Research Advances in Plant Golgi Apparatus
2021-08-02TingLUYuanyongGONGLihuaZHAOFeiYAN
Ting LU Yuanyong GONG Lihua ZHAO Fei YAN
Abstract Golgi apparatus, together with endoplasmic reticula, vacuoles and plasma membrane, constitutes the endoplasmic system of plant cells. It plays an important role in the secretion pathway of eukaryotic cells and is responsible for various intracellular events, such as protein classification, protein modification and glycosylation. At present, much less is known about plant Golgi proteins. The research on its function is still insufficient. In order to provide a comprehensive research background and research ideas for related researchers, this paper systematically and comprehensively evaluated the structure of plant endoplasmic system, the common endoplasmic reticulum-Golgi transport pathway in plant cells, various possible transport models between endoplasmic reticula and Golgi bodies, Golgi-associated specific proteins and functions, and Golgi biogenesis pathway. The latest research progress in this field was reviewed and analyzed in detail. This paper will provide an important reference for related researchers to carry out the research of plant Golgi.
Key words Golgi apparatus; Endoplasmic reticulum (ER); Golgin; Plant
Received: March 2, 2021 Accepted: May 6, 2021
Supported by Special Fund for the Research Team of Ecological Restoration and Governance Innovation of the Jinsha River Dry and Hot Valley (035200179); 2020 Doctoral Research Startup Fund of Panzhihua University (035200254).
Ting LU (1985-), male, P. R. China, lecturer, devoted to research about plant cytology.
*Corresponding author.
Golgi apparatus is the central organelle of the secretory pathway of eukaryotic cells. It is composed of flat membranes called cisternae, which are usually superimposed on each other to form a characteristic Golgi apparatus[1-2]. In most mammalian cells, Golgi stacks are connected laterally to form a Golgi apparatus zone, on both sides of which are tubular cis and trans Golgi networks, which serve as sites for entering and leaving the Golgi apparatus respectively[1-2]. Golgi apparatus is the main site for protein post-translational modification. Glycoproteins and glycolipids are added or modified with glycan chains in Golgi cisternae, and then transported from one side of the Golgi apparatus to the other under the action of various functional proteins of the Golgi apparatus[3]. In addition, Golgi apparatus is also the main sorting workshop, which is the place for packaging goods for delivery to various downstream destinations, and returning selected proteins to endoplasmic reticula through retrograde trafficking, which is the first stop of the secretory pathway[4-5]. In addition to the main membrane transport and glycosylation functions of Golgi apparatus, the Golgi apparatus of mammalian cells is also involved in the regulation of a series of cellular processes, including mitosis, DNA repair, stress response, autophagy, apoptosis and inflammation[6]. Plant Golgi plays an important role in protein glycosylation and sorting, and it is also the main biosynthetic organelle for synthesizing a large number of cell wall polysaccharides[7]. This review emphasized the unique aspects of the organization and function of the Golgi apparatus of plants from the following aspects, which can provide certain information for the specialized research of plant Golgi apparatus, and provide a certain reference value for the research of plant cell biology.
Plant Membrane System
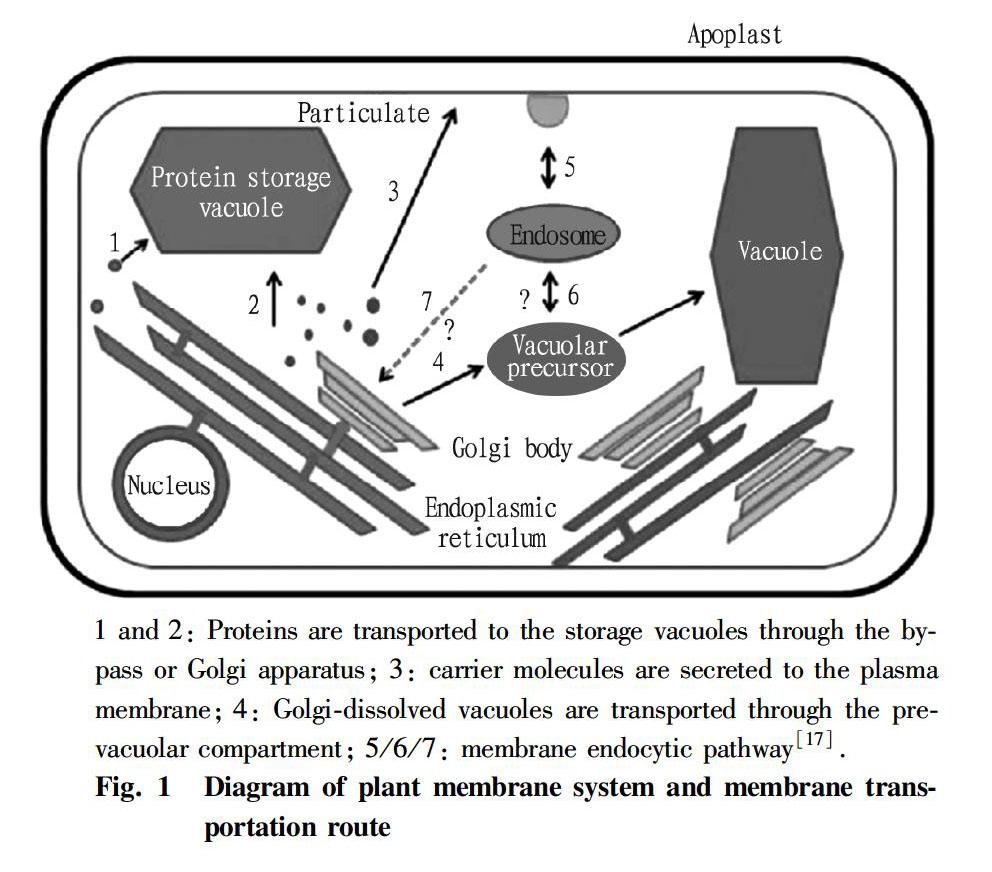
