Effects of Acetylacetone Solution Soaking on Agrobacterium-transformed Maize Seed Buds
2021-08-02MengyuLYUFushuangDONGShuoZHOUJianfangCHAIHeZHAOYongweiLIUFanYANGBoJIAOXiaoyuLIJunminZHANGHaiboWANG
Mengyu LYU Fushuang DONG Shuo ZHOU Jianfang CHAI He ZHAO Yongwei LIU Fan YANG Bo JIAO Xiaoyu LI Junmin ZHANG Haibo WANG
Abstract [Objectives] The effect of acetosyringone seed soaking on the transformation of maize seed buds was analyzed, so as to improve the genetic transformation efficiency of maize and to provide technical support for transgenic breeding of maize.
[Methods]The seeds of the "Zheng 58" maize inbred line were used as experimental materials. When the seeds were germinated, AS was added to the water at concentrations of 70, 140, 210, and 280 μmol/L, respectively, and the seeds germinated without the addition of AS served as the CK. The Agrobacterium-mediated method was used to transform bud growth points of maize seeds, and green fluorescent protein detection was performed on the young shoots transformed with EGFP (enhanced green fluorescent protein) gene. The effect of soaking seeds with acetosyringone solution on the transformation of maize bud growth points by Agrobacterium was studied according to the detection results.
[Results] Soaking seeds in acetosyringone solutions for germination had the effect of inhibiting the germination of maize seeds and inhibiting sprout elongation, and the higher the concentration of acetosyringone, the stronger the inhibition. When the concentration of acetosyringone solution was 280 μmol/L, the germination rate of seeds was only 36.2% of the CK, while soaking seeds with 70-140 μmol/L acetosyringone solution for germination could not only ensure a higher germination rate of maize seeds, but also significantly increased the transformation efficiency of maize bud growth points. When the seeds were soaked with 70 μmol/L acetosyringone solution for germination, the positive rate of transformed maize buds was the highest, reaching 32.1%.
[Conclusions]When maize bud growth points were used as the receptor of Agrobacterium transformation, soaking seeds with 70-140 μmol/L acetosyringone for germination basically did not affect the germination of seeds, and was beneficial to the activation of Agrobacterium, thereby promoting the transformation.
Key words Maize; Acetosyringone; Seed soaking; Transgenic efficiency
Received: March 3, 2021 Accepted: May 5, 2021
Supported by Natural Science Foundation of Hebei Province (C2017301071); "Science and Technology Innovation Project" of Hebei Academy of Agriculture and Forestry Sciences (F18C10002).
Mengyu LYU (1963-), male, P. R. China, researcher, devoted to research about agricultural biotechnology.
Fushuang DONG (1979-), female, P. R. China, associated professor, devoted to research about agricultural biotechnology.
# These authors contributed equally to this work.
*Corresponding author. E-mail: HBSLMY@126.com.
Maize is the most widely distributed food crop in the world and one of the most important crops in China[1]. As a major achievement of biotechnology industrialization, genetically modified maize has been widely used all over the world, and it is of great significance to study maize genetic transformation technology[2]. Maize bud growth points serving as transformation receptor have the characteristics of directly sprouting to form transformed plants without the need of tissue culture, less restriction by maize genotypes, and short transformation period. They are a kind of good receptor material, the use of which for studying the methods for improving the transformation efficiency of maize has important practical significance. Liu et al.[1] believe that Agrobacterium-mediated method is currently the most mainstream method of plant genetic transformation, which has the characteristics of low cost, low copy number of foreign genes, stable genetic expression of foreign genes, etc., and is more suitable for large-scale transgenic technology systems. Liu et al.[3] and Zhang et al.[4] believe that acetosyringone (AS) and HO-AS and other phenolic substances as main inducers are mainly synthesized in the cell wall of dicotyledonous plants, and are usually not present in monocotyledons, so artificial addition of AS during the transformation process can promote Agrobacterium tumefaciens to infect monocots. In the past, when Agrobacterium was used to transform monocot plants, an appropriate concentration of acetosyringone was usually added to the infection solution used, which played a positive role in improving the transformation efficiency of Agrobacterium. Guo et al.