Interactive effect of shade and PEG-induced osmotic stress on physiological responses of soybean seedlings
2021-07-24MuhammadAhsanASGHARJIANGHengkeSHUIZhaoweiCAOXiyuHUANGXiyuShakeelIMRANBushraAHMADZHANGHaoYANGYueningSHANGJingYANGHuiYULiangLIUChunyanYANGWenyuSUNXinDUJunbo
Muhammad Ahsan ASGHAR,JIANG Heng-ke,SHUI Zhao-wei,CAO Xi-yu,HUANG Xi-yu,Shakeel IMRAN,Bushra AHMAD,ZHANG Hao,YANG Yue-ning,SHANG Jing,YANG Hui,YU Liang,LIU Chun-yan,YANG Wen-yu,SUN Xin,3,DU Jun-bo,3
1 College of Agronomy,Sichuan Agricultural University,Chengdu 611130,P.R.China
2 Research Center for Modern Agriculture of the Middle East,Sichuan Agricultural University,Chengdu 611130,P.R.China
3 Key Laboratory of Crop Ecophysiology and Farming System in Southwest China,Ministry of Agriculture,Sichuan Agricultural University,Chengdu 611130,P.R.China
4 Department of Agronomy,University of Agriculture,Faisalabad (UAF)|UAF Sub-Campus,Burewala 61010,Pakistan
5 Department of Plant Breeding and Genetics,University of Agriculture,Faisalabad 38000,Pakistan
Abstract Intensively farmed crops used to experience numerous environmental stresses.Among these,shade and drought significantly influence the morpho-physiological and biochemical attributes of plants.However,the interactive effect of shade and drought on the growth and development of soybean under dense cropping systems has not been reported yet.This study investigated the interactive effect of PEG-induced osmotic stress and shade on soybean seedlings.The soybean cultivar viz.,C-103 was subjected to PEG-induced osmotic stress from polyethylene glycol 6000 (PEG-6000) under shading and non-shading conditions.PEG-induced osmotic stress significantly reduced the relative water contents,morphological parameters,carbohydrates and chlorophyll contents under both light environments.A significant increase was observed in osmoprotectants,reactive oxygen species and antioxidant enzymes in soybean seedlings.Henceforth,the findings revealed that,seedlings grown under non-shading conditions produced more malondialdehyde and hydrogen peroxide contents as compared to the shade-treated plants when subjected to PEG-induced osmotic stress.Likewise,the shaded plants accumulated more sugars and proline than non-shaded ones under drought stress.Moreover,it was found that nonshaded grown plants were more sensitive to PEG-induced osmotic stress than those exposed to shading conditions,which suggested that shade could boost the protective mechanisms against osmotic stress or at least would not exaggerate the adverse effects of PEG-induced osmotic stress in soybean seedlings.
Keywords:shade,PEG-induced osmotic stress,reactive oxygen species,antioxidant enzymes,soybean
1.Introduction
Intercropping can improve soil fertility and promote effective disease and weed control,and efficient use of inputs,i.e.,light,water,and fertilizers.Soybean (Glycine max(L.)Merr.) based intercropping system is one of the momentous sources of grain production in southwestern region of China(Duet al.2018a).A fundamental ecological feature that affects the intercropping system is that,maize plants reach to the maximum height and alter the climatic conditions for soybean seedlings,which leads to multiple abiotic stresses at once including water shortage and reduction in light energy.It has been reported that,shading environment influences plants’ physiology in several ways.The primary plant traits influenced by shading conditions are the elongation in hypocotyl length,hyponasty,altered flowering period,and consequently yield decline (Casal 2013;de Witet al.2016).Many studies revealed that shading stress influences morphological,physio-hormonal,biochemical,metabolic,and molecular modulations of soybean (Barroet al.1989;Duet al.2018b;Liet al.2018;Hussainet al.2019b).Previous studies have elucidated that shading stress might be an harmful environmental strain,however,it is based on its severity of insinuation (Huanget al.2008).Some of the ecophysiological and genetic studies have been conducted to elucidate multiple environmental factors;however,each factor was investigated seperately and their combined effects on plant responses have been rarely studied.A few studies have been done on the joint effects of drought and shade stresses under forest ecology as well (Prider and Facelli 2004;Duanet al.2009;Liet al.2011).However,research on the interactive effect of shade with other abiotic strains especially the drought stress in soybean at the seedling stage has not been reported so far.
Water deficit condition is one of the key detrimental environmental factors that severely limits crop yield through several means around the world (Chami and Moujabber 2016).Many previous studies described the individual effect of water shortage condition on morpho-physiological,biochemical,molecular,and metabolic modulations on soybean (Clementet al.2008;Iqbalet al.2018;Castroet al.2019).However,its joint effect with shade on soybean seedling growth has not been explicated until recently.Osmotic stress progressively reduces the performance of photosynthetic machinery as it confines the transport of energy from photosystem II (PSII) to photosystem I(PSI),reduces the leaf thickness,and produces palisade and spongy tissues,which ultimately causes stumpy chlorophyllafluorescence induction (Chen 2013;Wanget al.2018,2019).Under such contrary surroundings,plants follow the feed-forward mechanism through which they produce some signals in dry soil that lead to the reduction of stomatal openings,transpiration rate and shoot growth(Kang and Zhang 2004).These root-sourced chemical signals are the main players for the elevation of abscisic acid(ABA) level in plants that leads to oxidative destruction by excessive reactive oxygen species (ROS) production (Beis and Patakas 2015).Inactivity of plant defense system might damage its metabolism and physiology violently since ROS exaggerate the impacts of drought stress by deleteriously upsetting cell membrane properties,cause injuries to lipids,chlorophyll,and proteins,and consequently lead to cell death (Leprinceet al.1990;Chen 2013).Hence,plants stimulate antioxidant enzymes to diminish the ROS functioning (Fanet al.2017).Plants’ antioxidant defense system includes peroxidase (POD),superoxide dismutase(SOD) and catalase (CAT).Although the impact of limited water conditions at morpho-physiological,biochemical and molecular levels have been discussed broadly,it would be a novel issue of high priority when investigated along with shade stress.
