fl(2)d单等位基因的敲除显著降低小菜蛾的生殖力和育性
2021-07-30李飞飞王贝贝赖颖芳杨菲颖尤民生何玮毅
李飞飞,王贝贝,赖颖芳,杨菲颖,尤民生,何玮毅
单等位基因的敲除显著降低小菜蛾的生殖力和育性
李飞飞,王贝贝,赖颖芳,杨菲颖,尤民生,何玮毅
闽台作物有害生物生态防控国家重点实验室/福建农林大学应用生态研究所/福建农林大学教育部害虫生态防控国际合作联合实验室/福建农林大学农业农村部闽台作物有害生物综合治理重点实验室/海峡两岸特色作物安全生产省部共建协同创新中心,福州 350002
【】RNA甲基化是基因转录后水平表观修饰的主要形式,参与了众多重要的细胞学过程。小菜蛾()是危害十字花科蔬菜的重要寡食性害虫,与RNA甲基化相关基因的功能尚未见报道。本研究通过克隆小菜蛾的RNA甲基化蛋白同源基因,鉴定其表达模式,并敲除该基因以探究其生物学功能。通过小菜蛾基因组网站查找基因序列,PCR扩增其蛋白质编码序列(CDS);采用实时荧光定量PCR(qRT-PCR)技术,检测小菜蛾不同发育阶段个体以及成虫生殖腺中的相对表达量;运用CRISPR/Cas9结合卵的显微注射技术,对小菜蛾进行编辑;将被编辑过的成虫与野生型成虫杂交,并对其产生的后代进行近交,筛选突变品系;观测并比较突变体与野生型个体遗传特性、生物学参数和表型的差异,明确的功能。克隆得到长度为912 bp的CDS,在雌蛹、雌成虫和卵中的表达量较高,雄成虫和雄蛹的表达量较低,幼虫期的表达量最低,成虫卵巢中表达量显著高于精巢。通过向小菜蛾的卵注射靶向的向导RNA(sgRNA)和Cas9蛋白的混合物,对所产生的阳性后代进行10代的单对近交筛选,获得3种杂合的移码突变品系,分别缺失了4个(Δ213-4)、5个(Δ213-5)和7个(Δ214-7)碱基。在上述品系的筛选过程中,发现了6只缺失4个碱基的纯合突变个体,2只缺失5个碱基的纯合突变个体;缺失4个碱基的纯合个体成功配成了两对,近交未产卵;剩余的2只缺失4个碱基的雄性纯合个体和2只缺失5个碱基的雄性纯合个体分别与同世代的雌性杂合突变个体近交后仍未产卵。说明纯合突变的个体存活率极低,且可能无法产生后代。通过分析后代基因型的分离比,发现杂合突变个体近交以及杂合突变个体与野生型个体杂交产生的后代中,杂合突变个体与野生型个体的比例分别略小于2和1,说明杂合突变会影响小菜蛾正常的生长发育,并导致部分个体死亡。杂合突变体后代中含有突变的雌雄个体比例接近1﹕1(<0.05),推测小菜蛾可能与性别决定无关。只要是有突变品系小菜蛾所参与的交配,雌成虫产卵量和卵的孵化率均显著低于野生型(<0.01),所产的卵多数发育异常,表现为失水皱缩、不能正常孵化。通过对成虫的生殖腺进行解剖,发现在野生型雌成虫与突变体雄成虫交配后,卵巢内卵的附着量较未交配的个体明显减少;未交配的突变体雌成虫卵巢内卵的附着量亦少于野生型,而突变体雄成虫的精巢未见明显异常。部分能够孵化的杂合突变个体在整个发育过程会发生不同程度的畸变,导致不能正常完成整个世代;另外一些杂合突变个体未见异常,可以将突变类型遗传给后代。根据上述发现,提出了基于的小菜蛾遗传防控模型。参与小菜蛾的生殖过程和胚胎发育,突变后显著影响后代种群数量,是开展小菜蛾遗传控制的理想靶标。
小菜蛾;;杂合突变体;生殖力;育性
0 引言
【研究意义】小菜蛾()属鳞翅目(Lepidoptera)菜蛾科(Plutellidae),是危害十字花科蔬菜的重要害虫,全球每年用于其防治的费用及其造成的损失高达40—50亿美元[1]。在我国南方菜区,小菜蛾可终年发生危害,世代重叠严重、抗药性强,促使研究人员不断探索控制其种群增长的方法[2-3]。随着有害生物综合治理(integrated pest management,IPM)理念的提出,人们不再拘泥于传统的防治策略,而是致力于开发绿色、环保、有利于生态平衡的可持续治理方法[4-5]。当前,害虫灾变机理的研究已迈入组学时代,基于害虫遗传控制的原理和方法,研发小菜蛾绿色防控技术显示出了巨大的潜力和优势[6-8]。筛选合适的靶标基因,研究配套的遗传操作系统,将进一步提升害虫遗传控制的效率和稳定性[9-10]。RNA甲基化是基因转录后水平表观修饰的主要形式,rRNA、mRNA、tRNA甚至是非编码RNA都会出现超过100多种的修饰类型,其中以m6A(6-腺苷酸甲基化)最为常见[11-12]。m6A甲基化存在于绝大多数生物体内,包括哺乳动物、昆虫、细菌和病毒。m6A影响生物体基因表达、mRNA稳定性、RNA可变剪接、蛋白翻译效率和X染色体失活等重要的细胞学过程[13-14]。参与m6A甲基化过程蛋白的失活会影响生物体正常的生命活动,探明小菜蛾中m6A甲基化蛋白成员female- lethal-2-d(fl(2)d)的生物学功能,对今后以该基因作为靶标建立小菜蛾遗传防控系统具有重要意义。【前人研究进展】近年来,人们将RNA甲基化修饰抗体富集技术和高通量测序技术相结合,实现了对m6A甲基化在转录组水平的分布和丰度检测[15-16]。在人类和小鼠的基因组中,m6A甲基化位点高度保守,大多集中在终止密码子附近和长的内部外显子,说明这种修饰在基因表达的表观遗传调控中发挥了重要作用[17]。参与m6A代谢和信号途径的蛋白有三大类,分别是RNA甲基化蛋白(writers)、去甲基化蛋白(erasers)和甲基化阅读蛋白(readers),它们均以复合物的形式发挥功能[18]。一旦哺乳动物体内的这些蛋白出现异常,就会引发一系列的疾病,包括肿瘤、神经性疾病和发育迟缓等[15,19-20]。Writers主要包括methyltransferase-like 3(METTL3)、methyltransferase-like 14(METTL14)、fl(2)d、vir-Like m6A methyltransferase associated(KIAA1429/VIRMA)和RNA-binding motif protein 15(RBM15)[18,21-22],在昆虫中的报道较少。家蚕()中发现,和在滞育品系中的表达量高于非滞育品系,说明m6A甲基化介导的转录后表观遗传调控可能参与了家蚕滞育的发生[23];在细胞水平利用RNA干扰(RNAi)技术沉默和,会导致家蚕细胞周期进程停止、染色体联会和分离失败[24]。、和的表达水平以及m6A含量在工蜂和蜂王幼虫阶段不同时期发生显著变化,说明m6A甲基化影响了蜜蜂的幼虫发育和级型分化[25]。的研究常见于果蝇[26-28],该基因主要有3个靶基因:()、和,前两个基因是性别决定通路的上游基因,主要通过影响这两个基因前体mRNA的可变剪接影响果蝇的性别决定、剂量补偿、卵子形成与分化等[29-32]。也可以调控第3个靶基因的可变剪接,但对雌、雄两性的影响并无差异[33]。此外,在果蝇胚胎和卵巢中高表达,在卵子形成的早期,fl(2)d蛋白在不同的细胞核间移动,说明它是一种影响卵子发生的关键核蛋白[34]。【本研究切入点】昆虫RNA甲基化的研究目前仅见于家蚕、蜜蜂和果蝇,在小菜蛾上还未见报道。通过参与RNA甲基化过程影响了众多生物学过程,是开展害虫遗传控制潜在的靶标。由于鳞翅目昆虫中同源基因不具有决定昆虫性别的功能[35-36],利用基因编辑技术clustered regular interspaced short palindromic repeat sequences/CRISPR-associated protein 9(CRISPR/Cas9)探明小菜蛾同源基因是否参与性别决定、生殖及其他重要生长发育过程,可为今后利用该基因进行害虫的遗传防控打下理论基础。【拟解决的关键问题】小菜蛾的序列及其表达模式;建立小菜蛾突变品系;突变品系遗传特性、生物学参数和表型的变化;在小菜蛾生长发育中的功能。