Cell biomembrane system refers to a biomembrane system composed of cell membranes, nuclear membranes, endoplasmic reticula, Golgi apparatus, mitochondria and other organelles surrounded by membranes. The composition and structure of these biomembranes are very similar, and they are closely related in structure and function, which further reflects the coordination and cooperation between various structures in cells[8]. The cell biomembrane system plays an extremely important role in the life activities of cells. In addition, the study of cell biomembrane systems has broad prospects in medicine and production processes[8]. The inner membrane system of all eukaryotic cells is very complex, but well organized. It forms the central mechanism for the classification and secretion of proteins and other substances (such as lipids, glycans and polysaccharides). In plants, the inner membrane system consists of endoplasmic reticula (ER), vacuoles, nuclear membranes, plasma membranes and Golgi apparatus (Fig. 1). With the help of various fluorescent proteins, confocal laser microscopy, immunofluorescence labeling and other conventional biochemical techniques, the intramembrane system has been extensively studied in the past ten years[9]. The endoplasmic reticula in plant cells form a continuous tubular network in the cytoplasm. They have a highly dynamic structure that is constantly formed and changed. This dynamic characteristic is considered to be regulated by the actin cytoskeleton closely related to the ER network[10]. In the absence of actin filaments, the movement of endoplasmic reticula is disturbed. However, microtubules have no effect on endoplasmic reticulum dynamics. The secreted protein is produced by the ER binding ribosome as a peptide precursor, and must be correctly folded in the lumens of ER before being exported to other organelles[11]. The proteins and other carrier molecules processed by these endoplasmic reticulua may bind to specific classification receptors and concentrate at the exit of endoplasmic reticula, where they are transported by protein-encapsulated vesicles through receptor-mediated transport pathways, or they can be transported through a decentralized mechanism[11]. Golgi apparatus is the next stop for protein transport. The two main functions of the plant Golgi apparatus are protein glycosylation and the synthesis of cell wall polysaccharides and membrane lipids[12]. Proteins are transported by folding, and modified and non-resident proteins are classified and transported to plasma membrane or vesicle system. For a mature plant cell, vacuoles will occupy more than 30% of the cell volume (or up to 90% depending on the cell type), and are another kind of important organelle found in the intramembrane system, which is of great importance for maintaining intracellular balance and cell expansion and can also participate in programmed cell death through caspase[13]. According to the function of vacuoles in cells, they can be divided into two categories: protein storage vacuoles (PSV) and protein hydrolytic vacuoles (PHV). As the name implies, the protein storage vacuoles are storage rooms for proteins, which is more prominent in plant seeds, because the stored proteins can be used as a source of amino acids for the germination and development of seeds[14-15]. On the other hand, the function of protein proteolytic vacuoles is similar to that of lysosomes in animal cells. They play a major role in autophagy and are responsible for protein degradation, thereby providing nutrients to organisms under starvation conditions[15-16].
