Anti-inflammatory Activity and Mechanism of Total Flavonoids from the Phloem of Paulownia elongate S.Y. Hu in LPS-stimulated RAW264.7 Macrophages
2021-08-02LiyanLITaoHUANGChongLAN
Liyan LI Tao HUANG Chong LAN
Abstract The juice leaked from broken phloem of Paulownia elongate S.Y. Hu has been used to cure acute inflammation resulted from stung injury by poisonous insects for a long time in China, but its potential mechanism remains unclear. The present study was designed to evaluate anti-inflammatory activity and mechanism of total flavonoids from the phloem of P. elongate S.Y. Hu in RAW264.7 cells. Lipopolysaccharide (LPS)-induced nitric oxide (NO) was measured by Griess and mRNA of pro-inflammatory mediators was analyzed by q-PCR. Cell viability was measured using cell counting kit (CCK)-8 assay. The protein level was analyzed by Western blot. The results showed that the total flavonoids of P. elongate significantly inhibited the production of the pro-inflammatory mediators such as NO, interleukin (IL)-1β and interleukin (IL)-6 in LPS-stimulated RAW264.7 cells. Total flavonoids of P. elongate exerted potential anti-inflammatory activity through the regulation of several signaling pathways. Total flavonoids of P. elongate inhibited JAK/STAT by blocking JAK2 and STAT3 phosphorylation levels. This is the first report on anti-inflammatory activity of total flavonoids from the phloem of P. elongate, which suggests that the total flavonoids of P. elongate may have great potential for the development of anti-inflammatory drug to treat inflammatory disorders.
Key words Paulownia elongate; Total flavonoids; Anti-inflammatory activity; Mechanisms
Received: March 23, 2021 Accepted: May 6, 2021
Supported by Henan Provincial Department of Science & Technology (172102310165); Henan Provincial Department of Education (21B350001).
Liyan LI (1974-), female, P. R. China, associate professor, devoted to research about anti-tumor and anti-UV radiation.
*Corresponding author.
Inflammation is a complex physiological process triggered by harmful stimuli such as infection injury. The immune system will notice and remove the harmful stimuli and heal damages[1]. Several types of immune cells play a role in maintaining homeostasis by regulating the levels of inflammatory mediators. Macrophages are the most important inflammatory regulatory cells, which execute antigen presentation, phagocytosis and immune regulation functions through secreting various inflammatory cytokines and growth factors. In particular, lipopolysaccharide (LPS) stimulation on macrophages initiates a series of downstream signaling cascades, which eventually lead to an increase in inflammatory mediator levels, such as nitric oxide (NO), inducible NO synthase (iNOS) and pro-inflammatory cytokines, interleukin (IL)-1β, IL-6 and so on[2].
Nonsteroidal anti-inflammatory drugs are usually used to lower the levels of inflammatory mediators. While, concerning about its adverse side effects, the usage of herbs or traditional herbal medicines as complementary and alternative medicines for the management of inflammation has significantly increased in recent years[3].
For Paulownia Scrophulariaceae family, there are seven members and their biological activities had been developed in the past years. The juice leaked from the broken phloem of P. elongate is used to cure acute inflammation resulted from stung injury by poisonous insects in folk of China. It could be identified by Lius report[4] in which listed the effects of phloem of Paulownia, such as clearing heat, relieving pain and reducing swelling, and applied in treatment of skin disease. Even so, its potential mechanism remains unclear.
Therefore, the main purpose of the present study was to confirm the anti-inflammatory effects of total flavonoids from the phloem of P. elongate in vitro and identify the underlying mechanisms of its biological actions on RAW264.7 macrophages stimulated by LPS.
Materials and Methods
Materials, chemicals and reagents
The phloem of P. elongate was obtained on campus of Huanghe Science & Technology University. Aspirin was purchased from National Institute for the Control of Pharmaceutical and Biological Products (China). Other chemical reagents were all analytical reagents and purchased from Sinopharm Chemical Reagents Co., Ltd.
