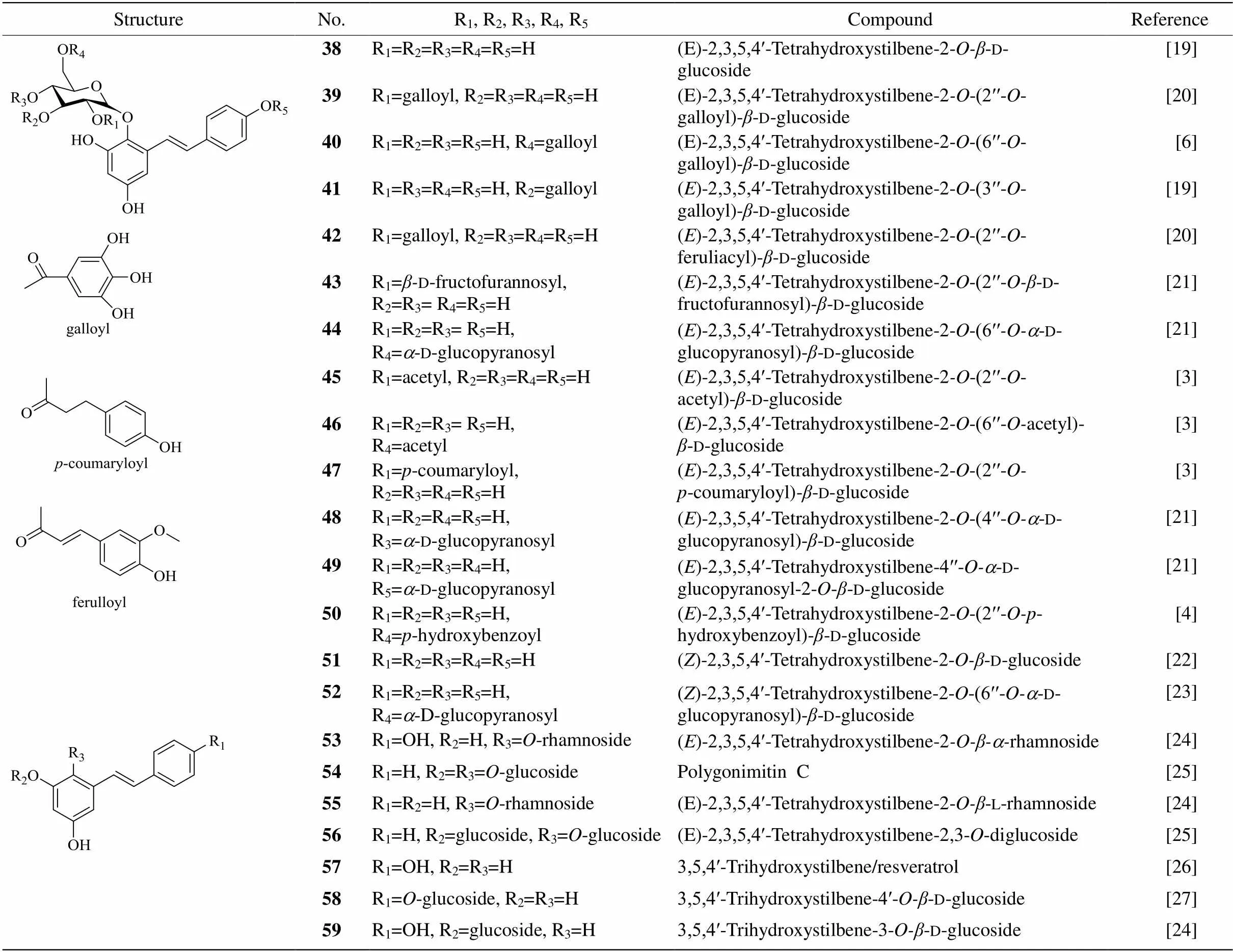
Table 3 Stilbenes (compounds 38-59) ofPleuropterus multiflorum
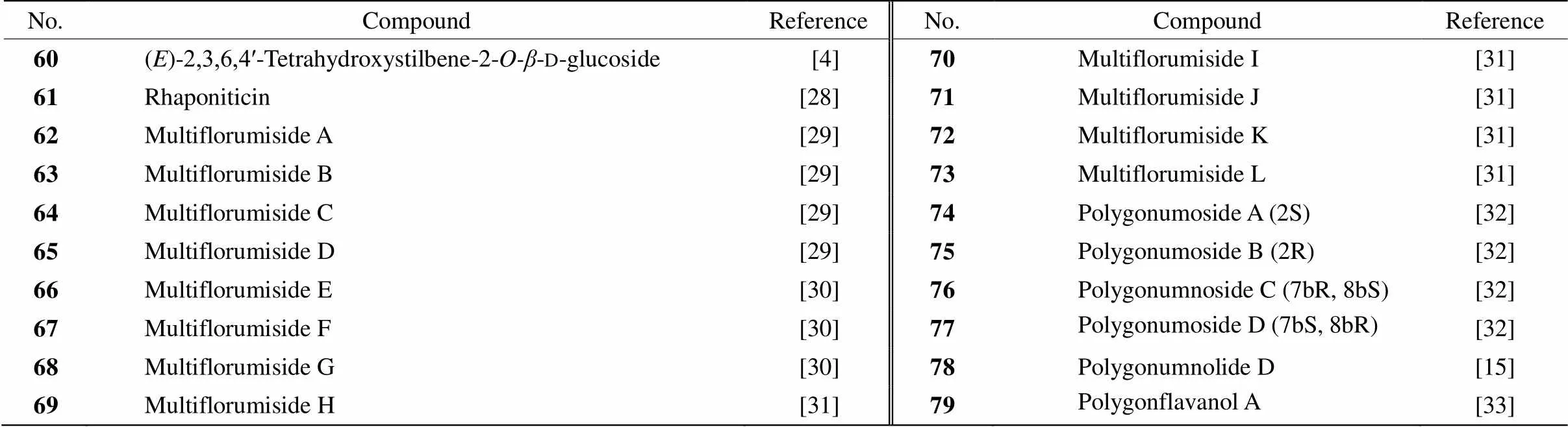
Table 4 Stilbenes (compounds 60-79) ofPleuropterus multiflorum
1.4 Flavones
There are 26 flavones (Table 5, 6, Fig. 2), one group of important natural compounds, have been isolated from PM[6–7,10,19,20,31,41–46], including rutin, kaempferol,quercetin and their derivatives. Modern medical research shows that flavones have multiple biological activities such as anti-diabetics, antioxidant,anti-cancer, anti-inflammatory activities.