Intramembrane Transport Pathway
In general, the intramembrane transport pathway is realized by vesicles that shuttle between the donor compartment and the accepter compartment. Vesicle budding is mediated by accessory proteins such as coat protein, and the mechanism of vesicle targeting and fusion is regulated by membrane tethering and fusion factors, such as Rab GTPases and SNARE proteins[18-20]. The endoplasmic reticulum-Golgi transport pathway is the center of protein transport. It consists of two different reverse pathways, namely the anterograde pathway and the retrograde pathway. These two pathways are regulated by cytoplasmic envelope complex II (COPII) and cytoplasmic envelope complex I (COPI), respectively. The coat protein not only participates in membrane deformation, but also participates in the formation of vesicle budding (budding), which provides an internal mechanism for the delivery of soluble cargo protein[18,20]. In animal cells, proteins transported from the endoplasmic reticula to the Golgi reticula pass through an intermediate layer called ERGIC (endoplasmic reticulum-Golgi reticulum intermediate layer). The intermediate layer is formed form small vesicles covered with COPII membrane, which gather mutually and undergo membrane fusion[21]. However, in plants, the endoplasmic reticulum-Golgi anterograde transport has nothing to do with the cytoskeleton, and no equivalent structure has been found yet. Therefore, the existence and function of COPII mechanism in plants need to be fully experimentally studied. On the contrary, the retrograde pathway that the COPI protein complex participates in can balance with the anterograde transport and recover the endoplasmic reticulum targeting protein secreted from Golgi bodies to endoplasmic reticula and the endoplasmic reticulum targeting protein outside the lipid membrane, so as to maintain the size of the two compartments[18,20]. The formation of the two coat protein complexes is controlled by a set of small GTPases, Sar1p for COPII and Arf1 for COPI. This is a common feature between plants and animals, because related homologous proteins have been identified in plants[22-23]. Studies in the model plant tobacco (Nicotiana tabacum) have shown that the punctiform stacking of Sar1p represents ER export sites (ER Export Sites, ERES), and these sites may represent the complex binding Sar1p-ER membranes, nascent COPII membranes, and COPII carriers in transportation. ERES can be induced by overproduction of Golgi membrane proteins instead of soluble carrier proteins. Almost no punctiform Sar1p sites independent of Golgi apparatus were observed, and these may be nascent ERES. Most secretory units formed by ERES move along the surface of ER along with the Golgi apparatus, but the movement does not affect the protein transport rate between these two organelles. These observations support the volume flow model of ER output of soluble proteins[24]. In addition, the volume flow model of protein transport can also be used for transport from Golgi apparatus to plasma membrane, because the exact signal substance that can regulate this process has not been determined[25]. The process of protein transport through Golgi apparatus to hydrolytic vacuoles is mediated by a set of Valsalva classification receptors and clathrin coat proteins. In some cases, proteins can be directly transported from endoplasmic reticula to storage vacuoles through the Golgi apparatus. In this case, the proteins aggregate into protein bodies before being transported to the destination[15, 27].
Golgi Function and Organization
The main function of Golgi apparatus is to add N-bond or O-bond sugar residues to proteins delivered from ER, and to post-translationally modify the proteins. This modification process is completed by various Golgi localization enzymes such as glycosidases and glycosyltransferases[12]. A unique function of plant Golgi apparatus is to synthesize polysaccharides for the construction of cell walls. In addition, Golgi apparatus is also the place for fat synthesis, but the mechanism of this process is still unclear[7,12]. Golgi bodies have a significant polarization structure, which can be divided into cis, middle and trans compartments. The cis Golgi bodies exchange proteins and lipids with the ER through COPI- and COPII protein-enveloped vesicles, and the trans Golgi bodies sort proteins to the plasma membrane and other subcellular organelles[27]. All secreted proteins pass through Golgi apparatus in a cis-to-trans manner, and are sequentially modified after translation. At present, there are two main models of transport between Golgi bodies, namely the stable chamber model and the intra-pool mature model. The former shows that the COPI-enveloped vesicles carry proteins or lipids from cis to trans transport, while each compartment remains stationary, while the latter believes that the entire pool passes forward through Golgi apparatus and the COPI vesicles work in the retrograde direction. Depending on the cell type, there is evidence to support each model. However, these two modes are likely to play a role in regulating Golgi transport at the same time[15,28]. In addition, the trans-Golgi network (TGN) is a compartment that is different in structure and function from Golgi bodies, but it is also considered to be a part of the Golgi system[15,29]. In animal cells, proteins (such as ligand receptors) enter the TGN from the plasma membrane circulation through the membrane compartment called endosome, and these proteins are sorted in the TGN and then transported back to the plasma membrane or sent to the late endosome to be degraded[15,29]. However, because it is difficult to define the TGN structure of plants experimentally, the existence of TGN in plant cells is still controversial[17].