Raw264.7 cell line was purchased from Cell Resource Center of Peking Union Medical College, China. Dulbeccos modified eagle medium (DMEM), penicillin-streptomycin solution, non-animal L-glutamine, dimethyl sulfoxide (DMSO), trypsin-EDTA, solution 1× (trypsin) were obtained from Life Technologies Company (USA). Fetal bovine serum (FBS) was purchased from Gibco company, USA. Phosphorylated protein extraction kit and BCA protein assay kit were purchased from Solarbio company, China. Primary antibodies and horseradish peroxidase (HRP)-conjugated secondary antibodies were purchased from Abcam company, USA. Griess reagent, IL-1β and IL-6 ELISA kits were purchased from Solarbio company, China.
Preparation of total flavonoids of P. elongate
The dried phloem of P. elongate was ground into fine powder in a blender. Petroleum ether was used to scavenge the lipid and pigment of the powder. Then the powder was reflux extracted twice by 70% ethanol at 60 ℃ for 1 h. The cooling supernatant was separated with the residue and then mixed. The ethanol extracts were filtered and concentrated in a rotor evaporator. The concentrated extract was isolated by means of chromatography on macroporous adsorption resin D101, eluted by 70% ethanol, the eluting fractions were vacuum concentrated at 40 ℃ and vacuum dried at 60 ℃. According to the rutin standard curve drawn, the extract rate of total flavonoids of P. elongate was 4.62%. The purified extract powder was stored at -20 ℃.
Cell culture
Raw264.7 cells, a murine macrophages cell line, were incubated in DMEM containing 15% FBS, 100 U/ml penicillin and 100 U/ml streptomycin at 37 ℃ in a humidified incubator with atmosphere of 5% CO2.
Cytotoxicity assay
CCK-8 assay was used to determine the cytotoxicity of total flavonoids of P. elongate in RAW 264.7 macrophages. The macrophages were grown in a 96-well plate (2×104 cells/well) followed by total flavonoids of P. elongate treatment (12.5, 25, 50 and 100 μg/ml) for 24 h. The macrophages were incubated for another 1 h after addition of 10% CCK-8 working solution. The absorbance was determined at 450 nm using a microplate reader (Multimode Plate Reader, Envision, PerkinElmer, Monza, Italy).
Determination of NO production
Cell density was adjusted to 2×105 cells/ml and cells were seeded in a 96 well plate as 100 μl/well for cytotoxicity assay. Overnight, the cells were then treated with LPS (0.5 μg/ml), total flavonoids of P. elongate (12.5, 25, 50 μg/ml) containing LPS (final concentration was 0.5 μg/ml), aspirin (20 μg/ml) as positive control containing LPS (final concentration was 0.5 μg/ml) for 24 h. The cells without any drugs were as control. Then the supernatant was collected by centrifuging at 1 000 rpm at 4 ℃ for 10 min for Griess assay.
As a major stable product of NO, the level of nitrite in the culture media was detected using Griess assay which was described by Sittisart et al.[5]. 50 μl of cell incubation supernatant mentioned above was mixed with an equal volume of Griess reagent, and incubated at room temperature for 15 min, and detected at 540 nm using a microplate reader. The quantity of nitric oxide in the samples was calculated using the linear sodium nitrite calibration curves at a concentration range of 3.125-100 μM. The cell incubation supernatant was replaced by NaNO2 as standard and draw standard curve according to the detection method mentioned above. The standard curve was made by absorbance as Y axis and molar concentration of NO2- as X axis, and y=0.046 9x+0.013 7 (R2=0.999 5).
Determination of IL-1β and IL-6 production
The levels of IL-1β and IL-6 induced by LPS and total flavonoids of P. elongate treatment in RAW264.7 were analyzed using ELISA kits according to the manufacture instructions. The cell supernatants were prepared according to the protocol in part Determination of NO production except in 48 well plates, and then kept in -86 ℃.
Pro-inflammatory cytokine levels (IL-1β and IL-6) in the cell supernatant were quantified by mouse IL-1β and IL-6 ELISA Kits (Solarbio, Beijing, China) according to the manufacture instructions.