The major determinant of plants’ resistance against adverse climatic conditions is photosynthesis (Olechowiczet al.2018).The extent of photosynthesis is largely condensed during limited water conditions.Nevertheless,the basic mechanistic for this decline remains unclear at the soybean seedling stage.Previously,numerous investigations accomplished to elucidate the photosynthetic characteristics,antioxidant,molecular and metabolic potential of plants,merely under water deficit conditions(Piperet al.2007;Clementet al.2008;Galméset al.2011;Guoet al.2018).However,these studies focused primarily on the morphophysiological,antioxidant activities and metabolic profiling.The purpose of this study was to determine how the interaction of PEG-induced osmotic stress and shade affected soybean photosynthesis and antioxidant enzyme activities.Therefore,this research evaluated the photosynthetic responses of soybean seedlings under the interactive effect of shade and PEGinduced osmotic stresses.This study also analyzed the response to the interactive effect of shade and PEGinduced osmotic stresses by controlling for physiological variables:the relative water contents,proline contents,malondialdehyde (MDA),hydrogen peroxide (H2O2),and antioxidant enzyme activities (SOD,POD and CAT).The aim of this study was to interpret the responses of soybean plants after shade (SH) and non-SH treatments coupled with multiple concentrations of PEG-6000 used to induce osmotic stress.
2.Materials and methods
2.1.Plant materials and growth conditions
The soybean genotype C-103 (shade and drought-sensitive)was grown in the greenhouse.Two light environments,non-SH and SH,were included in this experiment with four concentrations of PEG-6000,0,2,4,and 6%.Irradiance level in shade house was 30% for non-shaded conditions.Seeds were grown in two different light environments including non-SH (250±10 mean photosynthetic photon flux densities (PPFD) and red to far red (R:FR) ratio of 1.2) and SH ((75±10) µmol m−2s−1and R:FR ratio of 0.4–0.6) (Asgharet al.2020).The relative humidity in the growth room was strictly maintained at 60%.Seeds were kept at 22–25°C under 12 h light/12 h dark conditions by using sodium lamps((250±10) µmol m–2s–1PPFD) during germination.On the 6th day after sowing,plants were shifted to half-strengthened Hoagland solution.On the 12th day (the vegetative (V1)stage),soybean seedlings were divided into two groups.Non-shade+PEG (0,2,4,and 6%) were applied to soybean seedlings in the first group,and the second group seedlings were treated with shade+PEG (0,2,4,and 6%).The osmotic pressure for the studied 0,2,4,and 6% of PEG was 0,–0.14,–0.36,and–0.66 MPa,respectively (Michel and Kaufmann 1973).Sampling was done on the 4th day of the treatments.There were three biological repeats and each one was accomplished with three experimental repeats.Hereafter,T1,T2,T3,and T4 refer to 0,2,4,and 6% of PEG-6000,respectively.
2.2.Relative water content measurement
The relative water content (RWC) of fully expanded soybean leaves was calculated according to a previously described method (Turner 1981).
2.3.Pigment analyses
Soybean leaves were used for the measurement of chlorophyll pigments.Chlorophyll contents were extracted by grinding samples in 10 mL of 80% acetone and centrifuged at 1 000 r min–1at 4°C for 3 min.Then,these samples were placed in 10 mL of 80% aqueous acetone solution in the darkness at room temperature for 24 h.The supernatant was then separated and analyzed with a spectrophotometer (SpectraMax i3x from Austria) at wavelengths of 663 and 645 nm to compute the chlorophyllaandb,respectively (Hussainet al.2019a).The sampling for chlorophyll pigments analyses was done between 10:00 a.m.and 2:00 p.m.
2.4.Chlorophyll a fluorescence induction determination
Similar to photosynthetic characteristics determination,fully expended leaves of soybean seedlings were used to measure chlorophyllafluorescence induction measurements.The instrument used for those measurements was Technologia FluorImager Software,Technologia LTD (version 2.2.2.2)(Panet al.2017).The leaves were harvested and immediately well-kept in plastic bags that were placed in icebox to avoid direct light.The measurements are as follows:the maximum quantum yield in darkness (Fv/Fm),the maximum quantum yield under light (Fv´/Fm´),nonphotochemical quenching (NPQ),and photochemical quenching (qP).The chlorophyllafluorescence induction parameters were recorded between 10:00 a.m.and 2:00 p.m.
2.5.Gas exchange parameters determination
Fully expended soybean leaves were used for the measurements of photosynthetic characteristics.Among the photosynthetic traits,net photosynthetic rate (Pn),stomatal conductance (gs),transpiration rate (E),and intercellular CO2concentration (Ci) were measured,using the portable photosynthesis system (Model LI-6400,LI-COR Inc.,Lincoln,NE).The equipment settings used were as follows:stomatal ratio=0.5,flow=500 µmol mol–1,PARi=1 000 µmol m−2s–1,and reference CO2concentration=400 µmol mol–1.Aperture size measured for the instrument was about 6 cm2and the calculated leaf temperature was about 26°C.The gas exchange parameters were recorded between 10:00 a.m.and 2:00 p.m.
2.6.H2O2 contents measurement
The H2O2contents were calculated according to previously developed protocol (Velikovaet al.2000),with some modifications.A total of 0.5 g of fresh leaves was homogenized in an ice bath with 5 mL of 0.1% (w/v)trichloroacetic acid (TCA).After that,the homogenate was centrifuged at 12 000 r min–1at 4°C for 20 min.Thereafter,0.5 mL of the supernatant was added to 0.5 mL of 10 mmol L–1potassium phosphate buffer (pH 7.0) and 1 mL of KI.Then the absorbance of supernatant was computed at 390 nm.