Plant Endoplasmic Reticulum, Golgi Apparatus and Cytoskeleton Complex
The transport mechanism between endoplasmic reticula and Golgi apparatus in plant cells can be explained by three models. ① Vacuum cleaner model: The entire endoplasmic reticulum surface can be used as an outlet for secretions, and Golgi bodies move along the endoplasmic reticula to suck these secretions into ones own body[30]. ② Stop-and-go model: The endoplasmic reticula have a special area for the output of secretions, and Golgi bodies move from other places to this area to collect secretions[31]. ③ Secretion unit model: The exit positions of endoplasmic reticulum secretions and Golgi bodies move together as a common secretion unit[15,27,32]. However, the current data seems to support the secretion unit model more. The co-expression of fluorescent markers of plant endoplasmic reticula and Golgi bodies showed that these two kinds of organelles are closely related to each other, and Golgi bodies seem to be attached to the surface of ER when moving[33]. The co-expression of Golgi markers and the light-activated GFP fusion of endoplasmic reticulum membrane proteins showed that both endoplasmic reticulum membranes and Golgi apparatus move in the same direction at the same rate[33]. In addition, studies at the ultrastructure level have shown that the endoplasmic reticulum-Golgi interface maintains a certain degree of membrane continuity through some tubular membrane structures[34]. When Latrunculin B (LAT-B) (protoplast microfilament inhibitor) was treated, although Golgi bodies stopped moving, the protein transport between endoplasmic reticula and Golgi bodies was not affected. The results of the study indicated that the flow of Golgi bodies along the endoplasmic reticulum network is not necessary for protein transport[34]. Brefeldin A (BFA) reversibly blocks the protein transport between endoplasmic reticula and Golgi bodies by inhibiting the COPI mechanism. After cells are treated with BFA, Golgi apparatus is deconstructed, and most Golgi resident proteins are relocated to endoplasmic reticula or other compartments different from Golgi apparatus. However, this effect is reversible. When BFA is eliminated, Golgi apparatus will be re-formed, and the proteins related to Golgi apparatus and the plasma membrane will be restored again[25]. Studies on fluorescence recovery after photobleaching (FRAP) have shown that the recovery rate of some Golgi fluorescent markers is affected by ATP consumption, rather than cytoskeletal dysfunction[34]. In the presence of actin or microtubule inhibitors, the transport function of Golgi membrane proteins in Golgi apparatus still has the function at the BFA flushing stage[35]. All these experimental data indicate that the transport of membrane proteins between plant endoplasmic reticula and Golgi apparatus does not require the involvement of cytoskeleton. In animal cells, small microtubules and actins (such as dynein and kinesin) are necessary for endoplasmic reticulum-Golgi tissue and endoplasmic reticulum-Golgi transport. The ER-Golgi intermediate compartment (ERGIC) travels on the microtubule orbit, and the depolymerization of microtubules causes Golgi bodies to rupture, and the central Golgi body is broken down into small fragments and randomly distributed in the cytoplasm[36].
In plant cells, actin filaments cover endoplasmic reticula to form a network, and the movement of Golgi apparatus depends on these actin filament networks. The movement of Golgi apparatus is thought to be driven by myosin, because when the actin cytoskeleton is destroyed, although the morphology of endoplasmic reticula and Golgi apparatus remain unchanged, the movement of Golgi apparatus is blocked[35]. Members of the kinesin superfamily are microtubule-based movement proteins. AtKinesin-13A is an internal movement protein from Arabidopsis thaliana. The immunofluorescence results showed that AtKinesin-13A is located in the Golgi apparatus of plant cells, and AtKinesin-13A modifies Golgi-associated vesicles, and may be involved in the regulation of the formation of Golgi vesicles in A. thaliana root cap peripheral cells[37,38]. This result indicates that microtubules may also contribute to the stability of the morphology and function of the plant Golgi.
Agricultural Biotechnology2021
Golgi matrix protein
Golgi matrix proteins in animal cells play a very important role in the integrity of the Golgi structure. For example, the matrix protein GM130 could not be detected in the conditionally lethal mutants, in which the structural stability of Golgi apparatus was very sensitive to high temperature at this time, and if GM130 was reintroduced, the structural stability of Golgi apparatus could return to normal[39]. A similar effect on the structural stability of Golgi apparatus was also found in cell lines lacking Golgin-84[40]. In animal cells, a large number of Golgi matrix proteins (such as p115, Giantins, etc.) have been studied and identified. However, in contrast, people know much less about the plant Golgi matrix proteins. The BLAST analysis of the A. thaliana genome showed that most animal Golgi matrix proteins have homologous genes in plants[41], which supports the view that the stability of plant Golgi structure may be mediated by matrix proteins similar to those in animal cells. However, due to the differences between the mammalian system and the plant system, the functions of these plant homologs must be confirmed through experiments.