1 材料与方法
试验于2019—2020年在福建农林大学闽台作物有害生物生态防控国家重点实验室和应用生态研究所完成。
1.1 试验材料与试剂
虫源为本实验室长期保存的人工饲料饲养的小菜蛾品系(AD),饲养温度为(25±1)℃,光周期为16 h﹕8 h(L﹕D),相对湿度为70%—80%。基因克隆所用菌株为大肠杆菌DH5。
福州尚亚生物科技有限公司提供引物及测序服务。总RNA提取试剂盒、GoScriptTMReverse Transcription System逆转录试剂盒、GoTaq qPCR Master Mix实时定量PCR(qRT-PCR)试剂盒和无核酸酶水购于Promega;2×Hieff Canace® PCR Master Mix高保真酶购于翊圣生物科技有限公司;DNA提取试剂盒购于天根生化科技有限公司;胶回收试剂盒购于Omega;克隆载体PJET1.2和RNA体外转录试剂盒MEGAscript RNAi kit购于ThermoFisher;敲除试验的相关试剂购于金斯瑞生物科技有限公司。
1.2 RNA提取和cDNA合成
使用RNA提取试剂盒并按照说明书提取不同样品小菜蛾的总RNA,使用分光光度计检测RNA的质量,用1%(质量体积分数)的琼脂糖凝胶电泳检测RNA的完整性。取2 μg总RNA,参照逆转录试剂盒说明书合成第一条单链cDNA,-20℃保存备用。
1.3 基因克隆和序列分析
以小菜蛾卵的cDNA为模板,根据小菜蛾基因组数据库(http://iae.fafu.edu.cn/DBM)[8,37]提供的基因序列信息设计引物(表1),扩增蛋白质编码序列(CDS)区域。PCR程序:98℃ 3 min;98℃ 30 s,58℃ 30 s,72℃ 1 min,30个循环;72℃ 10 min。PCR扩增后进行胶回收纯化,将基因片段连接到克隆载体上并转化至大肠杆菌DH5感受态细胞中,挑取阳性克隆进行测序,获得目的基因序列信息。将获得的CDS序列与对应基因组序列进行比对,界定外显子与内含子区域。
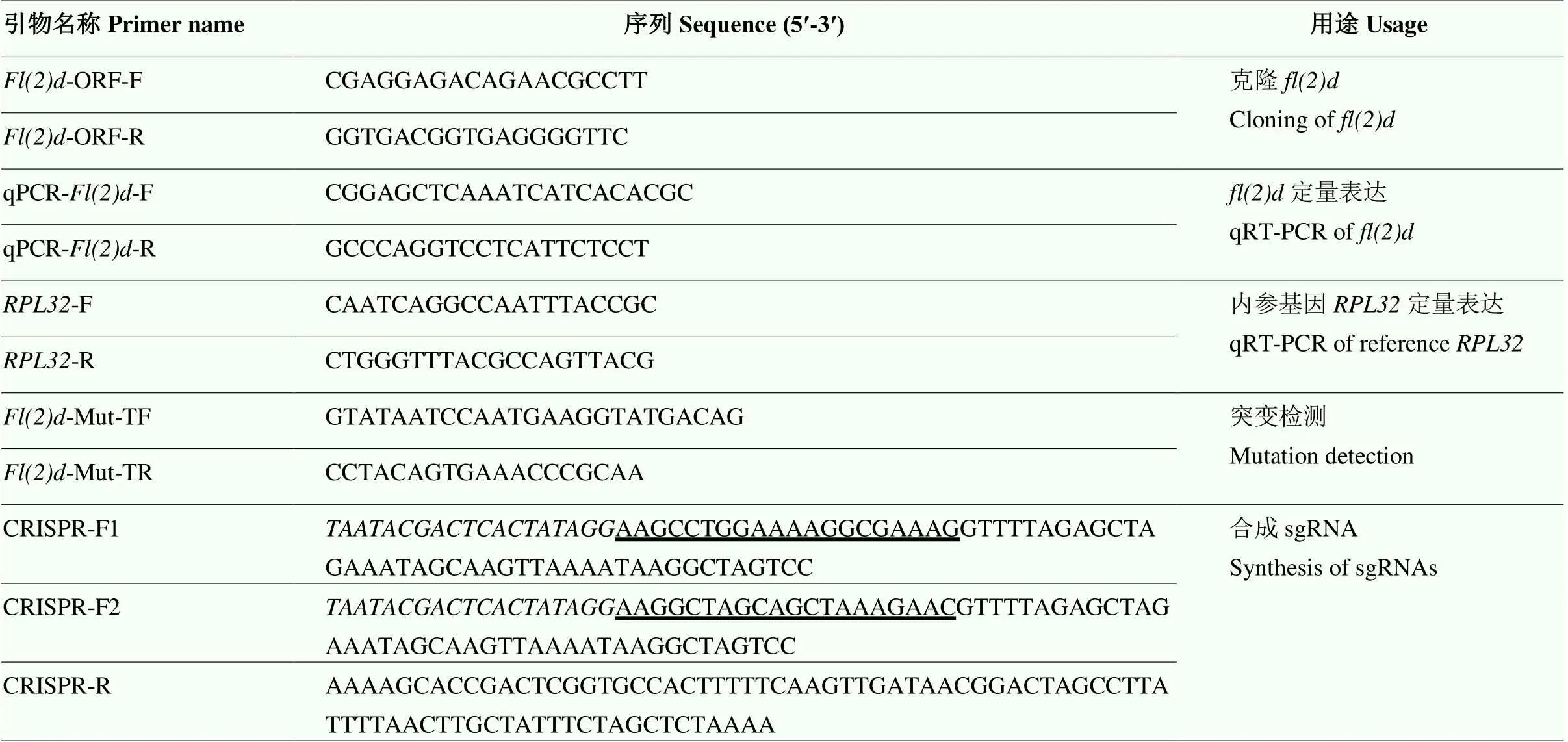
表1 本研究所用引物序列
下划线标记的序列表示sgRNA,斜体部分的序列表示T7启动子
The sequences underlined indicate the sgRNAs, and the sequences in italic denote the T7 promoter
1.4 实时荧光定量PCR
收集小菜蛾不同发育阶段的个体和成虫的生殖腺,每一样品各设置4个生物学重复,提取总RNA并逆转录成cDNA。其中收集交配过的雌虫所产2 h以内的卵,0.5 g/重复;4龄幼虫,5只/重复;化蛹后第2天的蛹,5只/重复;初羽化未交配的成虫(分雌雄),5只/重复,并分别解剖相应的卵巢和精巢,50个/重复。液氮处理后,-80℃保存备用。以小菜蛾核糖体蛋白基因ribosomal protein L32()为内参[38],设计实时荧光定量PCR引物(表1)。参照试剂盒说明书配制20 μL反应体系,在实时荧光定量PCR系统(ABI QuantStudioTM6 Flex)上进行反应,反应程序:95℃ 10 min;95℃ 15 s,60℃ 30 s,40个循环;95℃ 15 s,60℃ 1 min;95℃ 15 s。采用2-ΔΔCt的方法计算的相对表达量。
1.5 sgRNA设计及体外转录
参照Chen等[38]的方法,按照GGN19GG原则,在靠近5′端的外显子区域设计2条向导RNA(sgRNA),同时利用Cas9脱靶效应网站(http://www. rgenome.net/cas-offinder)进行检验。在2条sgRNA的两端设计另外2条检测引物,用于后续突变位点的检测(表1)。
上游引物CRISPR-F由三部分组成:T7聚合酶结合位点、sgRNA序列以及sgRNA骨架质粒的部分序列,下游引物CRISPR-R由与sgRNA骨架质粒互补的部分序列组成,扩增模板是sgRNA骨架质粒。对扩增后的PCR产物进行胶收纯化,使用试剂盒对PCR产物进行体外转录,最后用氯仿异戊醇纯化sgRNA。-80℃保存备用。
1.6 卵的显微注射
注射前,采用以下方法和体系进行Cas9蛋白和sgRNA的预处理:1 μL Cas9蛋白(200 ng·μL-1),2 μL sgRNA(250 ng·μL-1)、0.5 μL 10×Cas Nuclease Reaction Buffer,加水补足至5 μL,37℃条件下孵育15 min。选择小菜蛾雌成虫交配后第2天产的卵进行注射,将卵卡置于成虫产卵盒中30 min后拿出,然后使用显微注射系统(IM 300 Programmable Cell Microinjector)将Cas9蛋白和sgRNA混合物注射入卵。将注射完成的卵置于培养皿中,在25℃、相对湿度70%的条件下进行孵化,随后将孵化的幼虫转移至人工饲料上。
1.7 突变体筛选
将注射后的卵发育成的后代称为G0代,待其发育到成虫后,分别将单只与性别相反的野生型小菜蛾成虫杂交。产生子一代(G1)后,提取G0代成虫基因组DNA,利用突变体检测引物进行PCR扩增,对PCR产物进行测序。通过观察PAM结构附近有无突变,确定产生突变的群体。自G1代起,均采用同种群内随机近交的方式筛选突变体,待产出后代后,检测亲本的突变类型。对检测为突变的亲代所对应的后代进行保存,剔除亲本均为野生型的后代,直至筛选出2条染色体均被编辑的纯合突变品系(图1)。

圆圈表示细胞核,圈中长方形表示编辑的目标染色体,空白填充表示野生型,不同颜色的填充表示不同类型的突变
1.8 拍照记录及数据分析
用数码显微系统(VHX-2000C,Keyence)对突变品系中发育异常的卵进行拍照观察,并与野生型的卵进行比较。分别解剖15—20只杂合突变品系初羽化未交配的雌成虫卵巢和雄成虫精巢,以及与突变体雄成虫交配后12 h内未产卵的野生型雌成虫卵巢进行拍照观察,观察完成后提取DNA进行PCR检测,用于确定是否为突变个体,并与野生型初羽化未交配成虫的卵巢和精巢进行比较。产卵量数据的收集采用观察法,野生型观察了25对,除了缺失4个碱基的突变体类型观察了3—5对,其他突变体类型为10—18对。记录单对小菜蛾成虫交配后2 d内产的卵为第一次数据,换一次卵卡后交配2 d内产的卵为第二次数据,两次的数据相加即为最终产卵量。在每次收集卵卡的当天开始统计孵化幼虫的数量,连续统计3 d,两次统计得到的孵化幼虫总和除以产卵总数即为孵化率。采用Microsoft Office Excel(2016)软件对野生型和突变品系成虫的产卵量及卵的孵化率进行差异显著性检测并绘图;采用卡方(Chi-square)检验检测不同突变类型的遗传比率以及突变后代的性比。