[5] found that in the shoot tip transformation system, adding 2 μl of 150 μmol/L AS to the wound after infection with Agrobacterium achieved a better transformation efficiency than adding AS to the infection solution. Sun et al.[6] transferred the glyphosate-resistant EPSPS gene into the shoot tips of the maize inbred line Zheng 58 by the Agrobacterium-mediated method. After PCR detection, seven transformed plants were positive, and the transformation rate reached 7.14%, preliminarily proving that the exogenous gene had been integrated into the maize genome. Li et al.[7] took the shoot apex meristem of maize inbred line Qi 319 as the receptor, and applied the Agrobacterium-mediated method to transfer the soybean ferritin gene driven by the maize endosperm specific promoter gene 15 kDβ-Zein into maize. A total of 272 herbicide-resistant plants were screened out, of which 108 were positive by PCR detection and the transformation rate reached 3.6%, and it was preliminarily judged that the exogenous gene had been transferred into maize genome. In the past, the transformation of monocots such as maize with Agrobacterium was to promote the activation of Agrobacterium by adding AS to infection solutions. In this study, when Agrobacterium was used to transform maize seed bud growth points, acetosyringone was not only added to the infection solution, but also the water in which the maize seeds germinated during the germination stage of maize seeds to make the maize seed buds absorb acetosyringone, so that Agrobacterium could induce the activation of growth point cells when it came into contact with them, thereby improving the efficiency of transformation. This method has not been reported. In this study, with maize seed buds as the receptor material, the Agrobacterium-mediated method was used to transform maize bud growth points to obtain maize transgenic plants. Such method has the advantage of not undergoing tissue culture, and can shorten the transformation cycle and reduce dependence on genotype. However, maize is not a natural host of Agrobacterium, and without the induction of Agrobacterium by AS, Agrobacterium cannot complete the genetic transformation of maize. In this study, As penetrated into maize seeds during seed germination, and when Agrobacterium contacted the growth point cells, the AS absorbed into the seeds activated Agrobacterium, achieving an effect similar to the transformation of Agrobacterium on dicotyledonous plants. Therefore, the transformation efficiency of Agrobacterium to maize was improved, and the key problem that it is difficult to transform maize monocots with Agrobacterium was solved.
Materials and Methods
Experimental materials
Receptor material: With mature seeds of the maize inbred line "Zheng 58" (provided by the Institute of Biotechnology, Chinese Academy of Agricultural Sciences), the growth points of maize seeds were soaked and germinated as the receptor for transformation. PCR MasterMix was produced by Tiangen Biotech (Beijing) Co., Ltd., and the used instruments included PCR instrument (eppendorf, Germany), WD-9413A gel imaging analyzer (produced by Beijing Liuyi Instrument Factory), and fluorescent stereo microscope (Olympus SZX16, Japan).
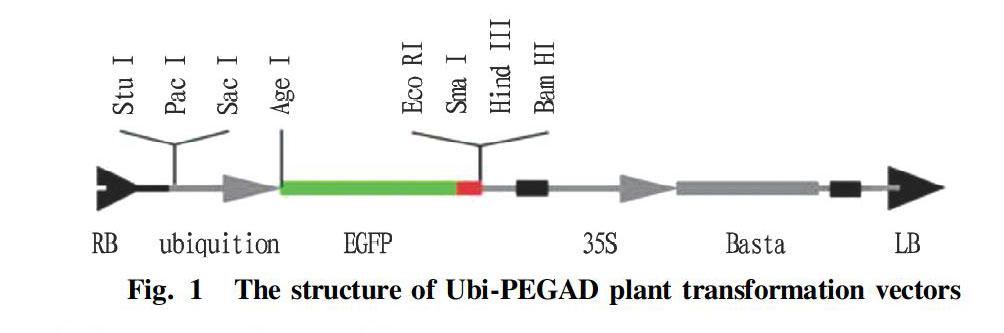
The vector PEGAD (ubi+EGFP) was obtained through the modification of vector PEGAD containing the enhanced green fluorescent protein gene (EGFP) provided by Hebei Normal University by replacing the 35S promoter with the ubiquition promoter (Fig. 1), and the used Agrobacterium strain was EHA105.
Experimental methods
Seed germination
After the maize seeds were cleaned, they were disinfected with 0.1% HgCl2 for 12 min, and then rinsed with sterile water 5 times. The sterilized seeds were placed in petri dishes (with a diameter of 9 cm) sterilized at a high temperature and laid with 2 layers of filter paper. Specifically, 20-21 maize seeds were added into each petri dish, and 8 ml of AS aqueous solutions of different concentrations were added into the petri dishes, respectively. The AS concentrations were 70, 140, 210, and 280 μmol/L, respectively, recorded as treatment 1, treatment 2, treatment 3, and treatment 4. The seeds germinated in the absence of AS served as the CK. Each treatment was done with 2 petri dishes. The petri dishes was placed in the dark at 23 ℃ for 1 d, and at 25 ℃ for 3 d in the dark.
