Effects of Oldenlandia diffusa Polysaccharides on the Structures of Chick Small Intestine Tissues
2021-08-02ZhihongHUANG
Zhihong HUANG
Abstract In order to explore the application effect of Oldenlandia polysaccharides (OPS) in the production of AA broilers, 200 7-day-old AA broilers were randomly divided into 4 groups, namely groups I, II, III, and IV, each including 50 chicks. The control group (group I) was fed with basal diet; and the 3 experimental groups were fed based on the basal diet with the addition of OPS at 0.4 g/kg (group II), 0.8 g/kg (group III), and 1.2 g/kg (group IV) feed for 1 week, respectively, and then fed the basal diet for 4 weeks. At the ages of 14, 28, and 42 d, 10 chickens were put into death in the control group and the experimental groups, respectively, and the duodenum and jejunum tissue samples were collected for the analysis of the effects of OPS on the structures of chick intestines. The results showed that at the age of 14 d, the villus lengths of duodenum and jejunum in groups II, III, and IV were significantly greater than those in group I (P<0.05), and the ratios of duodenal or jejunal villus length to crypt depth in groups II and III were significantly greater than that in group I (P<0.05); at the age of 28 d, the villus length of duodenum in group II was significantly greater than that in group I (P<0.05); and at the age of 42 d, the ratios of jejunual villus length to crypt depth in groups II, III and IV were significantly larger than those of group I (P<0.05). It showed that OPS could promote the small intestine development of AA broilers aged 14 to 42 d.
Key words Oldenlandia diffusa; Polysaccharide; Chicken; Intestinal villus length; Intestinal crypt depth
Received: December 12, 2020 Accepted: March 15, 2021
Zhihong HUANG (1963-), male, P. R. China, associate professor, master, devoted to research about basic veterinary medicine.
*Corresponding author. E-mail: hzhong601@163.com.
Oldenlandia diffusa or Hedyotis diffusa is a plant of Oldenlandia in Rubiaceae, which mainly contains flavonoids, polysaccharides, anthraquinones, alkaloids, terpenes, sterols, alkanes, organic acids, and cardiac glycosides[1-2]. The experimental results of Gao et al.[3] showed that O. diffusa decoction, crude polysaccharides, and total flavonoids all had the effect of promoting the proliferation of broiler lymphocytes. Chen et al.[4] confirmed that O. diffusa polysaccharides and flavonoids had the effect of enhancing the immune function of mice, and the effect of polysaccharides was the most significant. Wang et al.[5] found that O. diffusa polysaccharides could significantly improve the specific and non-specific immune function of mice, and promote the growth and development of mice. The morbidity and mortality of chicken colibacillosis, infectious bursal disease, infectious anemia, and coccidiosis remain high, and they often become the main causes of immunosuppression, slow weight gain and death in broilers. Additives such as antibiotics and anticoccidial drugs can cause adverse reactions in chickens, and there are problems such as drug residues and drug resistance. Therefore, it is of great significance to explore the use of plant polysaccharides with immunoregulatory and growth-promoting effects as feed additives for broilers. In this study, Oldenlandia polysaccharides were added to the chicken diet to preliminarily explore the effect of Oldenlandia polysaccharides on the structures of chick small intestine tissues, hoping to provide a reference for further exploring its growth-promoting effect.
Materials
Experimental agents
Oldenlandia polysaccharides (OPS) were extracted by Beijing Shengtaier Company, with an extraction rate of about 20% and a polysaccharide content of 45%.
Experimental animals
200 1-day-old healthy AA broilers were purchased from Chengdu Zhengda Co., Ltd., and the experiment was conducted after adaptive feeding for 1 week.
Methods
Grouping and administration of experimental animals
200 7-day-old AA broilers were randomly divided into a control group and 3 experimental groups, each with 50 chicks. The control group (group I) was fed with basal diet; and the 3 experimental groups were fed based on the basal diet with the addition of OPS at 0.4 g/kg (group II), 0.8 g/kg (group III), and 1.2 g/kg (group IV) feed for 1 week, respectively, and then fed the basal diet for 4 weeks. At the ages of 14, 28, and 42 d, 10 chickens were put into death in the control group and the experimental groups, respectively, and the duodenal and jejunal tissue samples were collected for use.