The most famous of the Golgi matrix protein family is the family called Golgins. They were first identified as self-antigens localized to Golgi apparatus, and are involved in the breakdown of Golgi during cell apoptosis or necrosis. However, subsequent studies have proved their importance in maintaining the structure of Golgi apparatus. Golgi proteins are a family of membrane proteins with long coiled-coil domains located on Golgi apparatus, and are involved in tethering events that occur on Golgi apparatus[42]. Their coiled-coil domains form rod-like structures that extend into the cytoplasm, so they can freely interact with membrane-like structures, such as cargo vesicles and neighboring cisterna, or form part of larger protein-binding complexes (tethering complexes)[43-44]. Most of the Golgi proteins rely on the small G proteins of the Arf family to recruit from the cytoplasm to the cytoplasmic surface of the Golgi membrane proteins, while the remaining Golgi proteins are embedded as complete membrane proteins[45]. More than 11 mammalian Golgi proteins have been found in the entire Golgi apparatus[45]. According to their positioning on Golgi apparatus, they can be divided into three types: ① transmembrane proteins, which directly chimerize with the Golgi membrane through the transmembrane domain at the carboxyl end of their own, such as CASP, Giantin and Golgin-84, ② peripheral membrane proteins, mainly GM130, whose carboxyl end is fixed on the Golgi membrane as a ligand of the PDZ domain of GRASP65, and ③ non-membrane proteins, among which GCC185, golgin-245, GCC88 and golgin-97 interact with Arf1 through the carboxy-terminal GRIP domain, while GMAP-210 has a similar GRAB domain that binds to Arf1, and TMF and golgin-160 are attached to the Golgi membrane through their carboxyl terminal by binding to Rab6 or Arf1, respectively[46]. Some Golgi proteins containing specific domains are also recruited into the Golgi membrane through interactions with other GTPases. The best example in plants is the AtGRIP protein, which is located on the Golgi membrane by combining the GRIP domain, which is highly similar to GCC185 and golgin-97, with the GTPase ARL1, which is similar to Arf[41]. The role of golgins is not only to maintain the structure of Golgi apparatus, but also to mediate the vesicles connected to the Golgi membrane. For example, p115, a helical protein, can interact with cis-Golgi COPII vesicles with the help of GM130 and Giantins[47]. However, the homologous proteins of GM130 and giantins do not exist in plants, so the function of plant p115 has not been determined[48]. AtCASP is a cis-Golgi localized Golgi protein and a good candidate protein that binds Golgi bodies to ER exit sites[49-50]. AtCASP is a type II transmembrane domain protein with a topological structure similar to animal CASP protein[51]. Its N-terminal coiled-coil domain will form a rod-like structure into the cytoplasm, while its C-terminal contains a transmembrane domain sufficient to achieve Golgi targeting[49] and multiple double-acid DXE motifs required for ER output[52]. New research shows that AtCASP plays a tethering effect at the ER-Golgi interface[53].
Another matrix protein family that is different from Golgins is multi-subunit complexes, including TRAPP (transport protein particle) complex, COG (conservative oligomeric Golgi) complex, GARP (Golgi associated retrograde protein) complex and Dsl1 complex, which function in regulating the structures of the membrane system and Golgi apparatus by interacting with SNAREs and small GTPase[47]. A BLAST search of the A. thaliana genome has identified possible homologs of animal multi-subunit complexes[47]. In addition, a protein called Spectrins has been shown to have a role in maintaining the Golgi structure in animal cells. It forms a network parallel to the Golgi membrane and is resistant to detergent extraction. However, the existence of plant Spectrin homologous proteins has not been fully confirmed[41].
Golgi biogenesis
Scholars hold two views on the biogenesis of Golgi bodies: template-mediated and endoplasmic reticulum de novo formation. People use different methods to study and verify these two models. One method is to use BFA treatment. The above has shown that this treatment will cause the structural decomposition of Golgi apparatus and the redistribution of the Golgi localization enzyme into endoplasmic reticula; and the same effect can also be achieved by Sar1p mutants, by the GTP lock-in form or the GDP lock-in form[35]. Studies have shown that not only the Golgi localization enzyme, but also the Golgi matrix protein will actively transfer to endoplasmic reticula after BFA treatment. When the BFA is cleaned off, Golgi bodies can still be re-formed[54-55]. Therefore, the formation of Golgi apparatus does not seem to require mediation by pre-existing templates. On the other hand, when the entire Golgi apparatus is removed from a cell with an intact endoplasmic reticulum, a new Golgi apparatus cannot be re-formed from the endoplasmic reticulum[56].
The biological process of Golgi apparatus in animals and plants is still unclear. Like other cytoplasmic organelles, Golgi apparatus must be replicated and passed to daughter cells before cell division; and alternatively, it may be regenerated after cell division. Animal cell research has established two models to explain this process[57]. At the beginning of cell division, the Golgi stacks break and divide. The point of contention is how the Golgi bodies are reconstructed from the fragments of the resulting daughter cells. One model indicates that Golgi bodies are transformed by direct vesicle fusion, while the other model believe that the Golgi body fragments are first merged into the ER, and then re-formed into a new Golgi body from the ER. In addition, studies on algae and protozoa have shown that Golgi apparatus can be isolated, and half of the original Golgi apparatus is inherited by each daughter cell[57]. When plant cells divide, the function of Golgi apparatus is not disturbed and the structure remains intact[25]. Plant cells need active secretion for cytokinesis, which is achieved through cell plates. The formation of cell plates requires new membrane and wall materials supplied by the Golgi-derived vesicles to the membranous plasma membrane, so the destruction of Golgi bodies by BFA during mitosis will lead to binuclear cells[58]. A careful stereological study of A. thaliana bud top meristem cells by electron microscopy showed that the number of Golgi stacks doubled in the G2 stage before mitosis, but the mechanism is still unknown[59].