Preparation of solution for Agrobacterium-mediated transformation
A single colony of Agrobacterium was picked and inoculated into 50 ml of LB liquid medium containing 50 mg/L kanamycin sulfate+40 mg/L rifampicin, and cultured with shaking at 28 ℃ and 210 rpm to OD600=0.5. The culture was centrifuged at 4 000 rpm and 5 ℃ for 5 min to collect the bacteria, and the supernatant was discarded. The bacteria were added with 0.5 ml of hypertonic infection base solution and shaken well to give the solution for Agrobacterium-mediated transformation. The formula of hypertonic infection base solution was: 1/10 MS medium salt+68.5 g/L sucrose+30 g/L glucose+400 mg/L MES+100 μmol/L acetosyringone+100 mg/L pluronic F68, pH=5.4-5.6.
Transformation treatment and detection
The maize seeds were subjected to transformation treatment after being soaked and germinated for 4 d. The germinated seeds were cold treated at 4 ℃ for 21 h, and then placed on the workbench for 10 min. The growth points were first peeled off with a scalpel and air-dried on a clean workbench for 30 min, and then transformed with the Agrobacterium infection solution. After the transformation, 7-10 seeds were placed in a petri dish with 2 layers of filter paper, which was then added with 1 ml of sterile water, covered and sealed, and placed in the dark at 23-25 ℃ for 3 d. Next, 10 ml of water was added to each petri dish, and the seeds were cultivated with light for 6 d. The plantlets were transplanted after training. After the transformation and germination, fluorescent protein detection was performed on the transformed buds with a fluorescence microscope, and the transformation effects of various treatments were compared. When the transformed plants grow out 5-6 leaves, the total DNA of the plant leaves was extracted by the CTAB method, and the EGFP gene was detected by PCR. The EGFP primers were EGFP-R: 5′-GCTTCAGCCGCTACCC-3′, EGFP-F: 5′-ACCTTGATGCCGTTCT -3′, and the fragment length was 280 bp. The PCR program was started at 94 ℃ for 5 min, followed by 35 cycles of 94 ℃ for 45 s, 60 ℃ for 45 s, and 72 ℃ for 1 min, and completed at 72 ℃ for 10 min. The products were stored at 4 ℃.
Results and Analysis
Effect of soaking seeds in acetosyringone on germination of maize
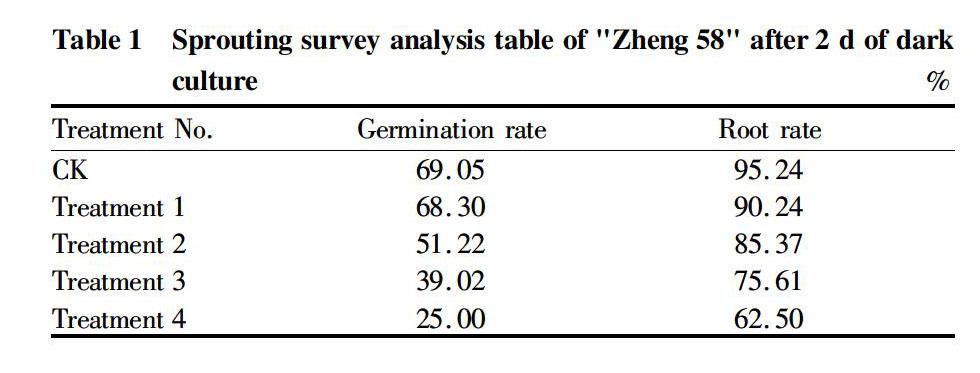
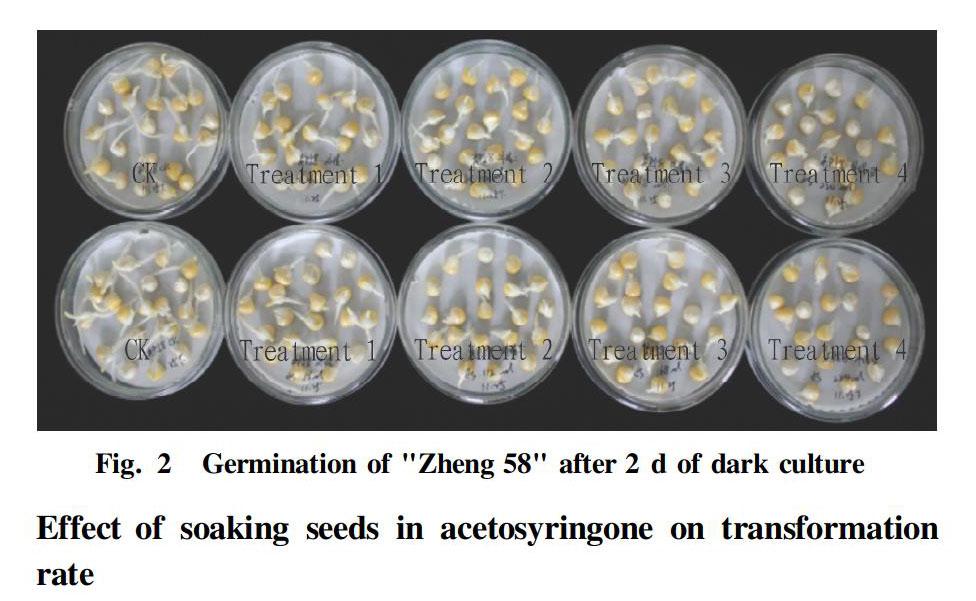