1.5 Phospholipids
Phospholipids were also isolated from PM. Pho- spholipids are one of the most important constituents of brain tissue and cranial nerve and the main materials of cytomembrane synthesized by erythrocyte and other cells of organisms. Phospholipids fromPM included phosphatidyl choline, phosphatidyl ethanolamine, lysophosphatidyl choline, phosphatidiyl glycerol, 7- hydroxy-4-methyl coumarin-5--glucoside, 7-hydroxy- 3,4-dimethyl coumarin-5--glucoside and phosphate- dylinositol. A new aliphatic ketone, 1,2-dihydroxyno- nadecone-3, was confirmed fromchloroform section of Radix Polygoni Multiflori Preparata[4]. Two new phospholipids,1--stearoyl-2--d-D4ʹ,7ʹ-dodecenoyl-3--phosphatodic acid---d-glucoside and 1--stearoyl-2--d-D4ʹ,7ʹ-dodecenoyl-3--phosphatodic acid--(6ʹ---d-glucose)--d-glucoside were isolated from Radix Polygoni Multiflori Preparata[33]. Other phospholipids from Radix Polygoni Multiflori Pre- parata, including dodecane, eicosane, hexanoic acid, hexadecanoic acid methyl ester, hexadecanoic acid ethyl ester, octadecanoic acid methyl ester, octade- canoic acid ethyl ester, ethyl oleate, docosanoic acid methyl ester, methyl palmitate, ethyl palmitate, tetra- decanoatic acid ethyl ester, diphosphatidiyl glycerol, copaene and squalene[15].

Fig. 1 Structures of compounds 60-79
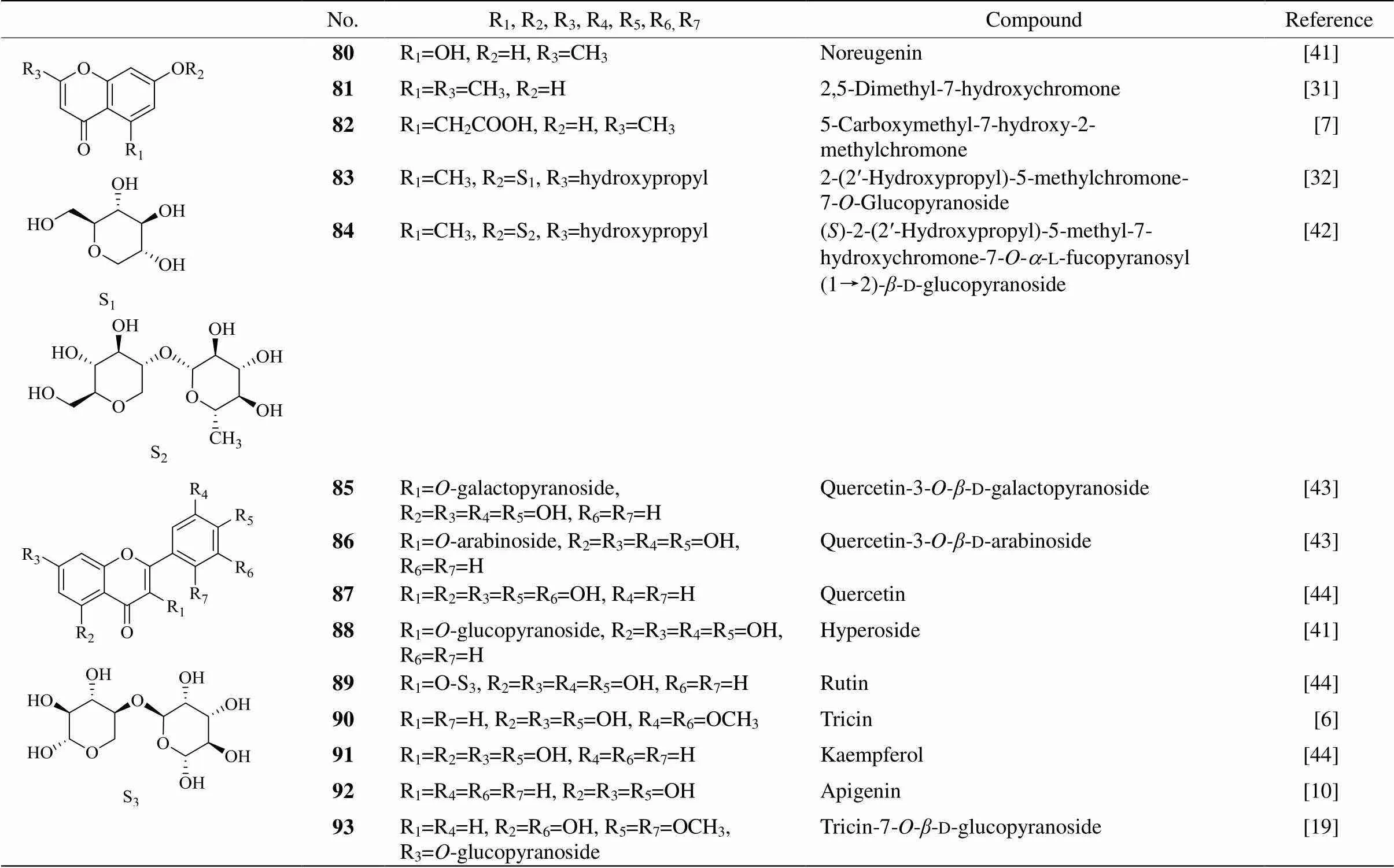
Table 5 Flavones (compounds 80-93) from Pleuropterus multiflorum
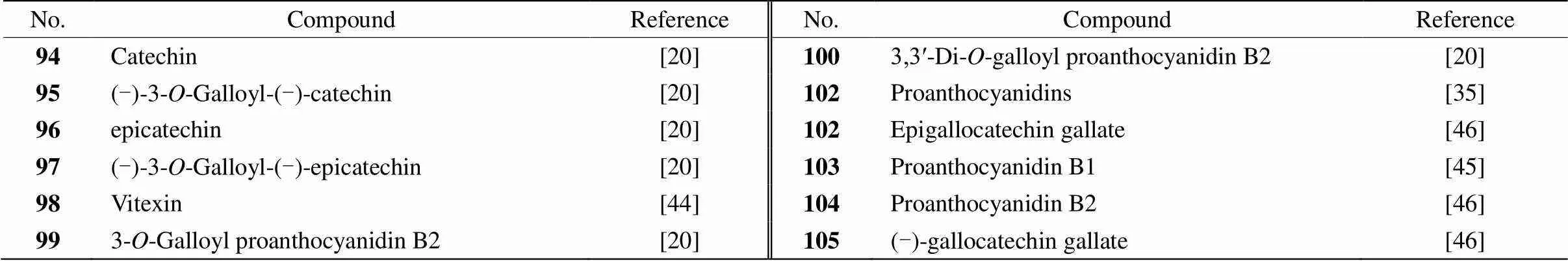
Table 6 Flavones (compounds 94-105) from Pleuropterus multiflorum

Fig. 2 Structures of compounds 94-105
1.6 Other chemical compounds
Additionally,-sitosterol, gallic acid, torachry- sone-8---d-glucopyranoside, daucosterol, torachry- chrysone-8--(6ʹ--acetyl)--d-glucopyranoside,--feruloyl tyramine,--feruloyl-3-methyldo- pamine, schizandrin, indole-3-(l--amino--hydroxy- propionicacid)-methyl ester, 7-hydroxyl-4-methylcou- marin-5---d-glucopyranoside, 7-hydroxyl-3,4-methyl-coumarin-5---d-glucopyrano-side, 1,3-dihydroxyl- 6,7-dimethyl xanthine-1---oxymethyl-7-hydroxyl-2- methyl chromone,-amtrin were and 1,2-dihydroxyl- nonadecanone-3, were isolated from PM[3,14,42].
2 Pharmacological activities
2.1 Anti-aging and antioxidant activities