2 结果
2.1 小菜蛾fl(2)d的鉴定及表达模式
克隆得到的CDS长度为912 bp(图2-A),预测编码304个氨基酸。通过与基因组序列进行比对,发现含有4个外显子,5个内含子。利用国际生物技术信息中心(NCBI)上的保守结构域数据库(CDD)网站(https://www.ncbi. nlm.nih.gov/Structure/ cdd)分析,发现其CDS在第85—237个氨基酸位置处有一个WTAP超家族保守功能域[31,39](图2-B)。qRT-PCR结果显示,在雌蛹、雌成虫和卵中的表达量较高,雄成虫和雄蛹的表达量较低,不同性别幼虫的表达量均最低(图2-C),卵巢中表达量显著高于精巢(图2-D),说明该基因可能与小菜蛾的生殖过程和胚胎发育有关。

A:fl(2)d CDS序列的克隆CDS cloning of fl(2)d;M:DNA分子标准DNA marker。B:预测的fl(2)d蛋白质序列Predicted protein sequence offl(2)d;红色字母:保守功能域Red letters indicate the conserved domain。C:fl(2)d在不同发育阶段的表达Expression of fl(2)d in different developmental stages;E:卵Egg;FL:雌幼虫female larva;ML:雄幼虫male larva;FP:雌蛹Female pupa;MP:雄蛹Male pupa;FA:雌成虫Female adult;MA:雄成虫Male adult;采用单因素方差分析法进行差异显著性检验,使用Tukey法进行多重比较,不同字母表示差异显著(P<0.05)Significant difference analysis was performed using one-way ANOVA followed by a Tukey’s HSD post hoc test. Different letters indicate significant difference at P<0.05 level。D:fl(2)d在成虫生殖腺的表达Expression of fl(2)d in adult gonads;O:卵巢Ovary;T:精巢Testis;采用t检验进行差异显著性分析,**表示检验性水平P<0.01 Significant difference analysis was performed using t test. Double asterisks indicate significant difference of P<0.01
2.2 CRISPR/Cas9介导的fl(2)d编辑及其突变类型
采用CRISPR/Cas9基因编辑技术,在的2号外显子上设计2条sgRNA,对小菜蛾卵进行Cas蛋白和sgRNA的混合注射。共注射了160个小菜蛾G0代卵,成功羽化64只。将成功羽化的成虫与野生型小菜蛾进行两两配对,待其产生后代后,提取G0代的64只成虫及3只干瘪蛹的基因组DNA,利用检测引物进行PCR扩增。对产物进行测序和比对分析,发现成功引起突变的sgRNA只有1条(图3-A),发生突变的成虫只有3只(突变率为1.88%,图3-B)、干瘪蛹1只。对发生突变的3只成虫产生的后代分别进行保种,将每个种群的成虫随机近交,筛选突变纯合体。经过10代的近交筛选,只获得了3种不同的杂合突变类型,分别缺失4个(Δ213-4)、5个(Δ213-5)和7个(Δ214-7)碱基(图3-C),均导致的移码突变,后续试验所用突变品系的小菜蛾均为这3种杂合类型。

A:fl(2)d基因结构及sgRNA靶点示意图Schematic diagram of the fl(2)d gene structure and sgRNA target site。B:G0代野生型(上)和突变体(下)成虫fl(2)d测序峰图;红色框是sgRNA,蓝色框是PAM结构Sequencing chromatograms of fl(2)d of wild-type (top) and mutant (bottom) adults; The edited site is indicated by a red rectangle and the protospacer adjacent motif (PAM) sequence is showed by a blue rectangle。C:突变序列,虚线表示缺失的序列Mutant sequences, the dashed lines represent the deleted bases caused by CRISPR/Cas9
2.3 小菜蛾fl(2)d基因突变的遗传效应
通过分析杂合突变个体近交后代共362只个体,在G3代和G4代中各获得了3只Δ213-4突变品系的纯合小菜蛾个体,且成功配成两对,但同为纯合突变个体近交后无卵产生。将剩余的两只雄性纯合突变个体,分别与同代雌性杂合突变个体近交后也无卵产生。同时,在G3代获得了2只Δ213-5突变品系的雄性纯合小菜蛾,分别与同代雌性突变个体近交后,仍无卵产生。除了在G3代和G4代出现了纯合突变品系,在10代的近交过程中均未再出现纯合突变个体。因此推测,纯合突变个体的存活率极低,且可能表现为不育,纯合突变的类型无法遗传。由于G1代近交产生的突变类型较多,且大多数突变类型或无法遗传或为同义突变,因此从G2代起,对筛选过程中各个世代3种突变类型的亲本和后代共992个个体的基因型开展分析。发现不同比例的基因型分离情况符合孟德尔遗传定律,经卡方检验达到显著水平(表2)。值得注意的是,亲本为杂合突变个体与野生型的近交后代中,杂合突变个体和野生型的比值略低于1,亲本均为杂合突变个体的近交后代中,杂合突变个体和野生型比值略低于2,说明突变基因型的存在可能影响了后代的存活率。
2.4 小菜蛾fl(2)d的生物学功能
通过统计单只配对的雌成虫产卵和卵孵化情况,发现无论是哪种突变类型,其亲本或均为杂合突变体,亦或亲本只有一方为杂合突变体,产卵量和孵化率显著低于野生型(<0.01,图4-A、4-B),产卵量和孵化率较野生型相比分别减少了49.17%—93.22%和69.49%—100%。值得注意的是,当雌雄亲本均为缺失4个碱基的杂合突变品系时,孵化率明显低于其他交配组合,推测其移码突变产生的新蛋白可能对小菜蛾胚胎正常的生长发育具有负面效应。同时,统计了突变亲本产生的后代中突变个体的性别比例,发现在G2到G10代的试验过程中,每一代突变个体的雌雄比例及G2至G10代的突变个体总和的雌雄比例均接近1﹕1,且经卡方检验达到显著水平(图4-C,<0.05,2=1.30),说明该基因可能不影响小菜蛾的性别决定。