Determination of villus length and crypt depth
The small intestine sections were made by conventional paraffin sectioning, and observed and photographed with an optical microscope (Motic B5 professional Series) after H. E. staining. Ten longest, straight and well-extended villi were selected from each section, and quantitative analysis was performed with an optical microscope photographic processing software and a pathological image analysis system to determine the intestinal villus length and crypt depth.
Statistical analysis of data
The SPSS 18.0 software was used to carry out a one-way analysis of variance on the experimental data, and the obtained data were expressed as "mean±standard deviation". The variance homogeneity test was used for analyzing the significance of the differences in the data, with P<0.05 indicating a significant difference.
Results and Analysis
Effect of Oldenlandia polysaccharides on intestinal villus length
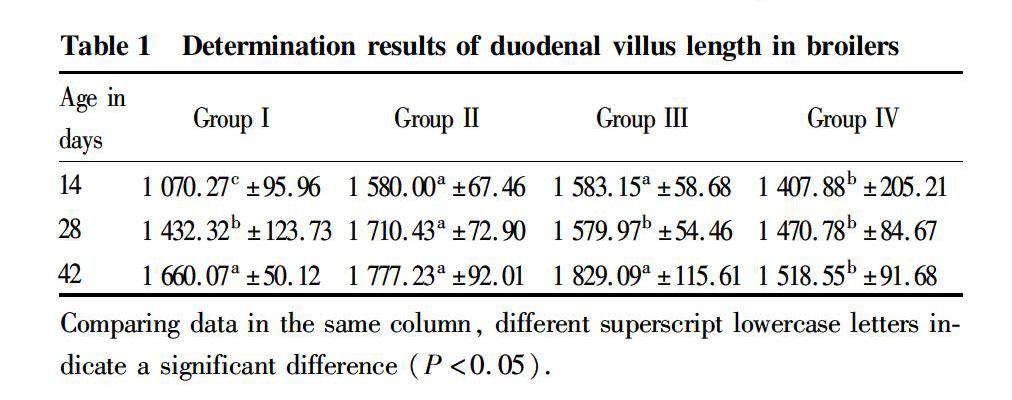
Effect of Oldenlandia polysaccharides on the villus length of duodenum
It can be seen from Table 1 that when AA broilers were 14 days old, the villus lengths of duodenum in groups II, III, and IV were significantly greater than that in group I (P<0.05), and the villus lengths of duodenum in groups II and III were significantly greater than that in group IV (P<0.05); at the age of 28 d, the villus length of duodenum in group II was significantly longer than those in groups I, III, and IV (P<0.05); and at the age of 42 d, although the villus lengths of duodenum in groups II and III increased compared with group I, the differences were not significant (P>0.05), while the villus lengths of duodenum in groups II and III were significantly higher than that in group IV (P<0.05). In summary, the villus lengths of duodenum of AA broilers in groups II, III, and IV were longer than that in the control group, but long-term increase of the polysaccharide dose could not make the duodenum villi of AA broilers develop better.