Real-time PCR (qPCR)
Total RNA of treated cells was isolated using a TRIzolk reagent (Servicebio, Wuhan, China) and cDNA was synthesized using a cDNA synthesis kit (G3330, Servicebio, Wuhan, China). The mRNA levels were analyzed by qPCR using a Magnetic Induction Cycler (BMS, Australia) as described previously[6]. The following primers were used: forward 5′-CCCCAATTTCCAATGCTCTCC-3′ and reverse 5′-CGCACTAGGTTTGCCGAGTA-3′ for IL-6; forward 5′-CCTCGTCCCGTAGACAAAATG-3′ and reverse5′-TGAGGTCAATGAAGGGGTCGT-3′ for IL-1β; forward 5′-GGAATTGTTTGTGCTGCTTTTG-3′ and reverse 5′-AGAGGACGAGTTCACG GTAGGC-3′ for JNK2; forward 5′-CCTCGTCCC-GTAGACAAAATG-3′ and reverse 5′-TGAGGTCAATGAAGGGGTCGT-3′ for GAPH. The following conditions were applied: 10 min at 95 ℃ and 40 cycles of 15 s at 95 ℃ and 60 s at 60 ℃. Relative fold change were calculated using the 2-ΔΔCT method, where ΔΔCT=ΔCT (a target sample)-ΔCT (a reference sample).
Western blot analysis
The cells were treated according to the protocol in part Determination of NO production except in 6 well plates and 1 μg/ml of LPS for 4 h. The cells were collected and washed twice by PBS, then lysed by RIPA lysate with 1 mM PMSF, proteinase inhibitor and phosphatase inhibitor on ice for 20 min according to the protocol of Phosphorylated Protein Extraction Kit (Solarbio, Beijing, China). Cytosolic protein was collected by centrifuge at 12 000 rpm for 30 min. The precipitate was lysed by nuclear protein lysate (Solarbio, Beijing, China) for 10 min and collected nuclear proteins by centrifuge at 16 000 rpm for 10 min. The proteins were then kept in -86 ℃ for western blot analysis.
The protein concentration was determined with a BCA protein assay kit according to the manufactures protocol. The cytosolic proteins were denatured by boiling in a loading buffer, and protein samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and electroblotted onto 0.45 μm polyvinylidene difluoride (PVDF) membrane for JAK2, STAT3. Subsequently, the membrane was blocked in blocking buffer (150 mM NaCl, 20 mM Tris-HCl, 0.1% Tween 20, and 5% nonfat milk) for 2 h at room temperature, followed by incubation with the primary antibodies (anti-JAK2, anti-STAT3, Abcam