PM could significantly reduce the activities of malondialdehyde (MDA), aspartate aminotransferase (AST) and alanine aminotransferase (ALT) in serum from d-galactose induced acute senescence rats, while improve the activities of glutathione peroxidase (GSH- px), superoxide dismutase (SOD)[47]. PM shown a protective effect on skin aging of mice, which could relate to increasing the dermis thickness of aging mice, reducing the level of insulin and IGF-1, and pro- moting the expression of collagen fibers[48]. Xu found that the extract of PMimproved the activity of SOD in rat heart and brain tissues, reduced the content of lipid peroxide and lipofuscin and showed significant anti- oxidant effects and a protective effect on the peroxi- dation damage of heart and brain tissues[49].
2.2 Improve immunity
The polysaccharide of(PPM) sig- nificantly inhibited cyclophosphamide-induced weight loss of immune organs and the decrease in the number of blood cells in mice and potentiated the immune- logical function in immunosuppressed mice. It signi- ficantly increased the phagocytic percentage and phagocytic index, the contents of serum hemolysin, the esterase positive rate of T-lymphocytes and Con A-induced proliferation of splenic T-lymphocytes, which indicated that PPM had the function of enhancing immunity[50].
2.3 Dyslipidemia regulation
The findings of a clinical study on 50 hyperlipi- demia patients demonstrated that the lipid-owering effect of PM may be related to regulating action of the genes involved in cholesterol synthesis and lipoprotein metabolism[51].
2.4 Neuroprotective activity
The therapeutic effect of PM on neurodege- nerative diseases is quite obvious and widely recog- nized[52]. An investigation about therapeutic activity of PM in Alzheimer’s disease (AD) by Chen et al. shown that the scores for the Ability of Daily Living Scale and the Mini-Mental State Examination were signifi- cantly improved in the treatment group compared with the Chinese herb and western medicine control groups[53]. In a randomized, piracetam-controlled, single- center clinical trial, PM was evaluated as monotherapy for vascular dementia (VaD). The results shown that the total clinical effective rate was 71.25% and that the herbal medicinal had obvious therapeutic effect on VaD, with no relative adverse drug reactions[54].
