Response of Vitellogenin in Tilapia Liver to 2, 2′, 4, 4′-Tetrabromodiphenyl Ether Stress
2021-08-02ShunlongMENGXiCHENZeqiuCHENLipingQIUChaoSONGLiminFANYaoZHENGJiachangCHENPaoXU
Shunlong MENG Xi CHEN Zeqiu CHEN Liping QIU Chao SONG Limin FAN Yao ZHENG Jiachang CHEN Pao XU
Abstract [Objectives] This study was conducted to understand the potential harm of BDE-47 to fish and aquatic ecosystems and obtain relevant toxicological data from the perspective of vitellogenin.
[Methods]Adopting the semi-static water exposure method, three exposure concentrations of 5, 50, and 500 μg/L and five sampling time of 1, 3, 7, 15, and 30 d were set to investigate the effect of BDE-47 on vitellogenin in tilapia liver.
[Results] The low concentration of BDE-47 (5 μg/L) had no effect on the level of vitellogenin in the liver of tilapia. When exposed to high concentrations of BDE-47 (50 and 500 μg/L), the VTG content of tilapia liver showed a trend of first decreasing, then returning to normal, and then increasing. An abnormal VTG content indicates that the endocrine system of tilapia is disturbed to a certain extent.
[Conclusions]This study plays a role in promoting the formulation of relevant water quality standards and the protection of aquatic living resources.
Key words 2, 2′, 4, 4′-Tetrabromodiphenyl ether; Tilapia; Liver; Vitellogenin
Received: January 16, 2021 Accepted: March 7, 2021
Supported by National Key R&D Program (2020YFD0900502); Special Project of National Characteristic Freshwater Fish Industry Technology System (CARS-46).
Shunlong MENG (1982-), male, P. R. China, researcher, PhD, devoted to research about environmental toxicology, fishery environmental protection, aquatic product quality and safety risk assessment.
*Corresponding author. E-mail: chenjz@ffrc.cn; xup@ffrc.cn.
Polybrominated diphenyl ethers (PBDEs) are brominated aromatic hydrocarbons, which are divided into 209 homologues based on the number of bromine atoms in their molecules. PBDEs began to be industrialized in the 1940s and 1950s. As excellent flame retardants, PBDEs are widely added to rubber, resin, polyurethane and other polymer materials to make fireproof materials. However, due to the strong stability of PBDEs and their release into the environment following the incineration of waste industrial products containing PBDEs, they have been widely detected in water, air, soil, sediments, animals and plants, and humans. Because PBDEs have the characteristics of environmental persistence, long-distance transmission, bioaccumulation, endocrine disrupting effects, etc., the environmental problems they cause have attracted more and more attention from the society, and they have been listed as new POPs and their production and use have been restricted.
Some pollutants at low concentrations will not cause fish death or growth abnormalities, but they will participate in the physiological and biochemical reactions at the molecular level in the fish body and interfere with normal physiological activities, such as too-high or too-low levels of sex hormones[1]. The pathway of fish VTG synthesis is as follows: fish ovaries synthesize estrogen, and transport it to the liver to bind with ERs in the liver, forming a hormone-receptor complex, which transforms its conformation to bind to the promoter sequence on RNA, which leads to the expression of the VTG gene, involving initiation of the synthesis of VTG polypeptide chains and finally synthesis of VTG; and VTG is transported into the ovary to produce vitellin then through related enzymatic reactions[2].
The sex differentiation of fish is easily affected by exogenous hormones, and even some fish have sexual reversal due to the stimulation of exogenous hormones. Therefore, environmental exogenous hormones will affect the normal reproduction process of fish and have a negative impact on the ecosystem. In this study, the effect of tetrabromodiphenyl ether (BDE-47) on the activity of VTG in the liver of tilapia was investigated, hoping to understand the potential hazards of BDE-47 to fish and aquatic ecosystems from the perspective of VTG, and obtain relevant toxicological data for promoting the formulation (revision) of relevant water quality standards and the formulation of limit standards and the protection of aquatic living resources.