When observing the germination of maize after 4 d of seed soaking, it can be seen that with the concentration of acetosyringone solution increasing, the germination rate of maize seeds showed a gradually decreasing trend, the sprout length of maize seeds became shorter, and the rooting rate of maize seeds decreased (Table 1). The results showed that the acetosyringone solution inhibited the germination and rooting of maize seeds, and the higher the concentration of acetosyringone, the stronger the inhibition. When the concentration of acetosyringone solution reached 280 μmol/L, the seed germination rate was 36.2% of the CK, and there were few maize seed buds that could be used for transformation. Furthermore, the high concentration of acetosyringone was not conducive to bud regeneration after transformation.
Mengyu LYU et al. Effects of Acetylacetone Solution Soaking on Agrobacterium-transformed Maize Seed Buds
Effect of soaking seeds in acetosyringone on transformation rate
The results of green fluorescent protein detection of transformed buds showed that when the concentration of acetosyringone in the seed soaking solution was 70 μmol/L, the positive rate of transformed maize buds reached 32.1%, which was 44.6% higher than the CK, and compared with the CK, the rate of regenerated buds was basically unaffected, so the concentration was an appropriate seed soaking concentration. From the expression of green fluorescent protein, when the concentration of acetosyringone was 140 μmol/L, the transformation degree was the largest, which was reflected in the highest brightness of the strongest expression of green fluorescent protein, but the buds were smaller and the ratio of regenerated buds after transformation was reduced. When the concentration of the acetosyringone solution exceeded 210 μmol/L, no transformation-positive buds were obtained in the test (Table 2), and the reasons need to be further studied. The results showed that the green fluorescent protein expression of the transformed buds obtained after the treatment with acetosyringone solutions was stronger than that of the transformed buds of the CK (Fig. 3), but green fluorescence was basically not observed in treatments 3 and 4, indicating that soaking seeds with an acetosyringone solution with a concentration in a certain range was beneficial to the transformation of maize buds by Agrobacterium. The suitable concentration range of AS for soaking the seeds of the "Zheng 58" maize inbred line was 70-140 μmol/L. Through the PCR detection on the T0 generation of maize seedlings, the results were basically consistent with the green fluorescent protein detection results, which also showed the reliability of the green fluorescent protein detection results.