2.5 Hepato-protective activity
andmodels, the hepatoprotective effects of ethanolic extract of PM were related to regulating the redox state in liver injury through Nrf2 activation and controlling hepatic bile acid homeo- stasis in obstructive cholestasis, through bile acid transporter expression modulation[55]. The study ofwater extract (PMW) by Wei et al. suggested that PMW accelerated bile acid entero- hepatic circulation and changed the composition of intestinal Bas lead to activation of Fxr-Fgf15 signal in intestines and further inhibition of the expression of Cyp7a1 in the liver[56].
2.6 Renal-protective activity
TSG from PM plays a concentration-dependent protective role in ameliorating the progression of an adriamycin (AD)-induced focal segmental glomerulo- sclerosis (FSGS) through activation of the Nrf2- Keap1 antioxidant pathway. TSG has the capacity of blocking angiotensin II (ANG II) signaling. In the streptozotocin (STZ)-induced diabetes model TSG was demonstrated the beneficial effect of therapy on renal damage though the inhibition of the RAS effectively to prevent renal injury in diabetic nephron- pathy[57].
2.7 Cardiovascular system
Tetrahydroxystilbene glucoside could induce relaxation of the superior mesenteric artery through an endothelium-dependent pathway that involves the inhibition of COX-2 activity and decreased in TXA2 and through an endothelium-independent pathwayopening of a voltage-dependent K+channel, blockade of Ca2+influx and release of intracellular Ca2+[58]. The cardioprotective effects of TSG have been demon- strated. TSG protected murine hearts against ischemia/ reperfusion injuryandby activating the Notch1/Hes1 signaling pathway and attenuating ER stress-induced apoptosis[59].
2.8 Anti-inflammatory and antibacterial activity
Lu et al.[60]reported that ethanol extract of(PME) had a significantly anti-inflame- matory effect. Chin et al.[61]found that TSG of PM could reduce periodontitis, gene expression of TNF-, interleukin-1 IL (IL-1) and IL-6, and inhibit the acti- vation of NK-kBand. Studies found that PM had the function of inhibiting human dysentery bacillus and mycobacterium tuberculosis. Especially, the anthraquinone derivatives of PM had inhibitory effect on bacteria, fungal influenza viruses and pathogens, such as paratyphoid rod 901, diph- theria bacillus, staphylococcus aureus, paratyphoid bacillus B, hemolytic streptococcus B and bacillus anthracis. Study confirmed that Raw Radixhad the effect of fighting staphylococcus aureus, whilepreparata had the effect of fighting staphylococcus albicans. And steamed and winepreparata had a better effect against diphtheria bacillus.
2.9 Other effects
A study on diabetes-related bone loss in mice shown that PMW significantly alleviated mouse body weight loss and hyperglycemia, and elevated serum levels of insulin, osteocalcin and bone- alkaline phosphatase. PMW might relieve diabetes- related bone disorders through regulating osteoclast- related genes and PM may be used as a preventive agent for diabetes-induced bone loss[62]. At the same time, tetra hydroxy stilbene glucoside from PM was also demonstrated the protected against diabetes- induced osteoporosis in mice with streptozotocin- induced hyperglycemia[63]. A study on PME in high- fat diet-induced obese mice demonstrated that PME might relieve obesity by inhibition of adipogenesis and lipogenesis and lipolysis and fatty acid oxide- tion[64].
3 Toxic effects
Although there are many reports referred to the toxicity of PM, especially for liver adverse reactions, mechanism of toxicity remains unclear. Liver injuries caused by PM have been reported and the incidences have increased in recent years[65–66]. Although the hepatotoxic chemicals attributing to the hepatic lesions of PM remain in dispute, the hepatotoxicity of emodin has been well documented in many studies[67–70]. Luteolin was reported to cause cytotoxicity in primary rat hepatocytes at dosages of 50mol/L or lower levels of concentration[71]. Apigenin was found to can significantly increase the accumulation of lipid droplets and cause fatty liver disease[72].He et al.[73]screen 25 ingredients in PM by a computational toxicology approach. Emodin, chrysophanol, rhein, danthron, aloe emodin, physcion, and apigenin could cause variable degrees of liver injury recorded in the literature.Emodin 8-glucoside, physcion-8--d-gluco- pyranoside, and luteolin were reported to possess potential hepatotoxicity. For the other 14 hepatotoxic ingredients, although direct evidence focused on their hepatotoxicity was not available, none of them was reported to be potential hepato-protector.Liu et al. hypothesized tannin, as another major component in PM, was one of the reasons for the induced liver damage[74]. While the components of PM responsible for the hepatotoxic effects have not yet been identified now, even many of the reports are contradictory, andthe mechanism involved in PM-induced liver damage are not comprehensive.Although many reports referring to toxicity of PM especially for liver adverse reactions have been reported, it might possible that PMW usage in TCM still keep safe currently, except high dose of HSW usage in a long term[75].
4 Conclusions
PM is an important medical plant wildly used in traditional Chinese medicine. There are abundant anthraquinones, stilbenes, flavones, phospholipids and dianthrone derivatives fromPM. At present these studies on PM mainly aimed at the medicinal effects, mechanisms and quality control. TSG, as the main component of PM, not only has many medicinal activities, but also has great potential exploitation for medicines. And with the concern about liver injury induced by PM, the model of safety monitoring and risk management of PM is very important based on quality control as one of the major safety problems in TCM drug safety concerns. The comprehensive know- ledge of PM by strengthening standardization of clinical applications, basic science research, quality control in manufacturing is significance. Measures should also be encouraged and implemented to promote healthy development of the TCM industry.