2.5 小菜蛾fl(2)d突变个体的生殖表型与利用
注射Cas9蛋白和sgRNA后的G0代卵,自孵化出幼虫起至成虫产卵后死亡,整个世代过程无明显异样。但通过使突变品系的成虫进行单对交配,即让杂合突变个体与野生型个体杂交或杂合突变个体近交,发现雌成虫产卵2 d后的总卵量明显少于野生型(图5-A、5-B),且部分卵随着发育时间的延长逐渐失水皱缩,无法孵化出幼虫(图5-C、5-D)。同时,还有部分卵在产出5 d后,虽然表型与野生型的初孵卵无异,但仍无法正常孵化出健康幼虫(图5-E、5-F)。通过解剖发现,未交配突变体雌成虫卵巢内卵的附着量比野生型显著下降,因此导致突变体雌成虫所参与的交配组合产卵量的下降。与突变体雄成虫交配过的野生型雌成虫,其卵巢内卵的附着量也明显少于交配前的数量(图5-G、5-H)。未交配的突变品系雄成虫生殖腺与野生型比较,无明显异常。试验过程中发现部分突变品系的个体在发育至幼虫、蛹及成虫阶段均会发生不同程度的畸变,暗示可能参与了小菜蛾的生长发育。另外一些杂合突变个体未见异常,可以将突变类型遗传给后代。

A:突变品系的产卵量The fecundity of mutant strains。B:突变品系的孵化率The hatchability of mutant strains。WT:野生型成虫杂交Hybridization between wild-type adults;F-4:杂合雌性Δfl(2)d213-4成虫与野生型杂交Hybridization of heterozygous female adult of Δfl(2)d213-4 with wild-type;M-4:杂合雄性Δfl(2)d213-4成虫与野生型杂交Hybridization of heterozygous male adult of Δfl(2)d213-4 with wild-type;-4:杂合Δfl(2)d213-4成虫近交Inbreeding between heterozygous adults of Δfl(2)d213-4;F-5:杂合雌性Δfl(2)d213-5成虫与野生型杂交Hybridization of heterozygous female adult of Δfl(2)d213-5 with wild-type;M-5:杂合雄性Δfl(2)d213-5成虫与野生型杂交Hybridization of heterozygous male adult of Δfl(2)d213-5 with wild-type;-5:杂合Δfl(2)d213-5成虫近交Inbreeding between heterozygous adults of Δfl(2)d213-5;F-7:杂合雌性Δfl(2)d214-7成虫与野生型杂交Hybridization of heterozygous female adult of Δfl(2)d214-7 with wild-type;M-7:杂合雄性Δfl(2)d214-7成虫与野生型杂交Hybridization of heterozygous male adult of Δfl(2)d214-7 with wild-type;-7:杂合Δfl(2)d214-7成虫近交Inbreeding between heterozygous adults of Δfl(2)d214-7。C:性别比例The sex ratios of mutant offspring;采用t检验进行差异显著性分析,**表示显著性水平P<0.01 Significant difference analysis was performed using t test. Double asterisks indicate significant difference of P<0.01
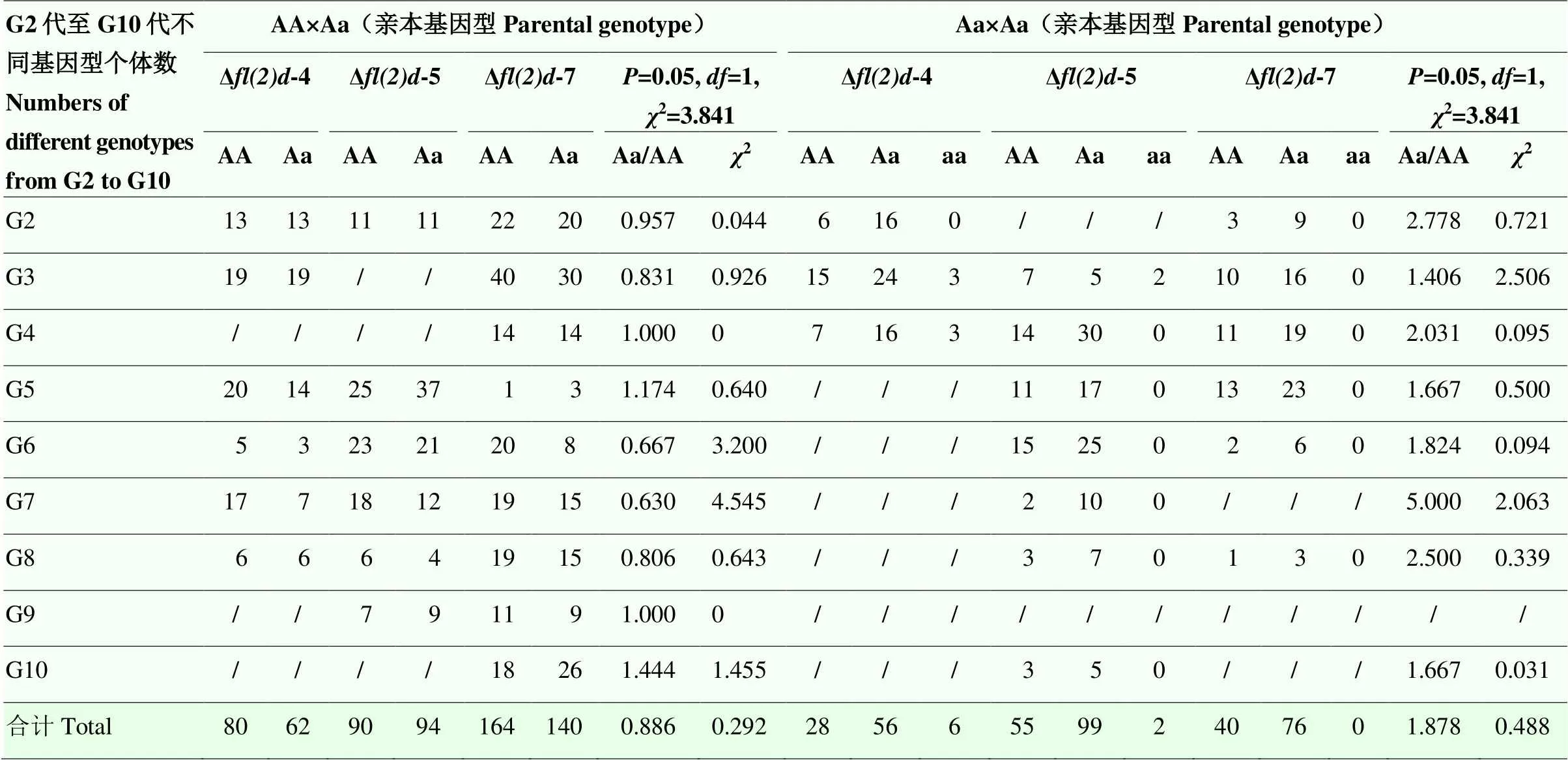
表2 G2—G10代fl(2)d基因型分离情况
斜杠表示未检测。亲本只统计基因型,不考虑性别The slash denotes the missing data. Only genotypes of the parents were recorded regardless of the sexuality。A:野生型基因型Wild-type genotype;a:突变型基因型Mutant genotype

A:野生型雌成虫产卵情况The fecundity of wild-type female adult。B:突变体雌成虫产卵情况The fecundity of mutant female adult。C、D:失水皱缩的卵The shrinking and dehydrated eggs。E:未能正常孵化的卵Eggs that do not hatch successfully。F:未能正常出卵的幼虫The larvae that fail to come out of eggs。G:未交配野生型雌成虫卵巢Ovary of the wild-type virgin female adult。H:与突变体雄成虫交配后野生型雌成虫卵巢Ovary of the wild-type female adult mated with the mutant male adult。除A图外,所观察的卵均为突变体雌成虫与野生型雄成虫单对交配后所产的后代,突变体类型为Δfl(2)d213-5 Except for figure A, the presented phenotypes of the eggs are all produced from the single-pair mating of mutant female adult and wild-type male adult, and the type of mutation is Δfl(2)d213-5
鉴于单等位基因的突变显著影响了小菜蛾的产卵量及孵化率但不影响性别比例的特性,笔者提出了一个防控模型(图6):首先构建两种基因组被编辑的小菜蛾品系,一种为被绿色荧光蛋白(GFP)基因插入单个基因座并发生碱基移码突变的小菜蛾,另一种为被红色荧光蛋白(RFP)基因插入到雌性特有的W染色体的小菜蛾。将两种编辑品系的小菜蛾进行杂交,通过观察后代卵的荧光类型,只保留发单一绿色荧光的小菜蛾雄性卵,大量饲养至成虫进行田间释放。从理论上讲,只需要周期性释放被编辑的小菜蛾雄成虫就能够达到预期的防控目的。

图6 基于fl(2)d的小菜蛾遗传防控模型
3 讨论