Prospect
The Golgi apparatus of plants plays an important role in protein glycosylation and sorting, and it is also the main biosynthetic organelle that synthesizes a large number of cell wall polysaccharides. This is also reflected in the existence of Golgi apparatus in the cytoplasm, which is a group composed of many individual functional individuals dispersed in the cytoplasm. Trans-staining shows that there are more polysaccharides in the trans-cell membrane direction and trans-arrangement; and synthesis of complex cell wall polysaccharides requires a large amount of glycosyltransferases. Microscopic scanning and biochemical analysis studies have shown that these enzymes are separated in stacks. Although there is no obvious trans-Golgi Network, the trans-Golgi network is often covered with clathrin, sometimes smooth and dense vesicles[7]. This review highlighted the unique aspects of the organization and function of plant Golgi apparatus. Basically similar processes may be the basis of Golgi tissue in all organisms. Therefore, the specialization of plant Golgi apparatus can provide general scientific information and important significance to plant cell biology. In A. thaliana, there must be some Golgi structural proteins, just like in other species, and A. thaliana homologues of certain matrix proteins from other species can be determined through database search. In the process of identifying other new proteins that may be involved in maintaining the Golgi structure of plants, it was discovered that some proteins of other species (such as Drosophila) affect the cell membrane system. Through bioinformatics search and homology comparison of these Drosophila proteins in the A. thaliana database, potential A. thaliana candidate genes were selected for further study. Further studies on the fusion of these proteins with confocal microscopy can further identify new plant Golgi proteins, and on this basis, we can better understand the biosynthesis and various physiological and biochemical functions of the Golgi apparatus of plants.
References
[1] KLUMPERMAN J. Architecture of the mammalian Golgi[J]. Cold Spring Harb Perspectives in Biology, 2011, 3(7): a005181.
[2] LOWE M. Structural organization of the Golgi apparatus[J]. Current Opinion in Cell Biology, 2011, 23(1): 85-93.
[3] STANLEY P. Golgi glycosylation[J]. Cold Spring Harb Perspectives in Biology, 2011, 3(4): a005199.
[4] SPANG A. Retrograde traffic from the Golgi to the endoplasmic reticulum[J]. Cold Spring Harb Perspectives in Biology, 2011, 5(6): a013391.
[5] GUO Y, SIRKIS D W, SCHEKMAN R. Protein sorting at the trans-Golgi network[J]. Annual Review of Cell & Developmental Biology, 2014, 30(1): 169-206.
[6] KULKARNI-GOSAVI P, MAKHOUL C, GLEESON PA. Form and function of the Golgi apparatus: scaffolds, cytoskeleton and signalling[J]. FEBS Letters, 2019, 593(17): 2289-2305.
[7] SCHOBERER J, STRASSER R. Plant glyco-biotechnology[J]. Seminars in Cell & Developmental Biology, 2018(80): 133-141.
[8] L WE M, KALACHEVA M, BOERSMA AJ, et al. The more the merrier: effects of macromolecular crowding on the structure and dynamics of biological membranes[J]. FEBS Journal. 2020, 287(23): 5039-5067.
[9] VISHWAKARMA K, MISHRA M, PATIL G, et al. Avenues of the membrane transport system in adaptation of plants to abiotic stresses[J]. Critical Reviews in Biotechnology, 2019, 39(7): 861-883.
[10] BRANDIZZI F, SAINT-JORE C, MOORE I, et al. The relationship between endomembranes and the plant cytoskeleton[J]. Cell Biology International, 2003, 27(3): 177-179.
[11] VITALE A, DENECKE J. The endoplasmic reticulum-gateway of the secretory pathway[J]. Plant Cell, 1999, 11(4): 615-628.
[12] ANDREEVA A, KUTUZOV M, EVANS D, et al. The structure and function of the Golgi apparatus: A hundred years of questions[J]. Journal of Experimental Botany, 1998, 49(325): 1281-1291.
[13] LAM E. Vacoular proteases livening up programmed cell death[J]. Trends in Cell Biology, 2005, 15(3): 124-127.
[14] VITALE A, HINZ G. Sorting of proteins to storage vacuoles: how many mechanisms[J]. Trends in Plant Science, 2005, 10(7): 316-323.
[15] SHIMADA T, TAKAGI J, ICHINO T, et al. Plant vacuoles[J]. Annual review of plant biology, 2018, 69(1): 123-145.
[16] MARTY F. Plant vacuoles[J]. Plant Cell, 1999, 11(4): 587-600.
[17] HAWES C. Cell biology of the plant Golgi apparatus[J]. New Phytologist, 2005, 165(1): 29-44.
[18] BONIFACINO JS, GLICK BS. The mechanisms of vesicle budding and fusion[J]. Cell, 2004, 116(2): 153-166.
[19] GILLINGHAM AK, MUNRO S. Long coiled-coil protein and membrane traffic[J]. Biochimica et Biophysica Acta, 2003, 1641(2-3): 71-85.