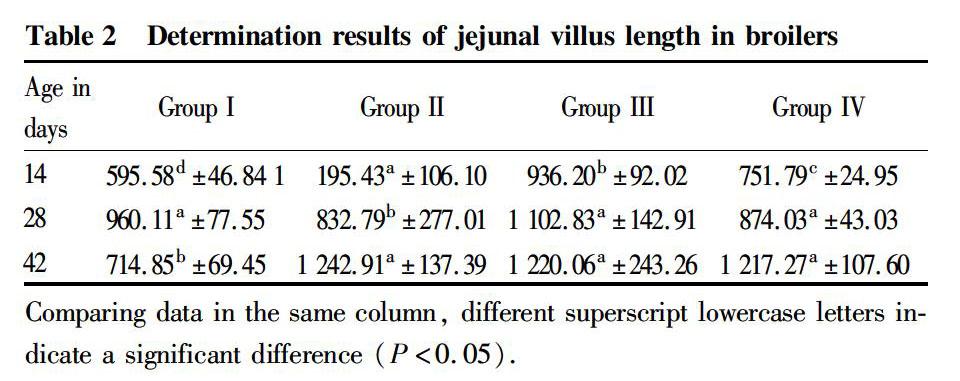
Effect of Oldenlandia polysaccharides on the villus length of jejunum
It can be seen from Table 2 that at the age of 14 d, the villus lengths of jejunum in groups II, III, and IV were significantly greater than that in group I (P<0.05), and the villus lengths of jejunum in the three groups decreased with the increase of polysaccharide dose, with significant differences (P<0.05); at the age of 28 d, the villus lengths of jejunum in groups III and IV were not significantly different from that in group I (P>0.05), but group III was significantly greater than group II (P<0.05); and at the age of 42 d, the villus lengths of jejunum in groups II, III, and IV were significantly greater than that in group I (P<0.05), while there were no significant differences between groups II, III and IV (P>0.05). The villus lengths of jejunum of AA broilers in groups II, III, and IV were greater than that in the control group at the ages of 14 and 42 d, but increasing the dose of polysaccharides could not make the jejunum villi of AA broilers develop better.
Effect of Oldenlandia polysaccharides on intestinal crypt depth
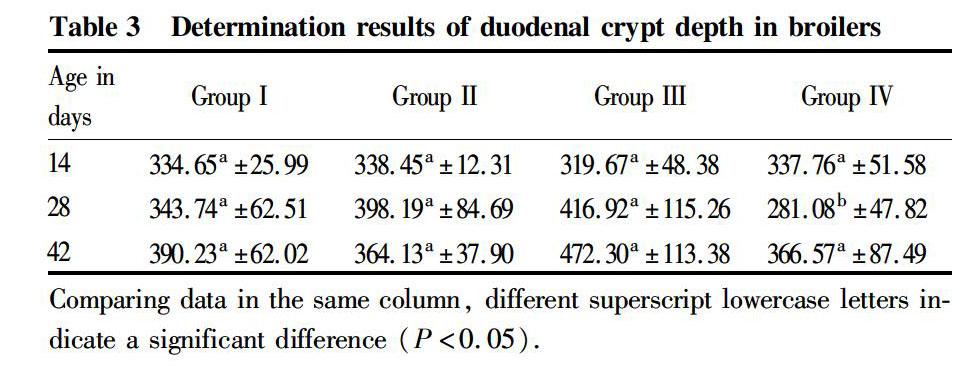
Effect of Oldenlandia polysaccharides on the crypt depth of duodenum
It can be seen from Table 3 that at the age of 14 d, there were no significant differences in the crypt depth of duodenum between the groups (P>0.05); at the age of 28 d, the crypt depth of duodenum in group IV was significantly smaller than those in groups II and III (P<0.05), and the differences between other groups were not significant (P>0.05); and at the age of 42 d, the differences in the crypt depth of duodenum between the groups were not significant (P>0.05). It can be seen that Oldenlandia polysaccharides had no significant effect on the crypt depth of duodenum in AA broilers.
Effect of Oldenlandia polysaccharides on the crypt depth of jejunum
It can be seen from Table 4 that at the ages of 14 and 28 d, the differences in the crypt depth of jejunum between the groups were not significant (P>0.05); and at the age of 42 d, the crypt depths of jejunum in groups II, III, and IV were significantly greater than that in group I (P<0.05). It can be seen that Oldenlandia polysaccharides could increase the crypt depth of jejunum in 42-day-old AA broilers.