2.7.MDA contents determination
The MDA levels of fresh soybean leaves were calculated by previously formulated protocol (Sunet al.2006).For this purpose,0.5 g of leaves was homogenized with 5%TCA followed by centrifugation at 3 000 r min–1for 10 min.Thereafter,2 mL of the supernatants were added into 2 mL of 0.67% TCA and boiled at 100°C for 15 min.After centrifuge at 4 000 r min–1for 10 min,the supernatants were subjected to analysis at 450 nm (OD450),532 nm (OD532) and 600 nm (OD600).The MDA contents were calculated by the following formula:
MDA (µmol L–1)=6.459(OD532–OD600)–0.569OD450
2.8.Antioxidants determination
The SOD,POD and CAT measurements were done with fresh soybean leaves.SOD enzyme determination was done with a prescribed method (Guoet al.2018).A total of 0.5 g of leaf sample was used to measure the SOD enzyme.The sample was ground in 5 mL phosphate-buffered saline(PBS) (0.2 mol L–1Na2HPO4and 0.2 mol L–1NaH2PO4)with the pH of 7.8 and then centrifuged at 8 000 r min–1for 10 min.Thereafter,40 µL of supernatant was added into 2 mL of reaction mixture that included methionine,yellow riboflavin,EDTA-Na2,and nitro-blue tetrazolium chloride(NBT).Later,the samples were kept in high resolution light.Lastly,the absorbance was computed at 560 nm by using the spectrophotometer (SpectraMax i3x,Molecular Devices,Austria).
CAT and POD enzymes were calculated by developed protocol (Yanget al.2008).For POD,0.076 mL guaiacol and a reaction mixture including 200 mL PBS (0.2 mol L–1PBS with pH 6.0) were prepared.Then,the samples (30 µL)were added in 2 mL of reaction mixture.The absorbance of supernatants was computed at 470 nm.
For CAT measurements,another reaction mixture that included 0.3092 mL of H2O2and 200 mL PBS was prepared.Thereafter,2 mL of the reaction mixture was added into 30 µL of sample.Later,the samples were kept at room temperature to complete the chemical reaction.Lastly,the absorbance was computed at 240 nm.
2.9.Free proline measurement
Leaves of soybean were used to determine the free proline contents by a previously developed method (Iqbalet al.2018).A total of 0.20 g of sample was extracted with 5 mL of 3% sulfosalicylic acid.A total of 2 mL of supernatant was added into 3 mL acid ninhydrin and 2 mL glacial acid,followed by boiling in water bath for 40 min.Thereafter,the 5 mL toluene was mixed and finally,samples were taken to compute the absorbance at 520 nm.
2.10.Saccharide contents determination
Leaves (100 mg) were extracted with 6 mL of 80% ethanol to measure saccharide content.The sample then was boiled at 80°C for 40 min,followed by centrifugation at 5 000 r min–1for 5 min.Thereafter,the supernatant was transferred into 50 mL tubes (main solution) to be used for further anslysis.
This practice was repeated twice,followed by addition of 80% ethanol to make it up to 50 mL.Later on,charcoal was added for one night until the color vanished.Then,the supernatant was filtered by filter papers for further use(Asgharet al.2020).
Starch determinationA total of 0.1 g of fresh soybean leaves was used to measure the starch contents.Leaves were extracted with 2 mL of 9.2 mol L–1HClO4and 6 mL of ddH2O was added followed by centrifugation at 3 000 r min–1for 20 min.Supernatants were taken and residues were reacted again with 2 mL of 4.6 mol L–1HClO4.After some time,the 6 mL of ddH2O was added,followed by centrifugation at 3 000 r min–1for 20 min.Supernatants were then taken again and combined.Thereafter,1 mL solution was added into 4 mL of 0.2% sulfate anthrone,followed by boiling for 15 min.Samples were then kept at room temperature for 15 min to normalize the temperature.Then,the samples were subjected to absorbance reading at 625 nm.
SucroseTo measure sucrose contents,0.9 mL extracted solution (the solution which was extracted from 80% ethanol)was added to 0.1 mL of 2 mol L–1NaOH and boiled for 10 min.After cooling down,3 mL of 10 mol L–1HCl and 1 mL of 0.1% resorcinol were added.Samples were then boiled at 80°C for 30 min.Finally,the absorbance was computed at 480 nm.
Soluble sugarsFor the soluble sugar measurement,1 mL extracted solution was added into 4 mL of 0.2% sulfate anthrone.Thereafter,the samples were boiled for 15 min.After cooling down,the absorbance was computed at 480 nm.
Reducing sugarsFrom main solution,1.5 mL was added into 0.5 mL of ddH2O and 1.5 mL 5-(dimethylamino)naphthalene-1-sulfono hydra zide (DNS),followed by boiling at 80°C for 10 min.Finally,the absorbance was computed at 520 nm.
2.11.Statistical analyses of data
Statistix 8.1 (Statistics 8.1.Tallahassee,FL,USA) was used to analyze the data.Treatment effects were measured by using the analysis of variance (ANOVA) test.Furthermore,Microsoft office (version 2010) was executed to draw figures or graphs with standard deviations.
3.Results
3.1.Combined effect of PEG-induced osmotic stress and shade on RWC
Under both light regimes,significant differences were observed in relative water content (RWC) of soybean seedlings (Fig.1).Under the SH treatment,soybean seedlings showed significantly higher RWC than those under non-SH.In comparison to non-shade,the T1,T2,T3,and T4 of shade treatment were improved by 0.55,5.66,10.44,and 11.33%,respectively.The findings demonstrated that the SH treatment had significantly alleviated the harsh effects of PEG-induced osmotic stress on soybean seedlings.