Materials and Methods
Materials
The test fish used in this experiment was Oreochromis niloticus. The fish were taken from the test site of the Freshwater Fisheries Research Center of the Chinese Academy of Fishery Sciences. After 4 weeks of domestication, the fish with strong activity were selected to start the experiment. The average body length and weight were measured before the start of the experiment. The results showed that the average body weight was (38.30±3.98) g (n=40), and the average body length was (13.57±0.50) cm (n=40).
Experimental water
The experimental water was ordinary tap water after three days of aeration. During the experiment, the water temperature was 24.3-25.5 ℃, the dissolved oxygen was 6.3-7.0 mg/L, and the pH value was 6.5-7.5. The experimental water met the fishery water quality standard (GB11607-89).
Main reagents and instruments
Main reagents: Tetrabromodiphenyl ether (Wuhan Kaimeike Chemical Technology Co., Ltd.); dimethyl sulfoxide (DMSO) (Jiangsu Qiangsheng Functional Chemical Co., Ltd.).
Kit used: VTG enzyme-linked immunoassay kit, purchased from Shanghai Lengton Biotechnology Co., Ltd.
Main instruments: Glass homogenizer; constant temperature water bath; low temperature refrigerated centrifuge (Sigma 26K); multi-function microplate reader (Spectra Max M5); constant temperature box; pipettes of various specifications; distilled water; test tubes; beakers; absorbent paper; measuring cylinder.
Preparation of reagent mother liquor: A certain amount of BDE-47 (50 mg) was accurately measured, dissolved in DMSO, and diluted to constant volume in a 25 ml brown volumetric flask. The prepared BDE-47 mother liquor had a concentration of 2×103 mg/L and stored in the dark at 4 ℃ for later use.
Experimental design
This experiment was set with three concentration gradients: 5, 50, and 500 μg/L, and a blank control and a solvent control were also set (the solvent concentration was the same as the solvent concentration in the highest concentration of poisoning solution). The poisoned groups and the control were all set with three parallels. 300 L aquaria were equipped with 200 L of the above-mentioned solutions in various concentrations, respectively, and 40 healthy tilapias were randomly added in each parallel group. The tilapia were fed once in the morning and once in the evening every day during the temporary rearing and experiment with a total feeding amount of 3% of the tilapia body weight, and oxygenation was performed with air compressor. The renewing static water quality contact test method was used. During the experiment, half of the contaminated water was discharged from each water tank every day, and the drug and water was added to make the total poisoning solution volume and drug concentration unchanged. The experimental period was 30 d.
In order to obtain the actual content of BDE-47 in each test group during the experiment, we referred to the method provided by Sha Jingjing et al. to sample and determine the experimental water. After analysis, the BDE-47 concentrations after 24 h of exposure were as follows: 0, 0, 4.86, 57.28, and 343.92 μg/L, respectively. The results showed that the actual concentration in the high-dose concentration groups were lower than the concentrations set in the experimental design. Relevant data show that due to its high lipophilicity, PBDEs have very low water solubility in actual water bodies and are easy to adsorb on container walls and in bottom sludge. Therefore, it was normal for the measured concentrations of the water samples to be slightly lower than the experimental design concentrations during the experiment.
Sample collection and testing
The tilapia samples of each concentration group were collected on the 1st, 3rd, 7th, 15th, and 30th d after exposure. Feeding was stopped the day before sampling. At the time of sampling, two test fish were randomly selected from each group of three parallels, and measured for their body lengths and weights, and then tilapia were quickly put to death to take 1 g of fish liver. Three parts of liver were preserved, and the remaining three parts of liver entered the next step of the experiment. The liver tissue was rinsed with normal saline at 4 ℃ to remove blood clots on it, wiped to remove water and put into a small beaker. First, a pipette was used to transfer 2/3 of the pre-cooled normal saline [the total volume of normal saline (ml) was 9 times the tissue weight (mg)] into the beaker, and the liver tissue was cut with scissors while keeping the temperature low. The liver fragments were poured from the beaker into a homogenizer, and then the beaker was flushed with the remaining 1/3 of normal saline, which was then poured into the homogenizer. The liver fragments were fully ground to a homogenate (the homogenization process needed to be kept at low temperature). Centrifugation was performed at 3 000 r/min and 4 ℃ for 10 min in a low-temperature centrifuge. The supernatant was added in a centrifuge tube and stored in a refrigerator at 4 ℃.