Effect of Oldenlandia polysaccharides on the ratio of intestinal villus length to crypt depth
Effect of Oldenlandia polysaccharides on the ratio of duodenal villus length to crypt depth
It can be seen from Table 5 that at the age of 14 d, the ratios of duodenal villus length to crypt depth in groups II, III, and IV were significantly greater than that in group I (P<0.05); and at the ages of 28 and 42 d, the ratios of duodenal villus length to crypt depth in groups II, III, and IV were not significantly different from that in the control group (P>0.05). It can be seen that Oldenlandia polysaccharides could increase the ratio of duodenal villus length to crypt depth in 14-day-old AA broilers.
Effect of Oldenlandia polysaccharides on the ratio of jejunal villus length to crypt depth
It can be seen from Table 6 that at the age of 14 d, the ratios of jejunal villus length to crypt depth in groups II and III were significantly greater than that of group I (P<0.05); and at the ages of 28 and 42 d, there were no significant differences in the ratio of jejunal villus length to crypt depth between various groups (P>0.05). It can be seen that Oldenlandia polysaccharides could increase the ratio of jejunal villus length to crypt depth in 14-day-old AA broilers.
Discussion
The intestinal villus length and crypt depth are important indices to measure the digestion and absorption functions of the small intestine[6]. Zhou et al.[7] studied the effect of cyclophosphamide on mouse intestinal mucosal immunity and found that villus length was positively correlated with the number of epithelial cells. When the villi become shorter, the number of epithelial cells decreases and the surface area of villi decreases, which ultimately leads to a decline in digestion and absorption capacity. When the villi are invaded or stressed by external adverse factors, they will be damaged and shortened, and the epithelial cells will also decrease[8-9]. The crypt depth of the small intestine mainly reflects the production rate of epithelial cells, and the decrease of cell production rate will make the crypts shallow[10]. The ratio of villus length to crypt depth can comprehensively reflect the functional status of the small intestine. A decrease in the ratio indicates a decline in the digestion and absorption function, which is often accompanied by diarrhea, and the growth and development of the animal will be inhibited; an increase in the ratio indicates the digestion and absorption function is enhanced, the diarrhea rate decreases, and the growth and development speed up[11-12]. He[13] found that galactomannan oligosaccharide, chitosan oligosaccharide and galactomannan oligosaccharide + lactic acid bacteria could increase the villus length and mucosal thickness of rat duodenum and jejunum. Tao et al.[14] added 0.4% and 0.6% Astragalus polysaccharides (APS) to broiler basal diet, which increased the height and width of intestinal villi, mucosal thickness, ratio of villus length to crypt depthand villus surface area in broiler chickens, but with the increase of broiler age, the effect was gradually weakened. The experimental results of Zhou[15] showed that sodium butyrate could maintain the structural integrity of the epithelial cells of the small intestinal mucosa, thereby promoting the digestion and absorption of the small intestine of broilers. The experimental results of Chen et al.[16] showed that the addition of Pseudostellaria heterophylla stem and leaf polysaccharides to weaned piglets diet could increase the villus height of duodenum and the ratio of duodenual villus height to crypt depth, thereby promoting piglets digestion and absorption of nutrients. From the changes in the length of the small intestine of the test broilers and the ratio of villus length to crypt depth, OPS could promote the development of the small intestine of AA broilers, and as the age increased, the effect of promoting the development of the small intestine decreased. Therefore, it is recommended to add OPS to the diet of young broilers. Wang et al.[17] found that Astragalus polysaccharides could enhance the immune function of the duodenal mucosa of broilers, and whether Oldenlandia polysaccharides can enhance the function of small intestinal mucosal immunity remains to be further studied.
Oldenlandia polysaccharides have a variety of physiological and pharmacological activity. The experimental results of Fan et al.[18] showed that Oldenlandia polysaccharides could promote the development of spleen, thymus and bursa of Fabricius in AA chickens. Chen et al.[19] found that Oldenlandia polysaccharides and total flavonoids had the effect of enhancing the immune function of the body. Wang et al.[5] found that Oldenlandia polysaccharides could significantly improve the specific and non-specific immune functions of mice, and promote the growth and development of the body. The study of Jiang[20] showed that Oldenlandia polysaccharides had a strong antioxidant capacity and a scavenging rate of 1,1-diphenyl-2-picrylhydrazyl free radicals as high as 82.66%. The effect of Oldenlandia polysaccharides on the development of chicks small intestines may be related to its immune enhancement and anti-oxidative stress function.