company, USA) overnight at 4 ℃, and anti-alpha-tubulin as internal standard except anti-Lamin B1 for anti-NF-κB p65 antibody (Abcam company, USA). The blot was then washed 3 times with TBST (150 mM NaCl, 20 mM Tris-HCl, 0.1% Tween 20) and incubated with the appropriate secondary antibody (goat anti rabbit, HRP) for 2 h at room temperature. Immunoreactive bands were visualized by Chemi-Doc XRS system using the enhanced chemiluminescence kit, and analyzed using the Quantity one software package (Bio-Rad).
Statistical analysis
Values were expressed as mean±SEM. The analysis of Variance (ANOVA) and Tukey test were used to assess biological activity data, with P<0.05 established as statistically significant.
Results and Discussion
Cytotoxicity assay
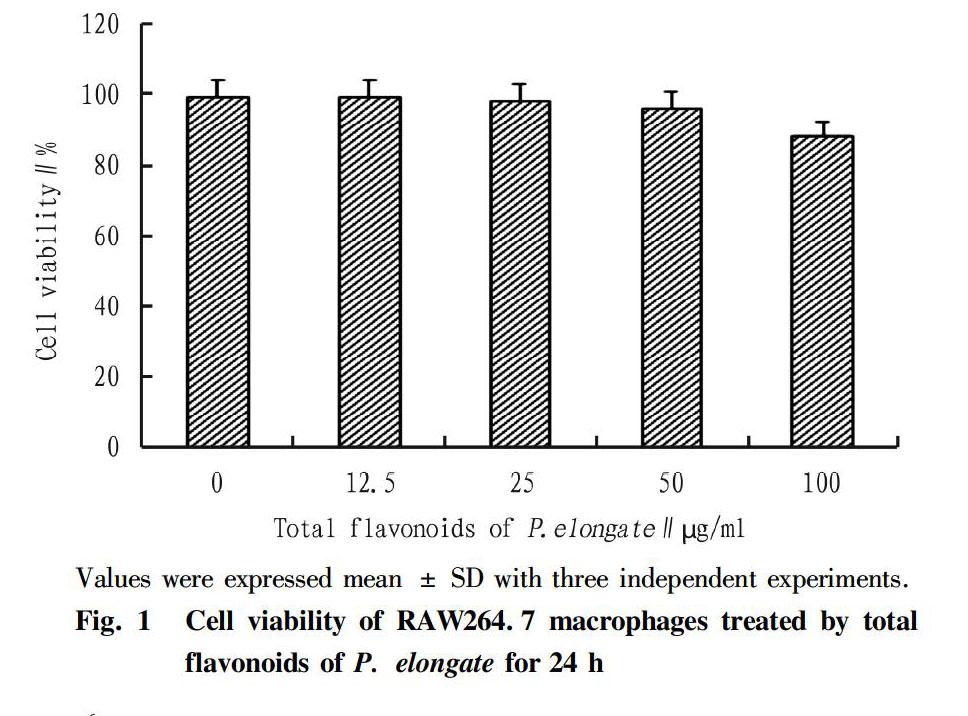
Prior to the evaluation of anti-inflammatory effect of total flavonoids from P. elongate in LPS-activated RAW 264.7 macrophage, the cytotoxic concentration of the extract was determined using CCK-8 assay. As shown in Fig. 1, total flavonoids of P. elongate showed almost no cytotoxic effect against RAW 264.7 macrophages at concentration of less 100 μg/ml. Therefore, 50 μg/ml of total flavonoids of P. elongate were chosen for the maximum test concentration.
Liyan LI et al. Anti-inflammatory Activity and Mechanism of Total Flavonoids from the Phloem of Paulownia elongate S.Y. Hu in LPS-stimulated RAW264.7 Macrophages
Effect of total flavonoids of P. elongate on NO production in LPS-stimulated RAW264.7 cells
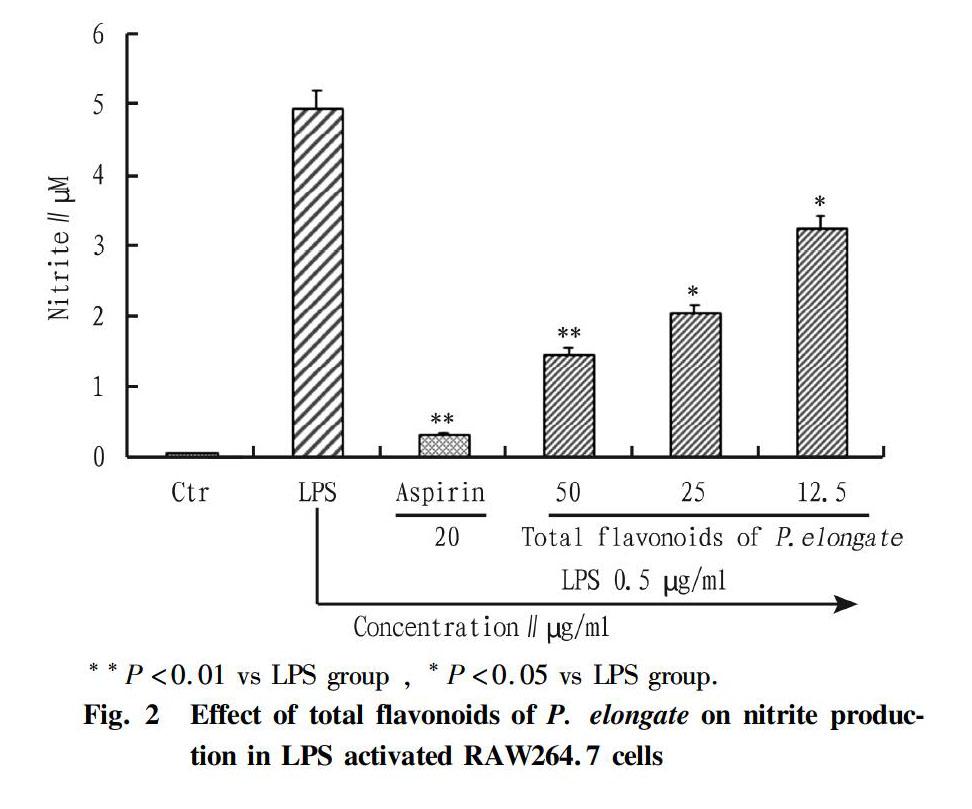
After the treatment with total flavonoids of P. elongate followed stimulation of LPS, NO concentration in the cultured medium was determined here. For control group, the basal concentration of NO in RAW264.7 macrophages was 0.095 μM, but this value was significantly increased to 4.96 μM after the treatment with 0.5 μg/ml of LPS, indicating inflammatory response in RAW264.7 macrophages. However, NO concentration by LPS treatment was significantly decreased by pretreatment with total flavonoids of P. elongate in a dose-dependent manner and it was1.5, 2.08 and 3.27 μM, respectively, and which was 0.35 μM in aspirin group (Fig. 2).