[20] GONG H, SENGUPTA D, LINSTEDT AD, et al. Simulated de novo assembly of golgi compartments by selective cargo capture during vesicle budding and targeted vesicle fusion[J]. Biophysical Journal, 2008, 95(4): 1674-1688.
[21] APPENZELLER-HERZOG C, HAURI HP. The ER-Golgi intermediate compartment (ERGIC): in search of its identity and function[J]. Journal of Cell Science, 2006, 119(11): 2173-83.
[22] TAKEUCHI M, UEDA T, YAHARA N, et al. Arf1 GTPase plays roles in the protein traffic between the endoplasmic reticulum and the Golgi apparatus in tobacco and Arabidopsis cultured cells[J]. Plant Journal, 2002, 31(4): 499-515.
[23] CLAIRELINE M, VALIRIE WB, GUILLAUME B, et al. The Qb-snare memb11 interacts specifically with arf1 in the golgi apparatus of arabidopsis thaliana[J]. Journal of Experimental Botany, 2015, 66(21): 6665-6678.
[24] DASILVA L LP, SNAPP EL, DENECKE J, et al. Dasilva, l.l.p. et al. er export sites and golgi bodies behave as single mobile secretory units in plant cells[J]. Plant Cell, 2004, 16(7): 1753-1771.
[25] HAWES C, SATIAT-JEUNEMAITRE B. The plant Golgi apparatus-Going with the flow[J]. Biochimica et Biophysica Acta, 2005, 1744(3): 466-480.
[26] HERMAN E, SCHMIDT M. Endoplasmic reticulum to vacuole trafficking of endoplasmic reticulum bodies provides an alternate pathway for protein transfer to the vacuole[J]. Plant Physiol, 2004, 136(3): 3440-3446.
[27] NEUMANN U, BRANDIZZI F, HAWES C. Protein Transport in Plant Cells: In and Out of the Golgi[J]. Annals of Botany, 2003, 92(2): 167-180.
[28] GLICK BS. Organization of the Golgi apparatus[J]. Current Opinion in Cell Biology, 2000, 12(4): 450-456.
[29] TRAUB LM, KORNFELD S. The trans-Golgi network: a late secretory sorting station[J]. Current Opinion in Cell Biology, 1997, 9(4): 527-533.
[30] BOEVINK P, OPARKA K, SANTA CS, et al. Stacks on tracks: the plant Golgi apparatus traffics on an actin/ER network[J]. Plant Journal, 1998, 15(3): 441-447.
[31] NEBENF HR A, FROHLICK JA, STAEHELIN LA. Redistribution of Golgi stacks and other organelles during mitosis and cytokinesis in plant cells[J]. Plant Physiology, 2000, 124(1): 135-151.
[32] RUNIONS J, BRACH T, KUHNER S, et al. Photoactivation of GFP reveals protein dynamics within the endoplasmic reticulum membrane[J]. Journal of Experimental Botany, 2006, 57(1): 43-50.
[33] MOREAU P, BRANDIZZI F, HANTON S, et al. The plant ER-Golgi interface: a highly structured and dynamic membrane complex[J]. Journal of Experimental Botany, 2006, 58(1): 49-64.
[34] BRANDIZZI F, SNAPP EL, ROBERTS AG, et al. Membrane protein transport between the endoplasmic reticulum and the Golgi in tobacco leaves is energy dependent but cytoskeleton independent: evidence from selective photobleaching[J]. Plant Cell, 2002, 14(6): 1293-1309.
[35] SAINT-JORE CM, EVINS J, BATOKo H, et al. Redistribution of membrane proteins between the Golgi apparatus and endoplasmic reticulum in plants is reversible and not dependent on cytoskeletal networks[J]. Plant Journal, 2002, 29(5): 661-678.
[36] WEHLAND J, HENKART M, KLAUSNER R, et al. Role of microtubules in the distribution of the Golgi apparatus: effect of taxol and microinjected anti-alpha-tubulin antibodies[J]. Proceedings of the National Academy of Sciences USA, 1983, 80(14):4286-4290.
[37] LIU L, LEE YR, PAN R, et al. An internal motor kinesin is associated with the Golgi apparatus and plays a role in trichome morphogenesis in Arabidopsis[J]. Molecular Biology of the Cell, 2005, 16(2): 811-823.
[38] WEI L, ZHANG W, LIU Z, et al. Atkinesin-13a is located on golgi-associated vesicle and involved in vesicle formation/budding in arabidopsis root-cap peripheral cells[J]. BioMed Central Plant Biology, 2009, 9(1): 138.
[39] VASILE E, PEREZ T, NAKAMURA N, et al. Structural integrity of the Golgi is temperature sensitive in conditional-lethal mutants with no detectable GM130[J]. Traffic, 2003, 4(4): 254-272.