The determination index was VTG In tilapia liver. The determination operation steps were based on the operation method of the kit.
Data processing
Considering that the experimental duration was 30 d, which is a relatively long time for tilapia with a relatively short life cycle, the enzyme activity and hormone level of the tilapia body might also change to some extent due to its own growth during the experiment. Therefore, in order to more scientifically evaluate the effects of BDE-47 on the liver enzyme activity and hormone levels of tilapia, the results of the experiment were expressed as relative values, namely: the measurement result of the treatment group at the same measurement time/the result of the blank measurement group×100%. The data results were expressed as mean±standard deviation (mean±sd) (n=3). The experimental data were analyzed for variance with SPSS 17.0 statistical software, and Tukeys multiple comparisons were used to analyze the differential effects of concentration and time.
Results and Analysis
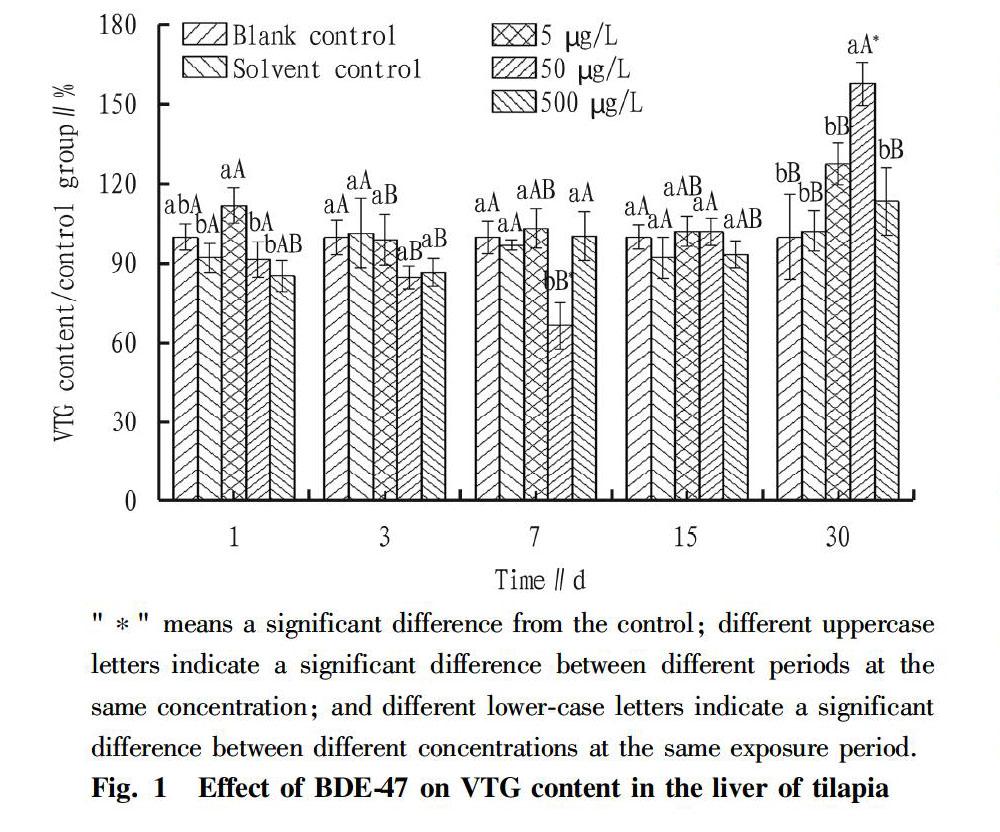
During the entire experimental period, there was no death of tilapia. The effect of BDE-47 on the VTG content of tilapia liver is shown in Fig. 1. During the entire period, the VTG contents of tilapia liver in the blank group and the solvent control group were relatively stable, and there was no significant difference between the two (P>0.05). The treatment of low-concentration BDE-47 (5 μg/L) exposure had no effect on the VTG content of tilapia liver, and the fish were relatively stable during the entire experimental period, and had no significantly difference from the control group (P>0.05). When exposed to high concentrations of BDE-47 (50 and 500 μg/L), the VTG content of tilapia liver showed a trend of first decreasing, then returning to the normal level, and then increasing. Specifically, on the 7th d after BDE-47 exposure, the VTG content in the liver of tilapia in the 50 μg/L group decreased significantly, showing an inhibitory effect with the inhibition rate reaching 30%, and the value was significantly lower than that of the control group (P<0.05); on the 15th d after BDE-47 exposure, the VTG content in each concentration group returned to the normal level, and the various groups were basically the same, with no significant differences (P>0.05); and on the 30th d after BDE-47 exposure, the VTG content of each concentration group showed an increasing trend, and the VTG content of tilapia liver in the 50 μg/L concentration group increased significantly, showing an induction effect reaching 50%, which was significantly higher than that of the control group (P<0.05).