[1] WANG S M, LONG S Q, WU W Y. Application of traditional Chinese medicines as personalized therapy in human cancers [J]. Amer J Chin Med, 2018, 46(5): 953–970. doi: 10.1142/S0192415X18500507.
[2] WANG J B, MA Z J, NIU M, et al. Evidence chain-based causality identification in herb-induced liver injury: Exemplification of a well- known liver-restorative herb[J]. Front Med, 2015, 9(4): 457–467. doi: 10.1007/s11684-015-0417-8.
[3] CHEN W S, YANG G J, ZHANG W D, et al. Two new compounds of Radix Polygoni Multiflori Preparata [J]. Acta Pharm Sin, 2000, 35(4): 273–276. doi: 10.3321/j.issn:0513-4870.2000.04.009. (in Chinese)
[4] ZHANG J X, CUI Y M. Chemical constituents from[J]. J Chin Mat Med, 2016, 41(17): 3252–3255. doi: 10. 4268/cjcmm20161721. (in Chinese)
[5] CHEN W S, FAN W, YANG G J, et al. Studies on the chemical constituents of Radix Polygoni Multiflori Preparata [J]. Acad J Sec Mil Med Univ, 1999, 20(7): 438–440.(in Chinese)
[6] LI J B, LIN M. Studies on the chemical constituents of tuber fleece- flower () [J]. Chin Trad Herb Drugs, 1993, 24(3): 115–118,166. (in Chinese)
[7] LI X E, LIU J Z, LIAO S T, et al. Chemical constituents from tubers ofThunb. [J]. J Trop Subtrop Bot, 2009, 17(6): 617–620. doi: 10.3969/j.issn.1005-3395.2009.06.018. (in Chinese)
[8] SUN J L, HUANG X L, WU H Q, et al. HPLC/IT-MS analysis of glycosides in RadixPolygoni Multiflori [J]. Nat Prod Res Dev, 2009, 21(5): 806–812.(in Chinese) doi: 10.16333/j.1001-6880.2009.05.032.
[9] YANG X W, GU Z M, MA C M, et al. A new indole derivative isolated from the root of tuber fleeceflower () [J]. Chin Trad Herb Drugs, 1998, 29(1): 5–11
[10] ZHANG Z G, LÜ T S, YAO Q Q. Advances in the study of[J]. Pharm J Chin PLA, 2008, 24(1): 62–64,97. (in Chinese)
[11] XING Y X, LI M H, TAO L, et al. Anti-cancer effects of emodin on HepG2 cells as revealed by1H NMR based metabolic profiling [J]. J Proteome Res, 2018, 17(5): 1943–1952. doi: 10.1021/acs.jproteome. 8b00029.
[12] DONG X X, ZENG Y W, LIU Y, et al. Aloe-emodin: A review of its pharmacology, toxicity, and pharmacokinetics [J]. Phytother Res, 2020, 34(2): 270–281. doi: 10.1002/ptr.6532.
[13] Zhou Y X, Xia W, Yue W, et al. Rhein: A review of pharmaco- logical activities [J]. Evid Based Compl Alternat Med, 2015, 2015: 578107. doi: 10.1155/2015/578107.
[14] YANG J B, YAN Z, REN J, et al. Polygonumnolides A1–B3, minor dianthrone derivatives from the roots ofThunb. [J]. Arch Pharm Res, 2018, 41(6): 617–624. doi: 10.1007/s12272-016- 0816-7.
[15] YANG J B, TIAN J Y, DAI Z, et al.-Glucosides inhibitors extracted from the root ofThunb. [J]. Fitoterapia, 2017, 117: 65–70. doi: 10.1016/j.fitote.2016.11.009.
[16] YANG J B, LI L, DAI Z, et al. Polygonumnolides C1-C4; minor dianthrone glycosides from the roots ofThunb. [J]. J Asian Nat Prod Res, 2016, 18(9): 813–822. doi: 10.1080/10286 020.2016.1171758.
[17] YANG J B, LIU Y, WANG Q. et al. Characterization and identification of the chemical constituents ofThunb. by high- performance liquid chromatography coupled with ultraviolet detection and linear ion trap FT-ICR hybrid mass spectrometry [J]. J Pharm Biomed Anal, 2019, 172: 149–166. doi: 10.1016/j.jpba.2019.03.049.
[18] WANG Q, WANG Y D, LI Y, et al. Identification and characterization of the structure-activity relationships involved in UGT1A1 inhibition by anthraquinone and dianthrone constituents of[J]. Sci Rep, 2017, 7(1): 17952. doi: 10.1038/s41598-017-18231-y.
[19] YUAN W, GAO Z P, YANG J B, et al. Chemical constituents from[J]. Chin Trad Herb Drugs, 2017, 48(4): 631– 634. (in Chinese) doi: 10.7501/j.issn.0253-2670.2017.04.002.
[20] NONAKA G I, MIWA N, NISHIOKA I. Stilbene glycoside gallates and proanthocyanidins from[J]. Phytoche- mistry, 1982, 21(2): 429–432.
[21] LI S G, CHEN L L, HUANG X J, et al. Five new stilbene glycosides from the roots of[J]. J Asian Nat Prod Res, 2013, 15(11): 1145–1151. doi: 10.1080/10286020.2013.837454.