[40] DIAO A, RAHMAN D, PAPPIN DJ, et al. The coiled-coil membrane protein golgi-84 is a novel rab effector required for Golgi ribbon formation[J]. Journal of Cell Biology, 2003, 160(2): 201-212.
[41] LATIJNHOUWERS M, HAWES C, CARVALHO C. Holding it all together? Candidate proteins for the plant Golgi matrix[J]. Current Opinion in Plant Biology, 2005, 8(6): 632-639.
[42] WONG M, MUNRO S. The specificity of vesicle traffic to the Golgi is encoded in the golgin coiled-coil proteins[J]. Science, 2014, 346(6209): 1256898.
[43] MALSAM J, S LLNER TH. Organization of SNAREs within the Golgi stack[J]. Cold Spring Harbor Perspectives in Biology, 2011, 3(10): a005249.
[44] CHIA PZ, GLEESON PA. Membrane tethering[J]. F1000Prime Reports, 2014, 6(74): 74.
[45] MUNRO S. The golgin coiled-coil proteins of the Golgi apparatus[J]. Cold Spring Harb Perspectives in Biology, 2011, 3(6): a005256-a005256.
[46] WITKOS TM, LOWE M. The Golgin Family of Coiled-Coil Tethering Proteins[J]. Frontiers in Cell and Development Biology, 2015(3): 86.
[47] SZTUL E, LUPASHIN V. Role of tethering factors in secretory membrane traffic. American Journal of Physiology-Cell Physiology, 2006, 290(1): C11-26.
[48] TAKAHASHI H, TAMURA K, TAKAGI J, et al. MAG4/Atp115 is a golgi-localized tethering factor that mediates efficient anterograde transport in Arabidopsis[J]. Plant & Cell Physiology, 2010, 51(10): 1777-1787.
[49] RENNA L, HANTON SL, STEFANO G, et al. Identification and characterization of AtCASP, a plant transmembrane Golgi matrix protein[J]. Plant Molecular Biology, 2005, 58(1): 109-122.
[50] LATIJNHOUWERS M, GILLESPIE T, BOEVINK P, et al. Localization and domain characterization of Arabidopsis golgin candidates[J]. Journal of Experimental Botany, 2007, 58(15-16): 4373-4386.
[51] GILLINGHAM AK, MUNRO S. Long coiled-coil proteins and membrane traffic[J]. Biochimica et Biophysica Acta, 2003, 1641(2-3): 71-85.
[52] HANTON SL, RENNA L, BORTOLOTTI LE, et al. Diacidic motifs influence the export of transmembrane proteins from the endoplasmic reticulum in plant cells[J]. The Plant Cell, 2005, 17(11): 3081-3093.
[53] OSTERRIEDER A, SPARKES IA, BOTCHWAY SW, et al. Stacks off tracks: a role for the golgin atcasp in plant endoplasmic reticulum-golgi apparatus tethering[J]. Journal of Experimental Botany, 2017, 68(13): 3339-3350.
[54] SAINT-JORE CM, EVINS J, BATOKO H, et al. Redistribution of membrane proteins between the Golgi apparatus and endoplasmic reticulum in plants is reversible and not dependent on cytoskeletal networks[J]. Plant Journal, 2002, 29(5): 661-678.
[55] LOWE M. Golgi Complex: Biogenesis de novo[J]. Current Biology, 2002, 12(2): 166-167.
[56] PELLETIER L, JOKITALO E, WARREN G. The effect of Golgi depletion on exocytic transport[J]. Nature Cell Biology, 2000, 2(11): 840-846.
[57] MUNRO S. More than one way to replicate the Golgi apparatus[J]. Nature Cell Biology, 2002, 4(10): E223-E224.
[58] REICHARDT I, STIERHOF YD, MAYER U, et al. Plant cytokinesis requires de novo secretory trafficking but not endocytosis[J]. Current Biology, 2007, 17(23): 2047-2053.
[59] SEGU-SIMARRO JM, STAEHELIN LA. Cell cycle-dependent changes in Golgi stacks, vacuoles, clathrin-coated vesicles and multivesicular bodies in meristematic cells of Arabidopsis thaliana: a quantitative and spatial analysis[J]. Planta, 2006(223): 223-236.
杂志排行
农业生物技术(英文版)的其它文章
- Anti-inflammatory Activity and Mechanism of Total Flavonoids from the Phloem of Paulownia elongate S.Y. Hu in LPS-stimulated RAW264.7 Macrophages
- Comparative Genomic Analysis of Boron Transport Gene Family in Arabidopsis and Five Crops
- Effects of Different Water-saving Irrigation Methods on Fruit Quality and Yield of Snow Melon
- Field Control Effects and Crop Safety Assessment of Triazole Fungicides on Apple Rust
- Effects of Acetylacetone Solution Soaking on Agrobacterium-transformed Maize Seed Buds
- Effects of Meteorological Factors on Overwintering Ability, Yield and Quality of Forage Rape