Agricultural Biotechnology2021
Discussion
Vitellogenin (VTG) is the precursor of vitellin in oviparous vertebrates[3]. The pathway of fish VTG synthesis is as follows: fish ovaries synthesize estrogen, and transport it to the liver to bind with ERs in the liver, forming a hormone-receptor complex, which induces the change of the conformation of the hormone receptors and binds to the promoter sequence on the VTG gene, leading to the expression of the VTG gene and the synthesis of VTG[4-6]. Under normal physiological conditions, fish VTG is only synthesized in the liver of sexually mature female fish, while male fish or juvenile fish cannot synthesize or the synthesis amount is very low. However, because the liver ERs of male fish or juveniles exposed to environmental estrogens can be activated by environmental estrogen, they can also be induced to synthesize VTG[7-9]. There have been many reports about male fish or juvenile fish being exposed to environmental estrogens to be induced to synthesize VTG[8,10]. Therefore, VTG content has become a highly sensitive biomarker, which is widely used to screen and indicate the environmental estrogen effects of pollutants[11-13], and VTG has been recommended by the International Organization for Economic Cooperation and Development (OECD) as an ideal biomarker for exposure to environmental estrogens[14]. This study showed that when tilapia was exposed to a concentration of 50 μg/L, BDE-47 showed a tendency to first inhibit and then induce the VTG content in the liver. An abnormal VTG content indicates that the endocrine system of tilapia is disturbed to a certain extent. Cionna et al.[15] studied the effect of estrogen and estradiol on the VTG content of male golden mullet, Folmar et al.[16] studied the effects of ethinyl estradiol, diethylstilbestrol and estradiol on the VTG content of male Cyprinodon variegatus, and Jung et al.[17] studied the effect of 4-renylphenol on the VTG content of male Gymnocorymbus ternetzi. They all observed a significant increase in the VTG content.
Conclusions
Exposure to the low concentration of BDE-47 (5 μg/L) had no effect on the VTG content of tilapia liver, and the fish grew relatively stable throughout the entire experimental period and was not significantly different from the control group. When exposed to high concentrations of BDE-47 (50 and 500 μg/L), the VTG content of tilapia liver showed a trend of first decreasing, then returning to the normal level, and then increasing. An abnormal VTG content indicates that the endocrine system of tilapia is disturbed to a certain extent.
References
[1] LI WM, YIN DQ, HU SQ, et al. Effects of two chloric-nitroanilines on serum sex steroids in carp (Carassius auratus)[J]. Journal of Nanjing University: Natural Sciences, 2001, 37(6): 707-712. (in Chinese)
[2] CHEN YP, QIAN XY, PAN N, et al. Effect of printing-dying sewage on vitellogenin and metallothionein level of Carassius auratus[J]. Journal of Hydroecology, 2011,32(6): 95-99. (in Chinese)
[3] UTARABHAND P, BUNLIPATANON P. Plasma vitellogenin of grouper (Epinephelus malabaricus): isolation and properties[J]. Comparative Biochemistry and Physiology Part C: Pharmacology, Toxicology and Endocrinology, 1996, 115(2): 101-110.
[4] BRION F, TYLER CR, PALAZZI X, et al. Impacts of 17β-estradiol, including environmentally relevant concentrations, on reproduction after exposure during embryo-larval-, juvenile-and adult-life stages in zebrafish (Danio rerio)[J]. Aquatic Toxicology, 2004, 68(3): 193-217.