The effect of O. diffusa on the intestinal mucosa is similar to those of Astragalus polysaccharides, galactomannan oligosaccharides, chitosan oligosaccharide, Radix pseudostellariae stem and leaf polysaccharides reported above, and its mechanism needs to be explored. Wu et al.[21] found that Atractylodes polysaccharides could increase the calcium level of small intestinal epithelial cells to promote cell migration and E-cadherin expression, which might be related to the role of A. macrocephala repairing gastrointestinal mucosal damage. Astragalus polysaccharides can increase the contents of bifidobacteria and lactobacilli in the intestinal tract of rats, but decrease the contents of enterococci and enterobacteria, and the intestinal flora of rats can thus restore the balance[22]. Poria polysaccharides can activate Peys node B lymphocytes and regulate the secretion of mouse intestinal secretory immunoglobulin A[23]. Shenqi Polysaccharide Oral Liquid can prevent the damage of cyclophosphamide to chicken jejunum mucosal structure and immune function[24]. Whether the crude polysaccharides of O. diffusa have effects similar to the above-mentioned plant polysaccharides and its mechanism need to be further explored.
References
[1] HOU SL. Research progress on the chemical constituents and pharmacological activity of TCM medicine[J]. Clinical Journal of Chinese Medicine, 2018, 10(6): 140-141. (in Chinese)
[2] PU FF, CHEN FX, XIA P. Research progress on chemic consistuent and anti-tumor activity of Oldenlandia diffusa willd[J]. Oncology Progress, 2019, 17(17): 1985-1988, 1996. (in Chinese)
[3] GAO YY, WANG GP, LI CL, et al. Effect of Oldenlandia extract on lymphocyte proliferation in chickens[J]. Progress In Veterinary Medicine, 2010, 31(10): 43-46. (in Chinese)
[4] CHEN H, XU J, YAN M, et al. Immunomodulatory effects of polysaccharides and total flavonoids in Oldenlandia diffusa[J]. Chinese Journal of Traditional Veterinary Science, 2008(2): 4-6. (in Chinese)
[5] WANG HG, TANG C, WANG H, et al. Effect of Hedyotis diffusa Willd polysaccharide on immune function and growth in mice[J]. Chinese Animal Husbandry and Veterinary Medicine, 2013, 40(10): 140-143. (in Chinese)
[6] LI KZ, LI N, LI JS, et al. Effects of short-chain fatty acids on the morphology and function of small intestine transplantation in rats[J]. World Chinese Journal of Digestology, 2002(6): 720-722. (in Chinese)
[7] ZHOU H, WANG PX, LIU L, et al. The influnence of cyclophosphamide on Peypers patches and intestinal mucosal associated lymphocytes in mice[J]. Chinese Journal of Immunology, 2001(4): 186-187, 189, 170. (in Chinese)
[8] LIU QD, ZHANG ZW, LIU FH, et al. Effects of herbal medicine on villus height and crypt depth in small intestine of canine[J]. Journal of Beijing University OF Agriculture, 2011, 26(3): 38-40. (in Chinese)
[9] WANG CR, LIU XF, CHENG YG. Protection of intestine function recovery decoction on intestinal mucosa injury for multiple organ dysfunction syndrom rats caused by infection[J]. Journal of Zhengzhou University: Medical Sciences, 2008(6): 1107-1111. (in Chinese)
[10] WANG ZX, SHE RP, CHEN Y, et al. Effect of different levels of zinc and selenium in diet on the structure of mucosa epithelium in broiler small intestine[J]. Chinese Journal of Veterinary Science and Technology, 2003(7): 18-21. (in Chinese)