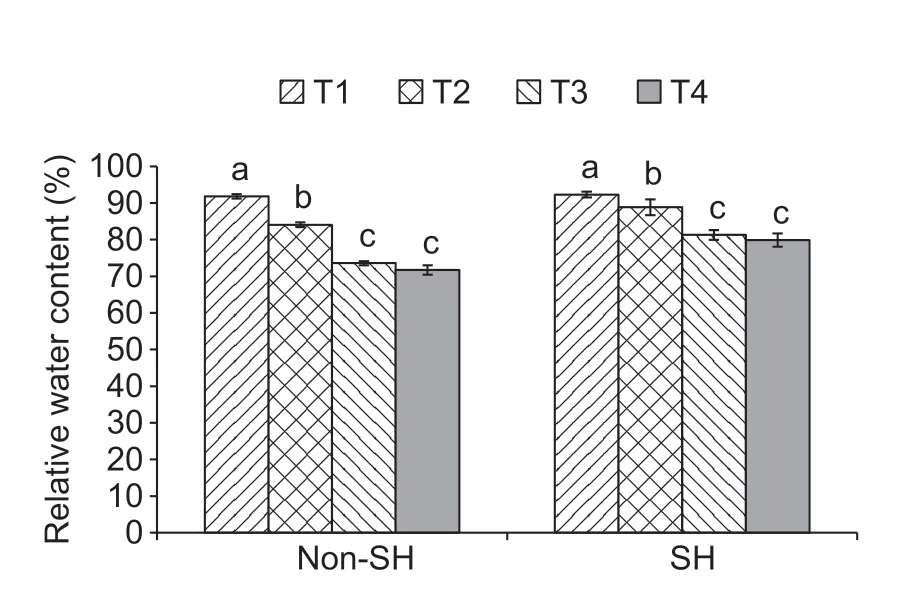
Fig.1 Combined effect of light regimes (non-shade (non-SH)and SH) and PEG-induced osmotic stress on relative water content of soybean seedlings.T1,T2,T3,and T4 refer to 0,2,4,and 6% of PEG,under both non-SH and SH,respectively.Bars mean SD (n=3).Different lowercase letters indicate significant difference (P<0.05) among treatments.
3.2.Interactive effect on chlorophyll contents of soybean
The experiment revealed that PEG-induced osmotic stress significantly affected the chlorophyll (chlorophyllaand chlorophyllb) contents of soybean under both light regimes in all the treatments (Fig.2).The results showed that the interactive effect of light regimes and PEG-induced osmotic stress on chlorophyllaand chlorophyllbwas insignificant.Especially,under SH,chlorophyllacontents were 25.50 and 7.80% lower at T1 and T3,respectively,and 11.71 and 49.47% higher at T2 and T4,respectively,than those under non-SH.A similar trend was observed for chlorophyllbas its contents under SH decreased by 17.84 and 13.45% at T1 and T2,respectively,and increased by 16.19 and 13.34% at T3 and T4,respectively,compared to under non-SH.Collectively,the chlorophyll contents indicated that the chlorophyll pigments were more affected by severe PEG-induced osmotic stress under non-SH than under SH,which suggested that SH enhanced PEG-induced osmotic stress resistance or at least did not impose drastic effects by reserving more chlorophyll pigments.
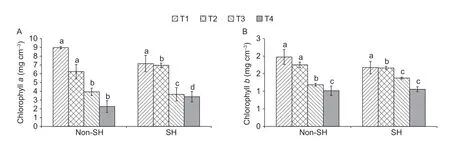
Fig.2 Combined effect of light regimes (non-shade (non-SH) and SH) and PEG-induced osmotic stress on chlorophyll a (A) and chlorophyll b (B).T1,T2,T3,and T4 refer to 0,2,4,and 6% PEG under both non-SH and SH,respectively.Bars are SD (n=3).Different lowercase letters indicate significant difference (P<0.05) between treatments.
3.3.Interactive effect on chlorophyll a fluorescence induction parameters
In this experimentation,PEG-induced osmotic stress significantly influenced the chlorophyllafluorescence induction parameters of soybean seedlings under both light environments (Fig.3).The interactive effect of light regimes and PEG-induced osmotic stress on all the chlorophyllafluorescence induction parameters were computed as insignificant.However,the osmotic treatments showed substantial differences.Fv/Fmand NPQ under SH decreased by 18.64 and 13.68% at T1,respectively,but improved by 1.00 and 13.28% at T2,6.06 and 14.82% at T3,and 5.91 and 8.04% at T4,respectively,compared to under non-SH.Fv´/Fm´ andqPshowed a similar trend as under SH they decreased by 8.13 and 15.5% at T1 and 20.75 and 11.02% at T2,respectively,but increased by 5.76 and 10.10% at T3,and 31.57 and 39.51% at T4,respectively,compared to under non-SH.Cumulative results about chlorophyllafluorescence induction parameters demonstrated that the shade treatment was able to combat the harsh consequences of PEG-induced osmotic stress or at least did not enhance the adverse effects of the stress.
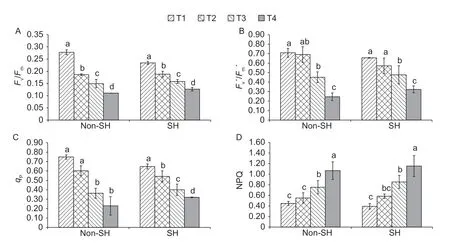
Fig.3 Combined effect of light regimes (non-shade (non-SH) and SH) and PEG-induced osmotic stress on photochemical parameters.T1,T2,T3,and T4 refer to 0,2,4,and 6% PEG under both non-SH and SH,respectively.Fv/Fm (A),Fv´/Fm´ (B),qP (C),and non-photochemical quenching (NPQ) (D) represent the maximum quantum yield in darkness,the maximum quantum yield in light,photochemical quenching,and non-photochemical quenching,respectively.Bars are SD (n=3).Different lowercase letters indicate a significant difference (P<0.05) among treatments.