尽管已经在许多物种中开展了与RNA甲基化相关的研究,但对昆虫的研究仅局限于其影响家蚕的滞育、蜜蜂的幼虫发育和级型分化以及果蝇的生殖过程、胚胎发育和性别决定[23,25,28]。本研究选择参与m6A甲基化进程的writers蛋白fl(2)d,通过qRT-PCR分析了该基因在小菜蛾体内的时空表达,发现表达量在雌蛹、雌成虫及卵期高于其他发育阶段,这与Lence等[28]的研究结果一致,表明该基因可能参与了小菜蛾的生殖发育。通过基因编辑技术CRISPR/ Cas9敲除,发现含有单个突变的等位基因的小菜蛾其生殖力和育性显著低于野生型,这种影响与编辑类型和突变亲本的性别无关,说明参与了小菜蛾的生殖过程和胚胎发育。在哺乳动物中,缺乏的小鼠胚胎表现出显著的细胞增殖缺陷,影响了内胚层和中胚层的形成,从而导致胚胎在发育早期便死亡[40-42]。笔者发现,当突变雌成虫与野生型雄成虫杂交以及突变成虫之间自交,雌成虫产卵量和卵孵化率的下降是由于卵巢发育受到了突变的影响。同样地,参与m6A甲基化过程的另一writers蛋白对果蝇生殖能力也会产生影响,突变后雌成虫表现出卵巢异常且不育[43]或卵室数量减少且无法完成发育[44]。然而,当突变体小菜蛾雄成虫与野生型雌成虫杂交后,其产卵量和孵化率同含有突变体雌成虫的交配组合结果类似,但交配前突变体雄成虫精巢与野生型的并无明显差异。这一结果引发了笔者新的思考:在突变体雄成虫精巢与野生型精巢表型无差异的基础上,解剖与突变体雄成虫交配后野生型雌成虫的卵巢,发现卵巢内卵的附着量比未交配的个体显著减少,说明突变体雄成虫可能在交配过程中向雌虫卵巢提供了一些不利于卵子发育的物质。已有研究表明,雄成虫的精液蛋白能以多种方式促进后代的繁殖,包括促进精子储存和雌性产卵[45-47]。笔者推测,突变体雄成虫可能产生了异常的精液蛋白,导致精子的活力降低,在与野生型雌成虫交配的过程中,由于大多数精子不能与卵子发生正常的精卵结合,导致卵的发育受阻[48-50],其具体机理还有待进一步的研究。在筛选突变品系的试验过程中,发现有相当比例的突变个体在幼虫、蛹和成虫阶段均会出现畸形的现象,不能正常完成整个世代,说明该基因可能还参与了小菜蛾的生长发育。果蝇中的研究发现突变m6A甲基化过程中相关的writers蛋白,无论是纯合还是杂合突变体,其雌、雄成虫均表现出明显的行为缺陷,包括移动及飞行能力的下降和翅不能合拢于背部[28,44]。此外,本研究对小菜蛾突变品系后代中的突变个体进行了9个世代的性别比例统计,发现每一代中被编辑的雌雄个体比例及G2到G10代所有突变个体的雌雄比例基本都符合1﹕1的理论值,说明该基因可能与小菜蛾的性别决定无关。然而,果蝇的基因突变后,雌性的活力和受精能力显著下降,而雄性却不受影响[51]。在果蝇中,同时缺失writers基因和性别决定通路关键基因的单等位基因也会影响种群的性别比例,具体表现为雌性的存活率严重下降,而雄性不受影响,这可能是由于剂量补偿通路受损所导致[28]。
随着生物技术的不断发展,众多与害虫种群控制相关的概念相继被提出并运用于生产实践,其中就包括种群置换和种群抑制[52-53]。相较于种群置换,种群抑制并不影响靶标害虫在生态系统中的生态位,只是将其种群数量控制在经济损失所允许的水平之下。本试验通过CRISPR/Cas9结合卵的显微注射技术,对小菜蛾进行了编辑并成功诱发突变,进一步发现其纯合突变个体存活率极低且不育,单等位基因杂合突变个体的成虫生殖力和育性则显著下降,为作为潜在的靶标应用于小菜蛾的遗传防控打下了理论基础。由于该杂合突变品系的获得不需要进行多代的近交筛选,节约了大量的试验成本,一旦检测出该基因被成功编辑,便可以迅速应用于生产实践。同时,考虑到昆虫雌性个体具有繁殖后代的特性及其在饲养和田间释放方面所增加的经济成本,在发育的早期阶段筛选雄性个体是开展害虫遗传控制的关键步骤[54]。由于小菜蛾在4龄幼虫以前无法肉眼辨别性别,因此可以采用基因敲入技术[55],将不同的荧光蛋白基因分别整合到基因座和雌性特有的W染色体。理论上而言,前者可导致单等位基因的突变并产生一种荧光,用于区分后代中的野生型个体,而后者则可以使雌性个体产生另一种荧光。利用荧光显微镜筛选两种编辑品系小菜蛾杂交后的虫卵,便可快速地筛选出雄性突变个体,并排除野生型和雌性突变个体[55],以便进行大规模的饲养和田间释放。这种防治策略通过降低靶标害虫的产卵量和孵化率,从而“柔和”地控制其种群数量并保证该物种在自然界可以继续繁衍存在,不会像基因驱动系统那样“暴力”地降低种群数量,甚至可能导致该物种的灭绝并引起负面生态效应[56-57]。
4 结论
参与了小菜蛾正常的生长发育过程,主要表现在对生殖过程和胚胎发育的影响,但不影响小菜蛾的性别决定。单等位基因的突变显著降低了小菜蛾的生殖力和育性,是开展遗传控制的理想靶标。
[1] FURLONG M J, WRIGHT D J, DOSDALL L M. Diamondback moth ecology and management: problems, progress, and prospects. Annual Reviews of Entomology, 2013, 58: 517-541.
[2] LI Z Y, FENG X, LIU S S, YOU M S, FURLONG M J. Biology, ecology, and management of the diamondback moth in China. Annual Review of Entomology, 2016, 61: 277-296.
[3] GURR G M, REYNOLDS O L, JOHNSON A C, DESNEUX N, ZALUCKI M P, FURLONG M J, LI Z Y, AKUTSE K S, CHEN J H, GAO X W, YOU M S. Landscape ecology and expanding range of biocontrol agent taxa enhance prospects for diamondback moth management. A review. Agronomy for Sustainable Development, 2018, 38: 23.
[4] 曾宝胜, 许军, 陈树清, 谭安江, 黄勇平. 昆虫种群的遗传调控. 中国科学: 生命科学, 2013, 43(12): 1098-1104.
ZENG B S, XU J, CHEN S Q, TAN A J, HUANG Y P. Genetic regulation of insect populations.Scientia Sinica Vitae, 2013, 43(12): 1098-1104. (in Chinese)
[5] 徐雪娇, 何玮毅, 杨婕, 陈玮, 尤民生. 害虫遗传防控技术的研究与运用. 中国科学: 生命科学, 2019, 49(8): 938-950.