Conclusions and Discussion
Maize stem tips are a kind of acceptor material widely used in genetic transformation in recent years. Compared with acceptor materials such as immature embryos and embryogenic calli, this method is simple and easy to implement, and has short cycle, high efficiency, no seasonal restrictions of material selection and other advantages[7]. Since maize is not a natural host of Agrobacterium, it is necessary to activate Agrobacterium with acetosyringone. Previous studies only added acetosyringone to the infection solutions[3,5], and did not implement acetosyringone treatment during the germination of maize seeds. Du et al.[8] found that the transformation rate was only 0.41% when AS was not added to the bacterial liquid, and after adding different concentrations of AS, the transformation rate increased to varying degrees, and had significant differences from the control. When the concentration reached 200 μmol/L, the transformation rate increased by about 8 times, reaching more than 5.34%. In this study, in addition to adding acetosyringone to the infection solution, the seeds were germinated by soaking in acetosyringone solutions, the effect of promoting transformation was more obvious (Table 2). When maize seeds were soaked with 140 μmol/L acetosyringone solution for germination, the green fluorescent protein expression of the regenerated maize buds after transformation was the strongest, indicating that the absorption of acetosyringone by maize seeds was beneficial to the transformation of the bud growth point by Agrobacterium. From the perspective of transformation rate, when soaking the seeds with 140 μmol/L acetosyringone solution for germination, the transformation rate was lower than that of seeds soaked with 70 μmol/L acetosyringone solution. The reason was related to the inhibitory effect of high concentration of acetosyringone on germination, which affected the regeneration of buds after the growth points were transformed, and indirectly affected the increase of the transformation rate. On the other hand, because most of the transgenic plants obtained after infecting bud growth points of maize seeds by Agrobacterium were chimeras, when the proportion of positive cells after transformation was large, the expression of green fluorescent protein was strong (Fig. 3), and the chance of obtaining genetically modified seeds was greater. Considering this aspect, when seeds were soaked with an acetosyringone solution with a concentration of 140 μmol/L, although fewer transformed plants were obtained, the seeds of transgenic offspring were not necessarily less. Comprehensive analysis showed that 70-140 μmol/L was a more appropriate concentration range of acetosyringone for soaking seeds. It is recommended to use 100 μmol/L acetosyringone solution for soaking seeds for germination before transformation.
References
[1] LIU YJ, JIA ZW, LIU Y, et al. Establishment and application of large-scale transformation systems for maize[J]. Scientia Agricultura Sinica, 2014, 47(21): 4172-4182. (in Chinese)
[2] SHEN P, ZHANG QY, LIN YH, et al. Thinking to promote the industrialization of genetically modified corn of our country[J]. China Biotechnology, 2016, 36(4): 24-29. (in Chinese)
[3] LIU ZX, MA XQ, HE YY, et al. An improved assisting method for genetic trans formation via Agrobacterium tumefaciens[J]. Journal of Fudan University: Natural Science Edition, 1999, 38(5): 601-604. (in Chinese)
[4] ZHANG LJ, CHENG LM, DU JZ, et al. Establishment and optimization of Puna chicory genetic transformation system with Agrobacterium-mediated method[J]. Acta Agrestia Sinica, 2011, 19(6): 1042-1050. (in Chinese)
[5] GUO XM, CHE XM, PEI YH, et al. Effect of acetosyringone on genetic transformation of receptor system of different waxy maizes[J]. Southwest China Journal of Agricultural Sciences, 2013, 26 (3): 899-902. (in Chinese)
[6] SUN CB, LI HH, GUO J, et al. Study on Agrobacterium tumefaciens mediated transformation of EPSPS gene into shoot apical point of maize[J]. Biotechnology Bulletin, 2011(3): 91-93. (in Chinese)
[7] LI XL, WANG JJ, XUI RJ, et al. Genetic transformation of GmFerritin in maize (Zea mays L.)[J]. Journal of Anhui Agricultural University, 2012, 39(2):263-268. (in Chinese)
[8] DU JZ, SUN Y, HAO YS, et al. Production of herbicide-resistant plants transformed by bar gene and their basta-tolerance activity[J]. Acta Botanica Boreali-Occidentalia Sinica, 2012, 32(2): 0231-0240. (in Chinese)
杂志排行
农业生物技术(英文版)的其它文章
- Anti-inflammatory Activity and Mechanism of Total Flavonoids from the Phloem of Paulownia elongate S.Y. Hu in LPS-stimulated RAW264.7 Macrophages
- Comparative Genomic Analysis of Boron Transport Gene Family in Arabidopsis and Five Crops
- Effects of Different Water-saving Irrigation Methods on Fruit Quality and Yield of Snow Melon
- Field Control Effects and Crop Safety Assessment of Triazole Fungicides on Apple Rust
- Effects of Meteorological Factors on Overwintering Ability, Yield and Quality of Forage Rape
- Effect of Planting Density on Physiological Indexes, Agronomic Traits and Yield of Buckwheat (Fagopyrum esculentum Moench.)