Effect of total flavonoids of P. elongate on inflammatory factors
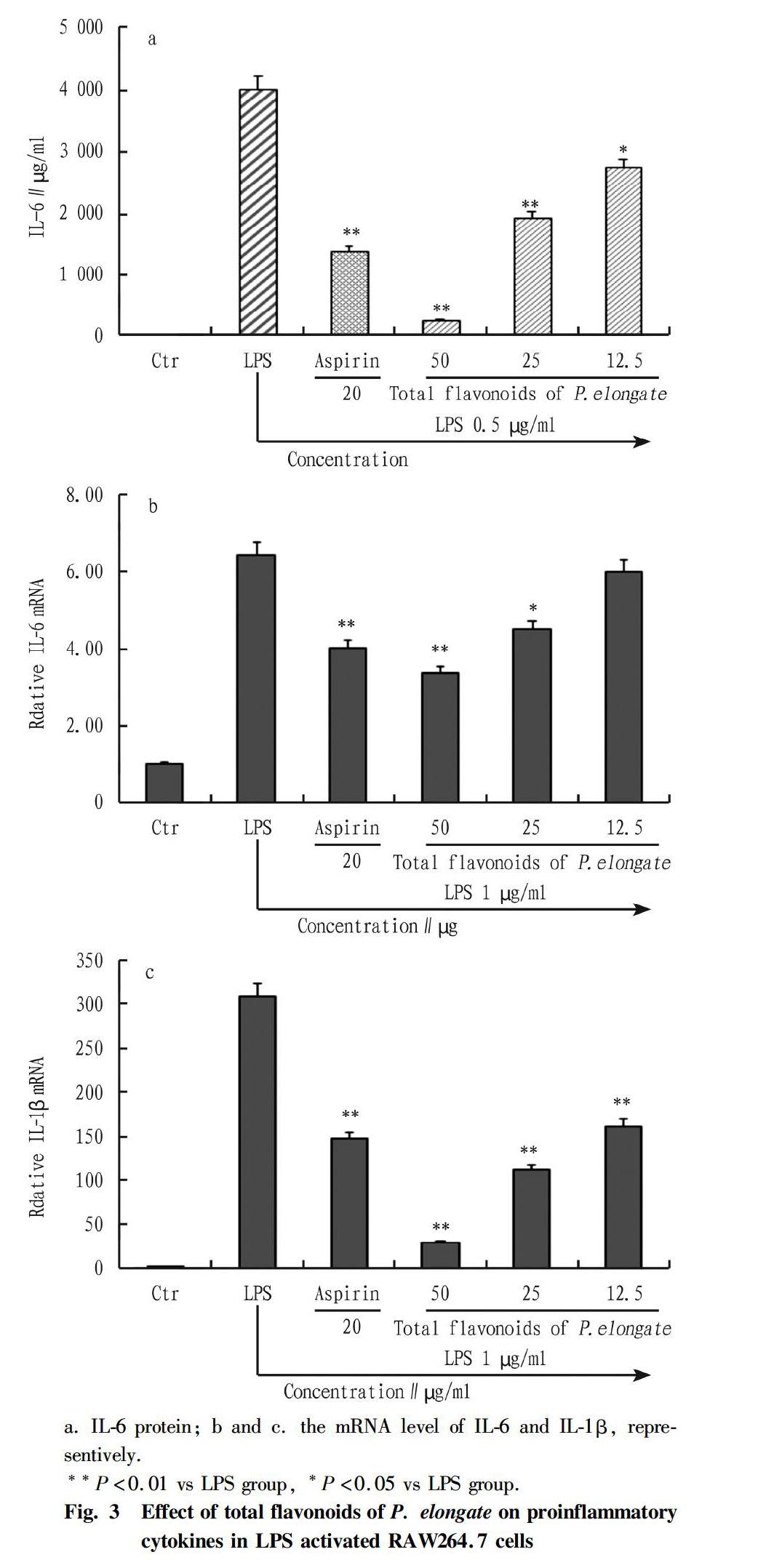
We also examined whether total flavonoids of P. elongate could regulate production of pro-inflammatory cytokines by ELISA assay. As shown in Fig. 3a-c, LPS treatment significantly increased the expression level of IL-6 and IL-1β compared with those of the nontreatment group, which was consistent with the results of mRNA expression (Fig. 3b and Fig. 3c) and protein level (Fig. 3a). While, the pretreatment of total flavonoids of P. elongate significantly inhibited the expression of proinflammatory cytokines in a dose-dependent manner. The present results indicate that total flavonoids of P. elongate may be beneficial for preventing inflammatory disorder.