XU X J, HE W Y, YANG J, CHEN W, YOU M S. Research and applications of genetics-based methods for pest control. Scientia Sinica Vitae, 2019, 49(8): 938-950. (in Chinese)
[6] CHEN W, YANG F Y, XU X J, KUMAR U, HE W Y, YOU M S. Genetic control ofin omics era. Archives of Insect Biochemistry and Physiology, 2019, 102(3): e21621.
[7] SHELTON A M, LONG S J, WALKER A S, BOLTON M, COLLINS H L, REVUELTA L, JOHNSON L M, MORRISON N I. First field release of a genetically engineered, self-limiting agricultural pest insect: evaluating its potential for future crop protection. Frontiers in Bioengineering and Biotechnology, 2020, 7: 482.
[8] YOU M S, YUE Z, HE W Y, YANG X H, YANG G, XIE M, ZHAN D L, BAXTER S W, VASSEUR L, GURR G M,. A heterozygous moth genome provides insights into herbivory and detoxification. Nature Genetics, 2013, 45(2): 220-225.
[9] JIN L, WALKER A S, FU G L, HARVEY-SAMUEL T, DAFA’ALLA T, MILES A, MARUBBI T, GRANVILLE D, HUMPHREY-JONES N, O’CONNELL S, MORRISON N I, ALPHEY L. Engineered female-specific lethality for control of pest Lepidoptera. ACS Synthetic Biology, 2013, 2(3): 160-166.
[10] HARVEY-SAMUEL T, MORRISON N I, WALKER A S, MARUBBI T, YAO J, COLLINS H L, GORMAN K, DAVIES T G, ALPHEY N, WARNER S, SHELTON A M, ALPHEY L. Pest control and resistance management through release of insects carrying a male-selecting transgene. BMC Biology, 2015, 13: 49.
[11] PAN T.6-methyl-adenosine modification in messenger and long non-coding RNA. Trends in Biochemical Sciences, 2013, 38(4): 204-209.
[12] ROUNDTREE I A, EVANS M E, PAN T, HE C. Dynamic RNA modifications in gene expression regulation. Cell, 2017, 169(7): 1187-1200.
[13] BATISTA P J, MOLINIE B, WANG J, QU K, ZHANG J J, LI L J, BOULEY D M, LUJAN E, HADDAD B, DANESHVAR K,. m6A RNA modification controls cell fate transition in mammalian embryonic stem cells. Cell Stem Cell, 2014, 15(6): 707-719.
[14] FU Y, DOMINISSINI D, RECHAVI G, HE C. Gene expression regulation mediated through reversible m6A RNA methylation. Nature Reviews. Genetics, 2014, 15(5): 293-306.
[15] DOMINISSINI D, MOSHITCH-MOSHKOVITZ S, SCHWARTZ S, SALMON-DIVON M, UNGAR L, OSENBERG S, CESARKAS K, JACOB-HIRSCH J, AMARIGLIO N, KUPIEC M, SOREK R, RECHAVI G. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature, 2012, 485(7397): 201-206.
[16] MEYER K D, SALETORE Y, ZUMBO P, ELEMENTO O, MASON C E, JAFFREY S R. Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons.Cell, 2012, 149(7): 1635-1646.
[17] DOMINISSINI D, MOSHITCH-MOSHKOVITZ S, SALMON- DIVON M, AMARIGLIO N, RECHAVI G. Transcriptome-wide mapping ofN-methyladenosine by m6A-seq based on immunocapturing and massively parallel sequencing. Nature Protocols, 2013, 8(1): 176-189.
[18] WU B X, LI L, HUANG Y, MA J, MIN J B, MIN J R. Readers, writers and erasers of6-methylated adenosine modification.Current Opinion in Structural Biology, 2017, 47: 67-76.
[19] ZHOU Z L, LICKLIDER L J, GYGI S P, REED R. Comprehensive proteomic analysis of the human spliceosome. Nature, 2002, 419(6903): 182-185.