3.4.Interactive effect on photosynthetic apparatus of soybean
The photosynthetic characteristics in soybean leaves were largely affected by the combined effect of PEG-induced osmotic stress and shade (Fig.4).Photosynthesis related parameters showed an exciting behavior,as light treatments were computed as insignificant underPnandCi.However,their interactive effect was found significant undergsandE.The same tendency was observed underPnandEas those under non-SH decreased by 9.49 and 4.47% at T1,respectively,and increased by 2.07 and 12.53% at T2,16.52 and 30.13% at T3,and 11.75 and 28.79% at T4,respectively,compared to those under SH.Moreover,ings,all the four treatments of SH were recorded higher than non-SH,and the highest improvement (21.35%) was shown at T4.An interesting trend was shown byCi;in contrast to non-SH,it decreased by 0.55 and 2.02% at T1 and T3,respecrively,but increased by 16.66% at T4,under SH.Overall,results of the gas exchange parameters showed that the SH treatment performed better than non-SH,which suggested that SH successfully mitigated the drastic effects of PEG-induced osmotic stress or at least did not exaggerate the stress.
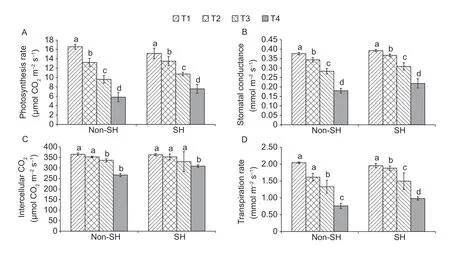
Fig.4 Combined effect of light regimes (non-shade (non-SH) and SH) and PEG-induced osmotic stress on gas exchange parameters,i.e.,photosynthetic rate (A),stomatal conductance (B),intercellular CO2 (C),and transpiration rate (D).T1,T2,T3,and T4 refer to 0,2,4,and 6% PEG under both non-SH and SH,respectively.Bars are SD (n=3).Different lowercase letters indicate a significant difference (P<0.05) among treatments.
3.5.Interactive effect on MDA and H2O2
This study examined the levels of MDA and H2O2under the interactive effect of PEG-induced osmotic stress and shade(Fig.5).PEG-induced osmotic stress had progressively enhanced the levels of MDA and H2O2in soybean seedlings.The overall MDA levels were significantly higher under non-SH treatment than under SH.Moreover,H2O2levels were computed as insignificant under different light conditions.The outcomes disclosed that under non-SH,MDA decreased by 3.14% at T1 but increased by 59.23,37.62 and 19.09%at T2,T3 and T4,respectively,as compared to under SH.Moreover,H2O2under non-SH increased by 0.68 and 18.84% at T2 and T4,respectively,but decreased by 2.53 and 46.11% at T1 and T3,respectively,as compared to under SH.These results suggested that the SH treatment boosted the PEG-induced osmotic stress resistance in soybean seedlings.
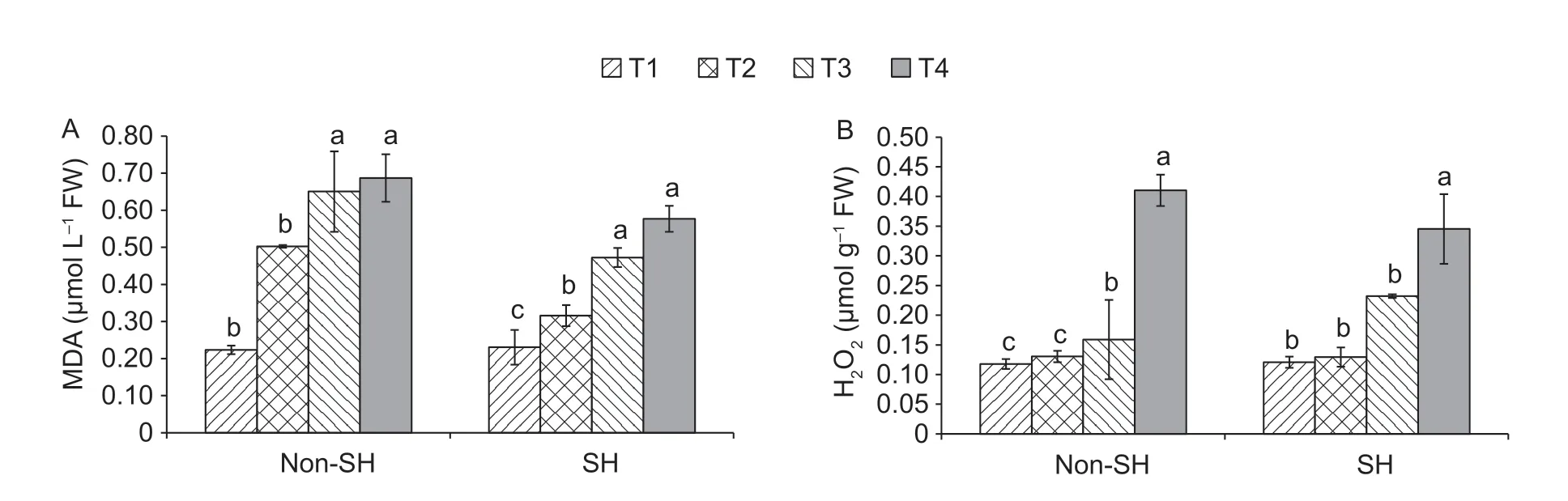
Fig.5 Combined effect of light regimes (non-shade (non-SH) and SH) and PEG-induced osmotic stress on malondialdehyde (MDA)(A) and hydrogen peroxide (H2O2) (B) of soybean seedlings.T1,T2,T3,and T4 refer to 0,2,4,and 6% PEG under both non-SH and SH,respectively.Bars mean SD (n=3).Different lowercase letters indicate significant difference (P<0.05) among treatments.