Western blot analysis
To determine whether the blockade of JAK/STAT signaling activation plays a role in mediating the anti-inflammatory effect of total flavonoids of P. elongate, we investigated the effects of total flavonoids of P. elongate on LPS-stimulated activation of p-JAK2 (Tyr1007/1008) and p-STAT3 (Tyr705) in macrophages by western blot. As illustrated in Fig. 4a, total flavonoids of P. elongate attenuated the phosphorylation of p-JAK2 induced by LPS (1 μg/ml) stimulation for 4 h in a dose-dependent manner. Subsequently, total flavonoids of P. elongate were also shown to attenuate LPS-induced STAT3 phosphorylation levels in a concentration-dependent manner (Fig. 4b). It suggested that total flavonoids of P. elongate prevented the JAK/STAT signaling pathway in LPS-treated inflammatory condition.
Discussion
The juice leaked from the broken phloem of Paulownia elongate has been used to cure acute inflammation resulted from stung injury by poisonous insects in folk of China for longtime. However, the mechanism through which the effective constituent exerts anti-inflammatory activity is not elucidated. Flavonoids in plant always show anti-inflammatory activity. In this study, therefore, we investigated the anti-inflammatory activity and mechanism of total flavonoids from phloem of P. elongate in LPS-stimulated murine macrophages, RAW264.7 cells.
The process of inflammation response include extensive leukocytes infiltration, secretion of inflammatory mediators, and abnormal activation of signaling pathways regulating inflammation, which may result in the development and ongoing of various diseases[7]. Macrophages are crucial cells that play a variety of roles in the inflammatory process, through produce a variety of proinflammatory mediators such as NO, IL-1β and IL-6 in response to LPS stimuli. Thus, we evaluated whether flavonoids of P. elongate exerts its anti-inflammatory properties by inhibiting other inflammatory mediators and cytokines, as well as NO, and investigate the signaling pathways involved in its mechanism in RAW 264.7 cells stimulated by LPS, which is widely used for searching proinflammatory agents.
In this study, we observed that total flavonoids of P. elongate significantly lowered LPS-induced NO levels, a key biomarker of oxidative stress in inflammatory reactions, in RAW264.7 cells[8]. Our study also indicate that total flavonoids of P. elongate displays great potency in deceasing LPS-stimulated proinflammatory cytokines IL-1β and IL-6 in RAW264.7 cells without exhibiting cytotoxicity. This suggested that it may be a promising candidate as a novel anti-inflammatory drug.
Furtherly, we investigated the possible mechanism of the anti-inflammatory effect of total flavonoids of P. elongate in RAW264.7 cells.
The transcriptional activation of JAK2/STAT3 signal pathway
has been reported to be essential in mediating inflammatory diseases[9-11]. The transcriptional activity of STATs could be activated by upstream inflammatory cytokines, and activated STATs protein mediates a positive feedback loop and results in the overproduction of inflammatory mediators and proinflammatory cytokines[12-13]. We observed a decreasing of phosphorylated JAK2 and phosphorylated STAT3 proteins expression level in LPS-activated macrophages following pretreatment with flavonoids of P. elongate, which in turn might inhibit the proinflammatory factors, such as IL-1β and IL-6.