[22] XU M L, ZHENG M S, LEE Y K, et al. A new stilbene glucoside from the roots ofThunb. [J]. Arch Pharm Res, 2006, 29(11): 946–951. doi: 10.1007/BF02969276.
[23] XIAO K, XUAN L J, XU Y M, et al. Novel stilbene glycosides from[J]. Acta Bot Sin, 2002, 44(12): 1491–1494. doi: 10.3321/j.issn:1672-9072.2002.12.016.
[24] YUAN W. Chemical constituents from[D]. Beijing: Beijing University of Chinese Medicine, 2017. (in Chinese)
[25] ZHOU L X, LIN M, LI J B, et al. Chemical studies on the ethyl cetate insoluble fraction of the roots ofThunb. [J]. Acta Pharmacol Sin, 1994, 29(2): 107–110. (in Chinese) doi: 10.16438/ j.0513-4870.1994.02.005.
[26] XU Y L, DONG Q, HU F Z. Simultaneous quantitative determination of eight active components inThunb. by RP- HPLC [J]. J Chin Pharm Sci, 2009, 18(4): 358–361.
[27] LUO Y Y. Studies on quality evaluation of[D]. Nanjing: Nanjing University of Chinese Medicine, 2016. (in Chinese)
[28] YI T, LEUNG K S Y, LU G H, et al. Identification and determination of the major constituents in traditional Chinese medicinal plantThunb. by HPLC coupled with PAD and ESI/MS [J]. Phytochem Anal, 2007, 18(3): 181–187. doi: 10.1002/pca.963.
[29] BAO N M, DAI J, LIAO N L, et al. Water-assisted/water-accelerated photoreaction of-2,3,4′,5-tetrahydroxystilbene-2---d-glucoside from the roots of[J]. J Agric Food Chem, 2020, 68(18): 5086–5092. doi: 10.1021/acs.jafc.9b04922.
[30] LI S G, HUANG X J, LI M M, et al. Multiflorumisides A-G, dimeric stilbene glucosides with rare coupling patterns from the roots of[J]. J Nat Prod, 2018, 81(2): 254–263. doi: 10.1021/ acs.jnatprod.7b00540.
[31] LI S G, HUANG X J, ZHONG Y L, et al. Stilbene glycoside oligomers from the roots of[J]. Chem Biodivers, 2019, 16(6): e1900192. doi: 10.1002/cbdv.201900192.
[32] YAN S L, SU Y F, CHEN L, et al. Polygonumosides A-D, stilbene derivatives from processed roots of[J]. J Nat Prod, 2014, 77(2): 397–401. doi: 10.1021/np400720y.
[33] CHEN L L, HUANG X J, LI M M, et al. Polygonflavanol A, a novel flavonostilbene glycoside from the roots of[J]. Phytochem Lett, 2012, 5(4): 756–760. doi: 10.1016/j.phytol.2012.08. 007.
[34] Pharmacopoeia Commission of the Ministry of Health of the People’s Republic of China. Pharmacopoeia of the People’s Republic of China [M]. Beijing: China Medical Science and Technology Press, 2010: 175–176. (in Chinese)
[35] SHEN J F, ZHANG Y Z, SHEN H, et al. The synergistic effect of 2,3, 5,4′-tetrahydroxystilbene-2-O--d-glucoside combined with adriamycin on MCF-7 breast cancer cells [J]. Drug Des Devel Ther, 2018, 12: 4083–4094. doi: 10.2147/DDDT.S186028.
[36] LÜ L S. Study on stilbene from roots ofThunb. antioxidant activities[J]. Food Sci, 2007, 28(1): 313–317. (in Chinese) doi:10.3321/j.issn:1002-6630.2007.01.081.
[37] ZHANG L L, CHEN J Z. Biological effects of tetrahydroxystilbene glucoside: An active component of a rhizome extracted from[J]. Oxid Med Cell Longev, 2018, 2018: 3641960. doi: 10.1155/2018/3641960.
[38] PARK S Y, JIN M L, WANG Z Y, et al. 2,3,4′,5-Tetrahydroxystilbene- 2--d-glucoside exerts anti-inflammatory effects on lipopolysaccha- ride-stimulated microglia by inhibiting NF-B and activating AMPK/ Nrf2 pathways [J]. Food Chem Toxicol, 2016, 97: 159–167. doi:10. 1016/j.fct.2016.09.010.
[39] YIN X M, CHEN C, XU T, et al. Tetrahydroxystilbene glucoside modulates amyloid precursor protein processingactivation of AKT- GSK3pathway in cells and in APP/PS1 transgenic mice [J]. Biochem Biophys Res Commun, 2018, 495(1): 672–678. doi: 10.1016/j.bbrc. 2017.11.059.
[40] LING S, XU J W. Biological activities of 2,3,5,4′-tetrahydroxy- stilbene-2-O-d-glucoside in antiaging and antiaging-related disease treatments [J]. Oxid Med Cell Longev, 2016, 2016: 4973239. doi: 10. 1155/2016/4973239.
[41] RAO G X, XUE Y M, HUI T T, et al. Studies on the chemical constituents of the leaves of[J]. J Chin Med Mat, 2009, 32(6): 891–893. doi: 10.13863/j.issn1001-4454.2009.06.051.