3.6.Interactive effect on proline contents
Under stressful environments,plants accumulate free proline.In this study,soybean seedlings exhibited significant differences in free proline contents (Fig.6).The free proline contents were noticed significantly higher under SH than under non-SH.Under SH,the free proline contents were higher,increasing by 40.46,28.42 and 22.02% at T1,T3 and T4,respectively,but decreased by 3.29% at T2,compared to under non-SH.These findings concluded that SH boosted up the PEG-induced osmotic stress resistance of soybean by accumulating more free proline contents as compared to under non-SH treatment in severe stressful conditions.
3.7.Interactive effect on enzymatic activities
Plants mobilize antioxidant defense system to eliminate ROS.Major enzymes,which scavenge ROS,are SOD,POD and CAT.In this experimentation,PEG-induced osmotic stress had regressively enhanced the SOD,POD and CAT levels under both light environments (Fig.6).Moreover,the interactive effect of SH and non-SH on SOD,POD and CAT was computed as insignificant.However,significant differences could be found in osmotic treatments.For instance,from non-SH to SH,SOD,POD and CAT decreased by 33.06,30.81 and 6.66% at T1 and 8.47,17.00 and 14.61% at T2,respectively,but increased by 38.56,2.66 and 5.37% at T3 and 26.12,27.24 and 28.72%at T4,respectively.Largely,the results of antioxidants strengthened the hypothesis of this study that shade could enhance the PEG-induced osmotic stress resistance of soybean seedlings or at least did not exaggerate the severe effects of the stress.
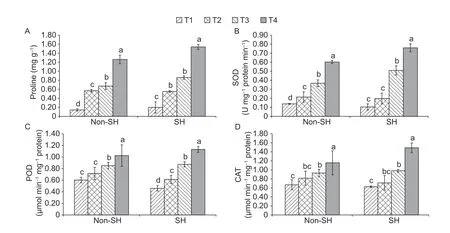
Fig.6 Combined effect of light regimes (non-shade (non-SH) and SH) and PEG-induced osmotic on free proline (A),superoxide dismutase (SOD) (B),peroxidase (POD) (C) and catalase (CAT) (D).T1,T2,T3,and T4 refer to 0,2,4,and 6% PEG under both non-SH and SH,respectively.Bars mean SD (n=3).Different lowercase letters indicate significant difference (P<0.05) among treatments.
3.8.Interactive effect on the saccharide contents
Both environmental cues have greatly influenced the saccharide contents of soybean under both light environments.Among the saccharide contents,sucrose,starch,reducing sugars and soluble sugars were explored in this study (Fig.7).The light environments showed significant differences in starch,sucrose and soluble sugars.However,an insignificant effect was found in the reducing sugars.Under SH,the starch level diminished by 29.41 and 18.69% at T1 and T2,respectively,whilst it increased by 1.07 and 45.58% at T3 and T4,respectively,compared to under non-SH.Sucrose and soluble sugars showed an interesting trend as they were higher under SH than under non-SH,increasing by 1.66 and 57.33% at T1,20.65 and 42.47% at T2,6.33 and 35.27% at T3,and 11.88 and 19.86% at T4,respectively.Moreover,the level of reducing sugars was recorded lower under SH than under non-SH at T1,decreasing by 7.59%,while at T2,T3 and T4,there was an inverse trend,with it under SH being 0.85,21.20 and 17.50% higher than under non-SH,respectively.The findings displayed more accumulation of sugars but less reduction of starch and sucrose were observed under SH than under non-SH under severe stressed conditions.These results suggested that the SH treatment improved the PEG-induced osmotic stress resistance in soybean by protecting the cells from rupture in the scenario of severe water unavailability.
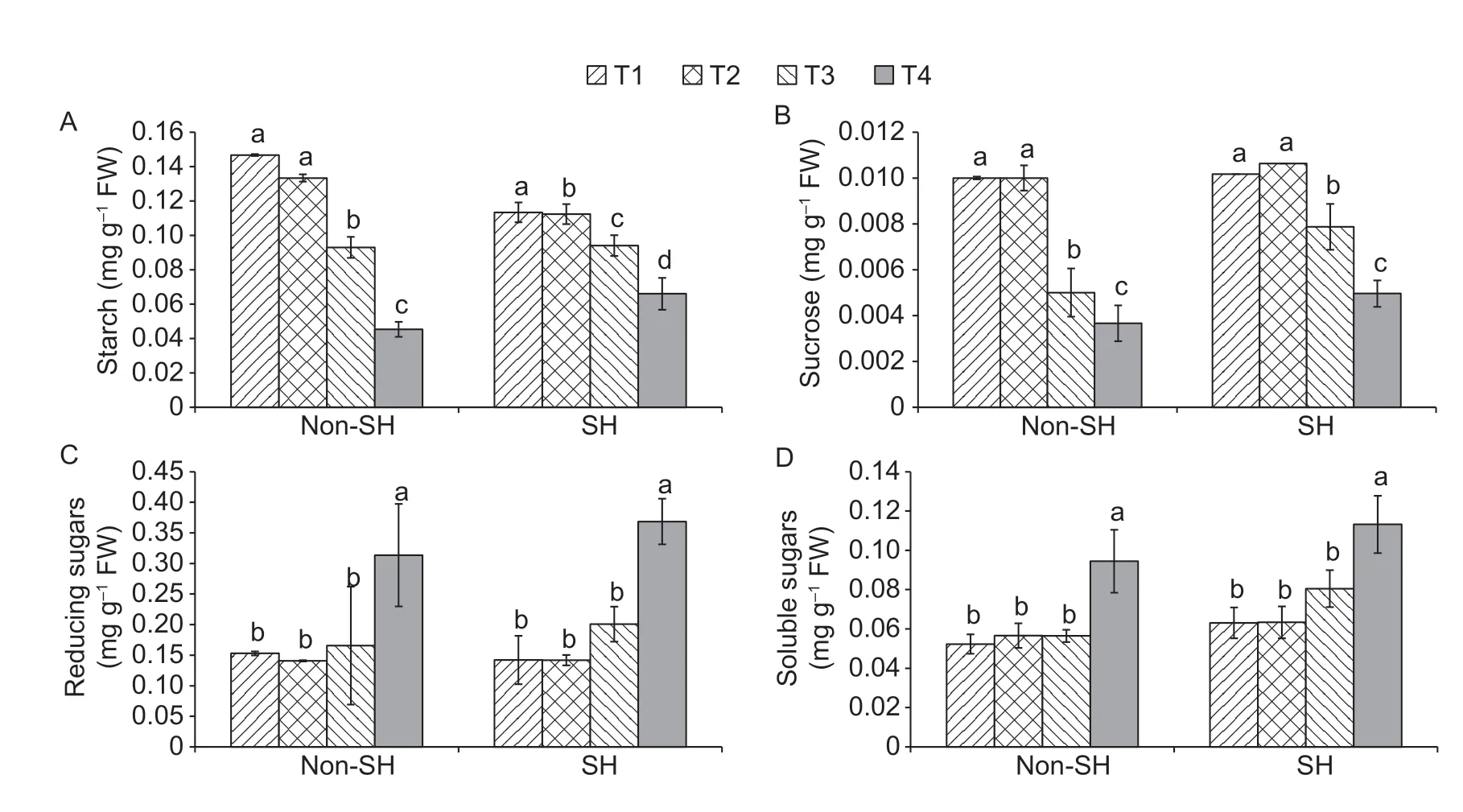