[11] HAN ZK. Nutritional physiology of livestock[M]. Beijing: China Agriculture Press, 1991. (in Chinese)
[12] LU DX. Introduction to system animal nutrition[M]. Beijing: China Agriculture Press, 2004. (in Chinese)
[13] HE YN. Effects of two types of oligosaccharides on the structure of gut mucosa and mucasal immuno-associated cell in rats[D]. Beijing: China Agricultural University, 2006. (in Chinese)
[14] TAO H, WEI BD, CHEN Q. Effect of astragalus polysaccharide on mucosal structure in small intestine of 14-day-old broilers[J]. Journal of Northeast Agricultural University, 2012, 43(3): 52-57. (in Chinese)
[15] ZHOU WQ. Effect of sodium butyrate on the structure of intestinal mucosa in broilers[J]. Chinese Journal of Animal Husbandry and Veterinary Medicine, 2015(2): 26. (in Chinese)
[16] CHEN LF, CAI XB, TAN XZ, et al. Effects of Radix pseudostellariae stem and leaf polysaccharide on intestinal immune function, intestinal mucosal morphology and cecum contents flora of weaned piglets[J]. Acta Zoonutrimenta Sinica, 2017, 29(3): 1012-1020. (in Chinese)
[17] WANG HF, GUO B. Modulator effects of astragals polysaccharides on duodenum mucosal immunity of broilers[J]. Acta Zoonutrimenta Sinica, 2015, 27(5): 1534-1539. (in Chinese)
[18] FAN HF, XIE MN, LIU JH, et al. Effects of Hedyotis diffusa polysaccharides on the development of immune organs in AA chickens[J]. Chinese Journal of Animal Husbandry and Veterinary Medicine, 2014(8): 19-20. (in Chinese)
[19] CHEN H, XU J, YAN M, et al. Immunomodulatory effects of polysaccharides and total flavonoids in Oldenlandia diffusa[J]. Chinese Journal of Traditional Veterinary Science, 2008(2): 4-6. (in Chinese)
[20] JIANG JP, XU M, LU YL, et al. Antioxidant activity of polysaccharide from Hedyotis diffusa Willd[J]. Chinese Archives of Traditional Chinese Medicine, 2012, 30(5): 1076-1078. (in Chinese)
[21] WU TT, LI RL, ZENG D, et al. Research of Atractylodes macrocephala polysaccharides in promoting cell migration and e-cadherin expression through regulating calcium ions[J]. Traditional Chinese Drug Research and Clinical Pharmacology, 2017, 28(2): 145-150. (in Chinese)
[22] LIANG JH, ZHENG KW, SUN LQ. Explore the regulative action of Astragalus polysaccharide for intestinal dysbacteriosis in ulcerative colitis rat models[J]. Studies of Trace Elements and Health, 2013, 30(2): 1-3. (in Chinese)
[23] WANG Q, HU MH, DONG Y, et al. Pachymaran up-regulates immunoglobulin a response and CD80, CD86 expression in intestines of mice[J]. Chinese Journal of Experimental Traditional Medical Formulae, 2011, 17(13): 127-129. (in Chinese)
[24] QIU FA, REN Z, ZHENG JY, et al. Protective effects of Shenqi polysaccharide oral liquid on jejunal mucosal immunity in chickens[J]. Veterinary Science in China, 2017, 47(11): 1441-1449. (in Chinese)
杂志排行
农业生物技术(英文版)的其它文章
- Anti-inflammatory Activity and Mechanism of Total Flavonoids from the Phloem of Paulownia elongate S.Y. Hu in LPS-stimulated RAW264.7 Macrophages
- Comparative Genomic Analysis of Boron Transport Gene Family in Arabidopsis and Five Crops
- Effects of Different Water-saving Irrigation Methods on Fruit Quality and Yield of Snow Melon
- Field Control Effects and Crop Safety Assessment of Triazole Fungicides on Apple Rust
- Effects of Acetylacetone Solution Soaking on Agrobacterium-transformed Maize Seed Buds
- Effects of Meteorological Factors on Overwintering Ability, Yield and Quality of Forage Rape