[42] ZHAO H N, CHEN L L, HUANG X J, et al. A new chromone glycoside from roots of[J]. J Chin Mat Med, 2014, 39(8): 1441–1444. doi: 10.4268/cjcmm20140816. (in Chinese)
[43] YOSHIZAKI M, FUJINO H, ARISE A, et al. Polygoacetophenoside, a new acetophenone glucoside from[J]. Planta Med, 1987, 53(3): 273–275. doi: 10.1055/s-2006-962703.
[44] LOU Z H, LV G Y, YU J J. Review on the research of the components, pharmacological actions and toxicity ofThunb. (Heshouwu) [J]. J Zhejiang Chin Med Univ, 2014, 38(4): 495–500. (in Chinese) doi: 10.16466/j.issn1005-5509.2014.04.030.
[45] WANG H Y, SONG L X, FENG S B, et al. Characterization of proan- thocyanidins in stems ofThunb. as strong starch hydrolase inhibitors [J]. Molecules, 2013, 18(2): 2255–2265. doi: 10.3390/molecules18022255.
[46] ZHOU Y Z, WANG G Q, LI D D, et al. Dual modulation on glial cells by tetrahydroxystilbene glucoside protects against dopamine neuronal loss [J]. J Neuroinflammation, 2018, 15(1): 161. doi: 10.1186/s12974- 018-1194-5.
[47] YANG J Q, HE Y Q, ZOU J Y, et al. Effect ofThunb. on liver fatty acid content in aging mice induced by d-galactose [J]. Lipids Health Dis, 2019, 18(1): 128. doi: 10.1186/s12944-019- 1055-y.
[48] ZHOU X X, GE L, YANG Q, et al. Thinning of dermas with the increasing age may be against by tetrahydroxystilbene glucoside in mice [J]. Int J Clin Exp Med, 2014, 7(8): 2017–2024.
[49] XU C S. The protective effects of the extracts ofThunb. on peroxide damage of rats [J]. Yantai Teachers Univ J (Nat Sci), 2000, 16(3): 197–199. (in Chinese)
[50] GE C L, LIU Y. Polysaccharide fromThunb. potentiates the immunological function in immunosuppressed mice [J]. Chin J New Drugs, 2007, 16(24): 2040–2042. (in Chinese) doi: 10. 3321/j.issn:1003-3734.2007.24.011.
[51] KE S L, XIE R G, ZHENG W R, et al. Treatment of hyperlipidemia with[J]. Guangdong Med J, 2000, 21(11): 977–978. (in Chinese) doi: 10.3969/j.issn.1001-9448.2000.11.046.
[52] LIU Y, WANG W P, SUN M Y, et al.-induced liver injury: Clinical characteristics, risk factors, material basis, action mechanism and current challenges [J]. Front Pharmacol, 2019, 10: 1467. doi: 10.3389/fphar.2019.01467.
[53] CHEN L, HUANG J Y, XUE L. Effect of compoundextract on Alzheimer’s disease [J]. J CS Univ (Med Sci), 2010, 35(6): 612–615. (in Chinese) doi: 10.3969/j.issn.1672-7347.2010. 06.012.
[54] LI C S, LI J, GUAN X H, et al. Clinical study on effect of shouwuyizhi capsule on vascular dementia [J]. Chin J Geriatr, 2008, 28(4): 369–371. (in Chinese)
[55] LIN E Y, CHAGNAADORJ A, HUANG S J, et al. Hepatoprotective activity of the ethanolic extract ofThunb. against oxidative stress-induced liver injury [J]. Evid Based Compl Alternat Med, 2018, 2018: 4130307. doi: 10.1155/ 2018/4130307.
[56] WEI J, CHEN J R, FU L L, et al.Thunb. suppress bile acid synthesis by activating Fxr-Fgf15 signaling in the intestine [J]. J Ethnopharmacol, 2019, 235: 472–480. doi: 10.1016/j. jep.2018.12.007.
[57] CHEN G T, YANG M, CHEN B B, et al. 2,3,5,4ʹ-Tetrahydroxystil- bene-2---d-glucoside exerted protective effects on diabetic nephron- pathy in mice with hyperglycemia induced by streptozotocin [J]. Food Funct, 2016, 7(11): 4628–4636. doi: 10.1039/c6fo01319h.
[58] JIA M, ZHOU X X, QIN Q H, et al. Tetrahydroxystilbene glucoside- induced relaxation of the superior mesenteric arteryboth endo- thelium-dependent and endothelium-independent mechanisms [J]. Microvasc Res, 2019, 123: 42–49. doi: 10.1016/j.mvr.2018.10.007.
[59] ZHANG M, YU L M, ZHAO H, et al. 2,3,5,4ʹ-Tetrahydroxystilbene- 2---d-glucoside protects murine hearts against ischemia/reperfusion injury by activating Notch1/Hes1 signaling and attenuating endo- plasmic reticulum stress [J]. Acta Pharmacol Sin, 2017, 38(3): 317–330. doi: 10.1038/aps.2016.144.
[60] LÜ J S, MENG D S, XIANG M F, et al. Preliminary study on the anti- inflammatory effect of[J]. China Pharmacy, 2001, 12(12): 712–714. (in Chinese) doi: 10.3969/j.issn.1001-0408.2001. 12.004.
[61] CHIN Y T, HSIEH M T, LIN C Y, et al. 2,3,5,4′-Tetrahydroxystilbene- 2--glucoside isolated from Polygoni Multiflori ameliorates the development of periodontitis [J]. Mediators Inflamm, 2016, 2016: 6953459. doi: 10.1155/2016/6953459.