[20] HORIUCHI K, KAWAMURA T, IWANARI H, OHASHI R, NAITO M, KODAMA T, HAMAKUBO T. Identification of Wilms’ tumor 1-associating protein complex and its role in alternative splicing and the cell cycle. Journal of Biological Chemistry, 2013, 288(46): 33292-33302.
[21] LIU J Z, YUE Y N, HAN D L, WANG X, FU Y, ZHANG L,JIA G F, YU M, LU Z K, DENG X, DAI Q, Chen W Z, HE C. A-complex mediates mammalian nuclear RNA6-adenosine methylation. Nature Chemical Biology, 2014, 10(2): 93-95.
[22] BANSAL H, YIHUA Q, IYER S P, GANAPATHY S, PROIA D, PENALVA L O, UREN P J, SURESH U, CAREW J S, KARNAD A B, WEITMAN S, TOMLINSON G E, RAO M K, KORNBLAU S M, BANSAL S. WTAP is a novel oncogenic protein in acute myeloid leukemia. Leukemia, 2014, 28(5): 1171-1174.
[23] JIANG T, LI J S, QIAN P, XUE P, XU J, CHEN Y R, ZHU J, TANG S M, ZHAO Q L, QIAN H Y, SHEN X J. The role of6- methyladenosine modification on diapause in silkworm () strains that exhibit different voltinism.Molecular Reproduction and Development, 2019, 86(12): 1981-1992.
[24] LI B Q, WANG X Y, LI Z Q, LU C C, ZHANG Q, CHANG L, LI W S, CHENG T C, XIA Q Y, ZHAO P. Transcriptome-wide analysis of6-methyladenosine uncovers its regulatory role in gene expression in the Lepidopteran. Insect Molecular Biology, 2019, 28(5): 703-715.
[25] WANG M, XIAO Y, LI Y, WANG X Y, QI S Z, WANG Y, ZHAO L W, WANG K, PENG W J, LUO G Z, XUE X F, JIA G F, WU L M. RNA m6A modification functions in larval development and caste differentiation in honeybee (). Cell Reports, 2021, 34(1):108580.
[26] GUO J, TANG H W, LI J, PERRIMON N, YAN D. Xio is a component of thesex determination pathway and RNA6-methyladenosine methyltransferase complex. Proceedings of the National Academy of Sciences of the United States of America, 2018, 115(14): 3674-3679.
[27] HAUSSMANN I U, BODI Z, SANCHEZ-MORAN E, MONGAN N P, ARCHER N, FRAY R G, SOLLER M. m6A potentiatesalternative pre-mRNA splicing for robustsex determination. Nature, 2016, 540(7632): 301-304.
[28] LENCE T, AKHTAR J, BAYER M, SCHMID K, SPINDLER L, HO C H, KREIM N, ANDRADE-NAVARRO M A, POECK B, HELM M, ROIGNANT J Y. m6A modulates neuronal functions and sex determination in. Nature, 2016, 540(7632): 242-247.
[29] GRANADINO B, SAN JUAN A, SANTAMARIA P, SANCHEZ L. Evidence of a dual function in, a gene needed forexpression in. Genetics, 1992, 130(3): 597-612.
[30] ORTEGA A, NIKSIC M, BACHI A, WILM M, SANCHEZ L, HASTIE N, VALCARCEL J. Biochemical function of female-lethal (2)D/Wilms’ tumor suppressor-1-associated proteins in alternative pre-mRNAsplicing.Journal of Biological Chemistry, 2003, 278(5): 3040-3047.
[31] GRANADINO B, CAMPUZANO S, SANCHEZ L. Thegene is needed for the female-specific splicing ofRNA. The EMBO Journal, 1990, 9(8): 2597-2602.
[32] GRANADINO B, PENALVA L O, SANCHEZ L. The geneis needed for the sex-specific splicing ofpre-mRNA but not forpre-mRNA in.Molecular and General Genetics, 1996, 253(1/2): 26-31.
[33] BURNETTE J M, HATTON A R, LOPEZ A J. Trans-acting factors required for inclusion of regulated exons in themRNAs of.Genetics, 1999, 151(4): 1517-1529.
[34] ORTEGA A. Localization of theproteinin somatic cells and female gonads. Cell and Tissue Research, 2005, 320(2): 361-367.
[35] TRAUT W, NIIMI T, IKEO K, SAHARA K. Phylogeny of the sex-determining genein insects. Genome, 2006, 49(3): 254-262.
[36] FUJII T, SHIMADA T. Sex determination in the silkworm,: a female determinant on the W chromosome and the sex-determining gene cascade. Seminars in Cell and Developmental Biology, 2007, 18(3): 379-388.
[37] TANG W Q, YU L Y, HE W Y, YANG G, KE F S, BAXTER S W, YOU S J, DOUGLAS C J, YOU M S. DBM-DB: the diamondback moth genome database. Database, 2014, 2014: bat087.
[38] CHEN W, DONG Y H, SAQIB H S A, VASSEUR L, ZHOU W W, ZHENG L, LAI Y F, MA X L, LIN L Y, XU X J, BAI J L, HE W Y, YOU M S. Functions of duplicated glucosinolate sulfatases in the development and host adaptation of. Insect Biochemistry and Molecular Biology, 2020, 119: 103316.
[39] PING X L, SUN B F, WANG L, XIAO W, YANG X, WANG W J, ADHIKARI S, SHI Y, LV Y, CHEN Y S,. Mammalian WTAP is a regulatory subunit of the RNA6-methyladenosine methyltransferase.Cell Research, 2014, 24(2): 177-189.
[40] FUKUSUMI Y, NARUSE C, ASANO M. WTAP is required for differentiation of endoderm and mesoderm in the mouse embryo. Developmental Dynamics, 2008, 237(3): 618-629.
[41] HORIUCHI K, UMETANI M, MINAMI T, OKAYAMA H, TAKADA S, YAMAMOTO M, ABURATANI H, REID P C, HOUSMAN D E, HAMAKUBO T, KODAMA T. Wilms’ tumor 1-associating protein regulates G2/M transition through stabilization of cyclin A2 mRNA. Proceedings of the National Academy of Sciences of the United States of America, 2006, 103(46): 17278-17283.
[42] NARUSE C, FUKUSUMI Y, KAKIUCHI D, ASANO M. A novel gene trapping for identifying genes expressed under the control of specific transcription factors. Biochemical and Biophysical Research Communications, 2007, 361(1): 109-115.
[43] HONGAY C F, ORR-WEAVER T L.inducer of MEiosis 4 (IME4) is required for Notch signaling during oogenesis. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(36): 14855-14860.
[44] KAN L J, GROZHIK A V, VEDANAYAGAM J, PATIL D P, PANG N, LIM K S, HUANG Y C, JOSEPH B, LIN C J, DESPIC V,. The m6A pathway facilitates sex determination in. Nature Communications, 2017, 8: 15737.
[45] SINGH A, BUEHNER N A, LIN H, BARANOWSKI K J, FINDLAY G D, WOLFNER M F. Long-term interaction betweensperm and sex peptide is mediated by other seminal proteins that bind only transiently to sperm. Insect Biochemistry and Molecular Biology, 2018, 102: 43-51.
[46] SLOAN N S, LOVEGROVE M, SIMMONS L W. Social manipulation of sperm competition intensity reduces seminal fluid gene expression. Biology Letters, 2018, 14(1): 20170659.
[47] WIGBY S, SIROT L K, LINKLATER J R, BUEHNER N, CALBOLI F C F, BRETMAN A, WOLFNER M F, CHAPMAN T. Seminal fluid protein allocation and male reproductive success. Current Biology, 2009, 19(9): 751-757.