Conclusions
Our study demonstrated that total flavonoids of P. elongate significantly inhibited the production of the pro-inflammatory mediators such as NO, IL-1β and IL-6 in LPS-stimulated RAW264.7 cells. Total flavonoids of P. elongate exerted potential anti-inflammatory activity through the regulation of several signaling pathways. Total flavonoids of P. elongate inhibited JAK/STAT by blocking JAK2 and STAT3 phosphorylation levels. These findings suggest that flavonoids of P. elongate may have great potential for the development of anti-inflammatory drug to treat inflammatory disorders. In further, the experiments in vivo need to be done to further identify to supply a probability of flavonoids of P. elongate to develop as an anti-inflammatory drug.
References
[1] CHEN L, DENG H, CUI H, et al. Inflammatory responses and inflammation-associated diseases in organs[J]. Oncotarget, 2017, 9(6): 7204-7218.
[2] FANG H, PENGAL RA, CAO X, et al. Lipopolysaccharide-induced macrophage inflammatory response is regulated by SHIP[J]. J. Immunol. (Baltimore, Md.: 1950), 2004, 173(1): 360-366.
[3] KHANSARI N, SHAKIBA Y, MAHMOUDI M. Chronic inflammation and oxidative stress as a major cause of agerelated diseases and cancer[J]. Recent Patents on Inflammation & Allergy Drug Discovery, 2009, 3(1): 73-80.
[4] LIU BX, DONG GP, LIU HY, et al. Traditional application and modern research and development of skin phytomedicine in dermatosis treatment[J]. China Journal of Traditional Chinese Medicine and Pharmacy, 2018, 33(12): 5654-5659.
[5] SITTISART P, CHITSOMBOON P, KAMINSKI NE. Pseuderanthemum palatiferum leaf extract inhibits the proinflammatory cytokines, TNF-α and IL-6 expression in LPS-activated macrophages[J]. Food Chem. Toxicol., 2016(97): 11-22.
[6] OH Y, AHN CB, YOON NY, et al. Protective effect of enzymatic hydrolysates from seahorse (Hippocampus abdominalis) against H2O2-mediated human umbilical vein endothelial cell injury[J]. Biomed. Pharmacother, 2018(108): 103-110.
[7] LEE DH, SHIN JS, KANG SY, et al. Iridoids from the roots of Patrinia scabra and their inhibitory potential on LPS-induced nitric oxide production[J]. J. Nat. Prod., 2018, 81(6): 1468-1473.
[8] XUAN YT, GUO Y, ZHU Y, et al. Mechanism of cyclooxygenase-2 upregulation in late preconditioning[J]. J. Mol. Cell. Cardiol., 2003, 35(5): 525-537.
[9] YU X, KENNEDY RH, LIU SJ. JAK2/STAT3, not ERK1/2, mediates interleukin-6-induced activation of inducible nitric-oxide synthase and decrease in contractility of adult ventricular myocytes[J]. J. Biol. Chem., 2003, 278(18): 16304-16309.
[10] GORINA R, FONT-NIEVES M, MBRQUEZ-KISINOUSKY L, et al. Astrocyte TLR4 activation induces a proinflammatory environment through the interplay between MyD88-dependent NFκB signaling, MAPK, and Jak1/Stat1 pathways[J]. Glia, 2011, 59(2): 242-255.
[11] BOLLI R, DAWN B, XUAN YT. Role of the JAK–STAT pathway in protection against myocardial ischemia/reperfusion injury[J]. Trends Cardiovas. Med., 2003, 13(2): 72-79.
[12] MORI T, MIYAMOTO T, YOSHIDA H, et al. IL-1β and TNFα-initiated IL-6-STAT3 pathway is critical in mediating inflammatory cytokines and RANKL expression in inflammatory arthritis[J]. Int. Immunol., 2011, 23(11): 701-712.
杂志排行
农业生物技术(英文版)的其它文章
- Comparative Genomic Analysis of Boron Transport Gene Family in Arabidopsis and Five Crops
- Effects of Different Water-saving Irrigation Methods on Fruit Quality and Yield of Snow Melon
- Field Control Effects and Crop Safety Assessment of Triazole Fungicides on Apple Rust
- Effects of Acetylacetone Solution Soaking on Agrobacterium-transformed Maize Seed Buds
- Effects of Meteorological Factors on Overwintering Ability, Yield and Quality of Forage Rape
- Effect of Planting Density on Physiological Indexes, Agronomic Traits and Yield of Buckwheat (Fagopyrum esculentum Moench.)