Fig.7 Combined effect of light regimes (non-shade (non-SH) and SH) and PEG-induced osmotic stress on saccharide contents(starch (A),sucrose (B),reducing sugars (C) and soluble sugars (D)).T1,T2,T3 and T4 refer to 0,2,4,and 6% PEG under both non-SH and SH and,respectively.Bars are SD (n=3).Different lowercase letters indicate significant difference (P<0.05) among treatments.
4.Discussion
4.1.Combined effect of shade and PEG-induced osmotic stress on RWC and photosynthetic attributes of soybean
Measurements of RWC in leaf tissues are commonly used to assess plants’ water status.In this study,PEG-induced osmotic stress significantly reduced RWC under both light regimes.More specifically,lower RWC was linked to non-shaded plants than to shaded ones (Fig.1).These outcomes are in accordance with the theory that less water is required by plants grown under shading conditions than those exposed to full-light environments (Holmgren 2000;Liet al.2011).Moreover,in the experimentation,the chlorophyll contents (chlorophyllaandb) were considerably reduced by PEG-induced osmotic stress.Similar findings have also been reported by previous researchers(Naderikharajiet al.2008;Abbaspour and Rezaei 2014).Lower values of chlorophyll were observed under SH than under non-SH (Fig.2).Chlorophyll reduction under the PEG-induced osmotic stress conditions might be due to chlorophyll degradation which eventually led to damaged photosynthesis activity.Decline in chlorophyll contents is an indication of oxidative stress in plants,which has been confirmed by previous studies (Guneset al.2008;Masoumiet al.2010).
Chlorophyllafluorescence induction is one of the key factors in photosynthetic regulation and plant responses to environmental conditions (Li and Kakubari 2001;Daiet al.2009).Former study concluded that the decline in plant development under drought was attributed to reduced energy absorbed by leaves and subsequently translocated to PSII (Rahbarianet al.2011).Similar findings have been revealed in current investigation.This study elucidated thatFv/Fm,Fv´/Fm´ andqPsignificantly diminished as PEGinduced osmotic stress was exaggerated (Fig.3).However,this inhibition was much weaker in shade-grown plants than in non-shaded ones under severely stressful conditions.Surprisingly,NPQ significantly increased by PEG-induced osmotic stress in both light environments and this increment was higher in shade-treated plants than non-shaded ones,indicating the increment of thermal energy dissipation at PSII.These results are in consistent with the previous study of Correiaet al.(2006).
It was reported that reduction in photosynthesis caused declines in crop productivity,and decreases inPnwas related to the reduction ofgsand intercellular CO2concentration in water deficit conditions (Chaveset al.2009).The findings of the present study are also consistent with a previous study,wherePn,gs,E,andCiwere significantly reduced under osmotic stress conditions (Duanet al.2009).The inhibition ofPn,gs,E,andCiunder osmotic stress may be attributed to stomatal closure,although direct effects on several biochemical and photochemical processes have also been reported (Cornic 2000;Ohashiet al.2006).Additionally,the reduction in all the studied photosynthetic characters was smaller in shaded plants than in non-shaded ones.This suggested that non-shaded plants were more susceptible to photo-inhibition than shaded ones,and that the overall photosynthetic activity was severely affected in non-SH plants (Fig.4).The reason behind this phenomenon could be that there was a greater amplification in ABA contents in shade-treated plants than non-shaded ones,which controlled stomatal modulation and eventually directed to the improved photosynthetic machinery (Kurepinet al.2007a,b;Asgharet al.2020).