[62] HAM J R, LEE H I, CHOI R Y, et al. Heshouwu (Thunb.) extract attenuates bone loss in diabetic mice [J]. Prev Nutri Food Sci, 2019, 24(2): 121–127. doi: 10.3746/pnf.2019. 24.2.121.
[63] ZHANG J J, CHEN X F, CHEN B B, et al. Tetrahydroxystilbene glucoside protected against diabetes-induced osteoporosis in mice with streptozotocin-induced hyperglycemia [J]. Phytother Res, 2019, 33(2): 442–451. doi: 10.1002/ptr.6240.
[64] CHOI R Y, LEE H I, HAM J R, et al. Heshouwu (Thunb.) ethanol extract suppresses pre-adipocytes differen- tiation in 3T3-L1 cells and adiposity in obese mice [J]. Biomed Pharmacother, 2018, 106: 355–362. doi: 10.1016/j.biopha.2018.06.140.
[65] WANG J B, MA Z J, NIU M, et al. Evidence chain-based causality identification in herb-induced liver injury: Exemplification of a well- known liver-restorative herb[J]. Front Med, 2015, 9(4): 457–67. doi: 10.1007/s11684-015-0417-8.
[66] JUNG K A, MIN H J, YOO S S, et al. Drug-induced liver injury: Twenty five cases of acute hepatitis following ingestion ofThunb. [J]. Gut Liver, 2011, 5(4): 493–499. doi: 10.5009/ gnl.2011.5.4.493.
[67] YANG X W, ZHANG Y H, LIU Y, et al. Emodin induces liver injury by inhibiting the key enzymes of FADH/NADPH transport in rat liver [J]. Toxicol Res (Camb), 2018, 7(5): 888–896. doi: 10.1039/c7tx00307b.
[68] WU L L, CHEN Y L, LIU H, et al. Emodin-induced hepatotoxicity was exacerbated by probenecid through inhibiting UGTs and MRP2 [J]. Toxicol Appl Pharmacol, 2018, 359: 91–101. doi: 10.1016/j.taap.2018. 09.029.
[69] JIANG L L, JIANG Y, ZHAO D S, et al. CYP3A activation and gluta- thione depletion aggravate emodin-induced liver injury [J]. Chem Res Toxicol, 2018, 31(10): 1052–1060. doi: 10.1021/acs.chemrestox.8b00117.
[70] DONG X X, NI B, FU J, et al. Emodin induces apoptosis in human hepatocellular carcinoma HepaRG cells via the mitochondrial caspase- dependent pathway [J]. Oncol Rep, 2018, 40(4): 1985–1993. doi: 10. 3892/or.2018.6620.
[71] SHI F G, ZHAO P, LI X B, et al. Cytotoxicity of luteolin in primary rat hepatocytes: The role of CYP3A-mediated-benzoquinone metabo- lite formation and glutathione depletion [J]. J Appl Toxicol, 2015, 35 (11): 1372–1380. doi: 10.1002/jat.3106.
[72] CHOI Y J, YOON Y J, CHOI H S, et al. Effects of medicinal herb extracts and their components on steatogenic hepatotoxicity in Sk-hep1 cells [J]. Toxicol Res, 2011, 27(4): 211–216. doi: 10.5487/tr.2011.27. 4.211.
[73] HE S B, ZHANG X L, LU S, et al. A computational toxicology approach to screen the hepatotoxic ingredients in traditional Chinese medicines:Thunb. as a case study [J]. Biomo- lecules, 2019, 9(10): 577. doi: 10.3390/biom9100577.
[74] LIU Z L, SONG Z Q, ZHANG L, et al. Influence of process methods on contents of chemical component Radix Polygoni Multiflori [J]. China J Chin Mat Med, 2005, 30(5): 336–340. (in Chinese) doi: 10. 3321/j.issn:1001-5302.2005.05.004.
[75] XIA X H, YUAN Y Y, LIU M. The assessment of the chronic hepatotoxicity induced by Polygoni Multiflori Radix in rats: A pilot study by using untargeted metabolomics method [J]. J Ethnopharmacol, 2017, 203: 182–190. doi: 10.1016/j.jep.2017.03.046.
何首乌化学成分及其药理活性的研究进展
沈晓静1,2, 张敢娟1, 吕奇1, 杨俊滔1, 王青1, 姜薇薇1*
(1. 云南农业大学理学院,昆明 650201; 2. 云南省天然药物药理重点实验,昆明医科大学,昆明 650500)
何首乌()是一种珍贵的多年生中药,为蓼科(Polygonaceae)何首乌属植物,主要分布在四川、云南、贵州以及山西和甘肃南部,可用于治疗肝损伤、癌症、糖尿病、脱发、动脉粥样硬化以及神经退行性疾病。近年来,国际上有报道称摄入何首乌会引起肝损伤,因此,建立何首乌的安全监测和风险管理模型对何首乌的开发利用具有重要意义。对何首乌的化学成分、药理活性及其毒副作用进行了综述,为何首乌的临床应用、科学研究和生产质量控制提供参考。
何首乌;化学成分;药理活性; 综述
10.11926/jtsb.4304
2020–09–08
2020–10–16
This work was supported by the Project for Scientific Research of Yunnan Education Department (Grant No. 2020J0241), the Joint Project for Agricultural Basic Research in Yunnan Province (Grant No. 2018FG001-037), and the Open Projects of Yunnan Key Laboratory of Pharmacology for Natural Products (Grant No. 70120030506).
Shen Xiao-jing (Born in1988), Female, experimentalist, interesting in research and development of natural products. E-mail: 690361382@qq.com
.E-mail: 17366529@qq.com