[5] NILSEN BM, BERG K, EIDEM JK, et al. Development of quantitative vitellogenin-ELISAs for fish test species used in endocrine disruptor screening[J]. Analytical and bioanalytical chemistry, 2004, 378(3): 621-633.
[6] CHEN JC, WANG ZR, JU JH, et al. The comparative effect between 1-naphthol and 17β-estradiol on estrogen effect in male tilapia (GIFT Oreochromis niloticus)[J]. Ecology and Environment, 2012, 21(4): 754-759. (in Chinese)
[7] KIME DE. A strategy for assessing the effects of xenobiotics on fish reproduction[J]. Science of the Total Eenvironment, 1999, 225(1): 3-11.
[8] OKOUMASSOUN LE, AVERILL-BATES D, GAGNé F, et al. Assessing the estrogenic potential of organochlorine pesticides in primary cultures of male rainbow trout (Oncorhynchus mykiss) hepatocytes using vitellogenin as a biomarker[J]. Toxicology, 2002, 178(3): 193-207.
[9] LIU X, JI K, JO A, et al. Effects of TDCPP or TPP on gene transcriptions and hormones of HPG axis, and their consequences on reproduction in adult zebrafish (Danio rerio)[J]. Aquatic Toxicology, 2013(134): 104-111.
[10] ARUKWE A, KULLMAN SW, HINTON DE. Differential biomarker gene and protein expressions in nonylphenol and estradiol-17β treated juvenile rainbow trout (Oncorhynchus mykiss)[J]. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 2001, 129(1): 1-10.
[11] ZHANG Z, HU J, JIN WA, et al. Induction of vitellogenin mRNA in juvenile chinese sturgeon (Acipenser sinensis gray) treated with 17β-estradiol and 4-nonylphenol[J]. Environmental Toxicology and Chemistry, 2005, 24(8): 1944-1950.
[12] WANG H, TAN JTT, EMELYANOV A, et al. Hepatic and extrahepatic expression of vitellogenin genes in the zebrafish, Danio rerio[J]. Gene, 2005(356): 91-100.
[13] JIN Y, WANG W, SHENG GD, et al. Hepatic and extrahepatic expression of estrogen-responsive genes in male adult zebrafish (Danio rerio) as biomarkers of short-term exposure to 17β-estradiol[J]. Environmental Monitoring and Assessment, 2008, 146(1-3): 105-111.
[14] SUMPTER JP, JOBLING S. Vitellogenesis as a biomarker for estrogenic contamination of the aquatic environment[J]. Environmental Health Perspectives, 1995, 103(S7): 173-178.
[15] CIONNA C, MARADONNA F, OLIVOTTO I, et al. Effects of nonylphenol on juveniles and adults in the grey mullet, Liza aurata[J]. Reproductive Toxicology, 2006, 22(3): 449-454.
[16] FOLMAR LC, HEMMER M, HEMMER R, et al. Comparative estrogenicity of estradiol, ethynyl estradiol and diethylstilbestrol in an in vivo, male sheepshead minnow (Cyprinodon variegatus), vitellogenin bioassay[J]. Aquatic Toxicology, 2000, 49(1): 77-88.
[17] JUNG JH, SHIM WJ, ADDISON RF, et al. Protein and gene expression of VTG in response to 4-nonylphenol in rockfish (Sebastes schlegeli)[J]. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 2006, 143(2): 162-170.
杂志排行
农业生物技术(英文版)的其它文章
- Anti-inflammatory Activity and Mechanism of Total Flavonoids from the Phloem of Paulownia elongate S.Y. Hu in LPS-stimulated RAW264.7 Macrophages
- Comparative Genomic Analysis of Boron Transport Gene Family in Arabidopsis and Five Crops
- Effects of Different Water-saving Irrigation Methods on Fruit Quality and Yield of Snow Melon
- Field Control Effects and Crop Safety Assessment of Triazole Fungicides on Apple Rust
- Effects of Acetylacetone Solution Soaking on Agrobacterium-transformed Maize Seed Buds
- Effects of Meteorological Factors on Overwintering Ability, Yield and Quality of Forage Rape