4.2.Interactive effect on ROS and antioxidants
To cope with climatic strains,ROS production is exponentially enhanced,performing multiple functions within the plant cells and leading to oxidative damage (Carvalho 2008;Suzukiet al.2012;Baxteret al.2013).In this investigation,H2O2and MDA greatly increased as the PEG-induced osmotic stress was enhanced under both light environments,indicating the pervasiveness of oxidative damage.More explicitly,higher values of MDA and H2O2were noticed under the non-shaded treatment than the shaded one in severely stressful conditions,which implied that the abovediscussed damage was less under the SH treatment than under non-SH (Fig.5).This finding is supported by a former study (Huanget al.2008).The reason may be that exogenous auxin production is enhanced under shaded conditions,which is keenly involved in scavenging of ROS(Shiet al.2014a).
Under stressful environments,plants usually do mobilize their defense system that is responsible for the detoxification of ROS.The stress amelioration defense system includes SOD,CAT and POD enzymes (Yanget al.2008).In this study,PEG-induced osmotic stress greatly increased the activities of the above-mentioned enzymes (SOD,POD and CAT) under both light treatments.It is also very important to note that the enzyme activities were ominously improved under the SH treatment as compared to non-SH(Fig.6).This might be due to the increased auxin level that immensely improved the antioxidant enzyme activity in shaded plants as it was principally engaged in abolition of plants’ ROS (Shiet al.2014).These results are in accordance with a previously published investigation (Duanet al.2005).However,there still exists a vast information gap that is how the plant defense system regulates the said mechanisms when plants are simultaneously exposed to multiple stresses.
4.3.Interactive effect on saccharide and proline contents
Under drastic climatic conditions,it is widely known that osmoprotectants are engaged in osmotic regulations of plants (Djilianovet al.2005;Le and McQueen-Mason 2006;Hossain and Fujita 2010;Filippouet al.2014;Singhet al.2015).More specifically,proline is considered to be one of the most important osmoticums and also acts as a compatible solute in a number of cellular processes(Mohammadkhani and Heidari 2008).Previous studies have also revealed that osmotic stress induces a massive proliferation in the accumulation of proline contents in soybean (Iqbalet al.2018).In the current investigation,the PEG-induced osmotic stress significantly enhanced the proline under both light environments.However,higher levels of proline were observed in plants grown under shaded conditions as compared to non-shaded ones(Fig.6),indicating that shaded plants were proficiently able to safeguard their cells from impairment.
It was reported that there was an increment in sugar accumulation when plants were exposed to abiotic stresses to fight against damaging effects (Pradoet al.2000).In accordance with previous studies on osmotic stresses(Pradoet al.2000;Mohammadkhani and Heidari 2008),this study also elucidated that PEG-induced osmotic stress progressively enhanced the sugar levels (soluble and reducing sugars) under both the studied light environments.However,this increment was more in shaded plants than in non-shaded ones (Fig.7).There could be two possible elucidations of sugar accumulation in shaded plants.First,it was due to the enrichment in ABA biosynthetic and signaling genes in shaded plants (Kurepinet al.2007a;Kohnenet al.2016).Secondly,it might be due to the increment of auxin levels.
Under fluctuating environmental conditions,plants remobilize starch and sucrose to work as osmoprotectants to alleviate the harsh effects of water deficit conditions (Gupta and Kaur 2005;Krasensky and Jonak 2012).In the present research,PEG-induced osmotic stress had negative effects on starch and sucrose contents as both of them decreased as the PEG-induced osmotic stress was amplified under both light conditions (Fig.7).Precisely,their inhibition was much less in shaded plants than in non-shaded ones,which specified that shaded ones accumulated profusely of reserves to efficiently operate the carbon and energy succession for the maintenance of normal plant growth.This might be due to the fact that PEG-induced osmotic stress diminished the activities of sucrose phosphate synthase,suggesting that the degree of sucrose synthesis was sturdily influenced by water deficit conditions (Haupt-Herting and Fock 2002;Xinget al.2018).
5.Conclusion
The current study demonstrated that shade treatment improved the PEG-induced osmotic stress resistance in soybean seedlings primarily by amplifying the antioxidant defense system and osmoprotectants which ultimately control the physiological modulations in response to combined stresses.Moreover,PEG-induced osmotic stress significantly inhibited the relative water contents,photosynthetic characteristics,chlorophyll contents,photochemical characters,and ROS of soybean seedlings.Apparent improvements could be made in response to PEGinduced osmotic stress by shading conditions in soybean seedlings as indicated by enhanced RWC,chlorophyll contents,photosynthetic characteristics,photochemical traits,ROS,osmoprotectants,and antioxidant enzymes functioning.The key findings of the current study suggested that shade could be helpful for soybean seedlings to reduce the severe consequences of PEG-induced osmotic stress or at least did not exaggerate PEG-induced osmotic stress’ negative effects.However,it fully depended upon the degree of both applied stresses.Lastly,deliberation of these findings would be helpful to future studies on how to manipulate these variations to increase yield by increasing the survival of soybean seedlings in stressful environmental conditions under the maize–soybean relay strip intercropping system.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31871552 and 31671445),the Sichuan Science and Technology Program,China (2018HH0108)and the Sichuan Innovation Team Project of National Modern Agricultural Industry Technology System,China(sccxtd-2020-20).
Declaration of competing interest
The authors declare that they have no conflict of interest.
杂志排行
Journal of Integrative Agriculture的其它文章
- Molecular characteristics and structure–activity relationships of food-derived bioactive peptides
- lntegrating the physical and genetic map of bread wheat facilitates the detection of chromosomal rearrangements
- Physiological response of flag leaf and yield formation of winter wheat under different spring restrictive irrigation regimes in the Haihe Plain,China
- Variation of carbon partitioning in newly expanded maize leaves and plant adaptive growth under extended darkness
- Effects of plant density and mepiquat chloride application on cotton boll setting in wheat–cotton double cropping system
- Does heat accumulation alter crop phenology,fibre yield and fibre properties of sunnhemp (Crotalaria juncea L.) genotypes with changing seasons?