[48] SIMMONS L W, LOVEGROVE M. Socially cued seminal fluid gene expression mediates responses in ejaculate quality to sperm competition risk. Proceedings of the Royal Society B: Biological Sciences, 2017, 284(1861): 20171486.
[49] NEUBAUM D M, WOLFNER M F. Matedfemales require a seminal fluid protein, Acp36DE, to store sperm efficiently. Genetics, 1999, 153(2): 845-857.
[50] SIROT L K, BUEHNER N A, FIUMERA A C, WOLFNER M F. Seminal fluid protein depletion and replenishment in the fruit fly,: an ELISA-based method for tracking individual ejaculates. Behavioral Ecology and Sociobiology, 2009, 63(10): 1505-1513.
[51] GRANADINO B, SAN JUÁN A B, SÁNCHEZ L. The geneis required for various-controlled processes infemales. Roux’s Archives of Developmental Biology, 1991, 200(3): 172-176.
[52] BOURTZIS K, LEES R S, HENDRICHS J, VREYSEN M J B. More than one rabbit out of the hat: radiation, transgenic and symbiont- based approaches for sustainable management of mosquito and tsetse fly populations.Acta Tropica, 2016, 157: 115-130.
[53] FLORES H A, O’NEILL S L. Controlling vector-borne diseases by releasing modified mosquitoes.Nature Reviews. Microbiology, 2018, 16(8): 508-518.
[54] MEZA J S, UL HAQ I, VREYSEN M J B, BOURTZIS K, KYRITSIS G A, CÁCERES C. Comparison of classical and transgenic genetic sexing strains of Mediterranean fruit fly (Diptera: Tephritidae) for application of the sterile insect technique. PLoS ONE, 2018, 13(12): e0208880.
[55] ZHANG Z J, NIU B L, JI D F, LI M W, LI K, JAMES A A, TAN A J, HUANG Y P. Silkworm genetic sexing through W chromosome- linked, targeted gene integration. Proceedings of the National Academy of Sciences of the United States of America, 2018, 115(35): 8752-8756.
[56] GANTZ V M, JASINSKIENE N, TATARENKOVA O, FAZEKAS A, MACIAS V M, BIER E, JAMES A A. Highly efficient Cas9-mediated gene drive for population modification of the malaria vector mosquito. Proceedings of the National Academy of Sciences of the United States of America, 2015, 112(49): E6736-6743.
[57] HAMMOND A, GALIZI R, KYROU K, SIMONI A, SINISCALCHI C, KATSANOS D, GRIBBLE M, BAKER D, MAROIS E, RUSSELL S, BURT A, WINDBICHLER N, CRISANTI A, NOLAN T. A CRISPR-Cas9 gene drive system targeting female reproduction in the malaria mosquito vector. Nature Biotechnology, 2016, 34(1): 78-83.
Knockout of single allele ofsignificantly decreases the fecundity and fertility in
LI FeiFei, WANG BeiBei, LAI YingFang, YANG FeiYing, YOU MinSheng, HE WeiYi
State Key Laboratory of Ecological Pest Control for Fujian and Taiwan Crops/Institute of Applied Ecology, Fujian Agriculture and Forestry/International Joint Research Laboratory of Ecological Pest Control, Ministry of Education, Fujian Agriculture and Forestry University/Key Laboratory of Integrated Pest Management for Fujian-Taiwan Crops, Ministry of Agriculture and Rural Affairs, Fujian Agriculture and Forestry/Ministerial and Provincial Joint Innovation Centre for Safety Production of Cross-Strait Crops, Fuzhou 350002
【】RNA methylation is the main form of epigenetic modification at post-transcriptional level, which is involved in many important cellular processes. The diamondback moth,, is an important oligophagous insect pest, causing serious loss on the production of cruciferous vegetables. However, the function of RNA methylation-related genes inis still unclear. The present study aims to identify and clone the homologous, one of the members of the RNA methylation protein complex (writers), to determine the expression pattern of, and to knockoutusing CRISPR/Cas9 for the investigation of its biological functions in.【】The sequence of homologouswas identified in the genome database of, which was used for PCR amplification of the coding sequence (CDS). Quantitative real-time PCR (qRT-PCR) was used to study the relative expression levels ofin different developmental stages and adult gonads of. Thewas edited using CRISPR/Cas9 combined with egg injection. Each of the adults that developed from the injected eggs was used to pair with a wild-type adult for reproduction. Offspring of the same population was forced to inbreed by single-pair mating to establish the mutant strains. The differences of genetic characters, biological parameters and phenotypes between mutants and wild-type individuals were recorded and compared to decipher the function of.【】The CDS ofwith length of 912 bp was isolated, the expression of which was high in female pupa, adult and egg, moderate in male adult and pupa, the lowest in larva, and significantly higher in ovary than in testis of adult. The sgRNAs targetingand the Cas9 protein were mixed to inject eggs, and the offsprings carrying mutant alleles were screened for homozygous strains based on single-pair inbreeding for 10 generations. Three types of heterozygous mutant strains both predicted to cause frameshift of the CDS were obtained, with the deletion of 4 (Δ213-4), 5 (Δ213-5) and 7 (Δ213-7) bases. During the screening process, six and two homozygous mutants from Δ213-4 and Δ213-5 strains were identified, respectively. The homozygous mutants of Δ213-4 successfully mated in two pairs, but no eggs were produced. Meanwhile, each two male adults of homozygous mutants of either Δ213-4 or Δ213-5 were mated with the same type of female heterozygous mutant, and also no eggs were produced.The results indicated that individuals with homozygousmutation may have extremely low survival rate and not be able to produce offspring. Through analyzing separation ratio of the genotypes of offspring from the inbreeding of heterozygous mutants and the hybridization between heterozygous mutants and wild-type, it was found that the ratio of heterozygous mutant individuals to wild-type was slightly less than 2 and 1, respectively, indicating that heterozygous mutation ofwould affect the normal growth and development of, and in some cases would lead to death. The offsprings of mutant individuals, which carry a mutant allele, showed a sex ratio close to 1﹕1 (<0.05). It was speculated that themight not be involved in sex determination in. For the mating consists of mutant adults, the fecundity and hatchability were significantly lower (<0.01) than the mating between wild-type adults. Most of the eggs produced from the mutant parents look abnormal, and could not hatch normally due to water loss and shrinkage. Based on the dissection of adult gonads, it was found that the number of attached eggs on the ovary of the mutant female adult and the wild-type female adult that has mated with mutant male adult was less than that of the wild-type virgin female adult, while no obvious abnormality was found for the testis of mutant male adult. Some of the hatched heterozygous mutants showed different degrees of distortion during the whole developmental process, resulting in the failure to complete life cycle. A small part of the heterozygous mutant individuals could develop normally, and thus transmit the mutant allele to their offspring. According to our findings, a model of genetic control ofbased onwas proposed.【】Theis involved in the reproductive process and embryonic development of, mutation of which significantly affects the population size of the offspring, making it an ideal target for the genetic control of.
;; heterozygous mutant;fecundity; fertility

10.3864/j.issn.0578-1752.2021.14.009
2020-11-06;
2021-01-15
国家重点研发计划(2017YFD0200400)、福建省自然科学基金(2019J21369)、福建省科技重大专项(2018NZ0002)
李飞飞,E-mail:lff0371@163.com。通信作者何玮毅,E-mail:wy.he@fafu.edu.cn。通信作者尤民生,E-mail:msyou@fafu.edu.cn
(责任编辑 岳梅)
