Variation of carbon partitioning in newly expanded maize leaves and plant adaptive growth under extended darkness
2021-07-24LIANGXiaoguiSHENSiGAOZhenZHANGLiZHAOXueZHOUShunli
LIANG Xiao-gui,SHEN Si,GAO Zhen,ZHANG Li,ZHAO Xue,ZHOU Shun-li,3,4
1 College of Agronomy and Biotechnology,China Agricultural University,Beijing 100193,P.R.China
2 School of Agricultural Sciences,Jiangxi Agricultural University,Nanchang 330045,P.R.China
3 Scientific Observation and Experimental Station of Crop High Efficient Use of Water in Wuqiao,Ministry of Agriculture and Rural Affairs,Wuqiao 061802,P.R.China
4 Innovation Center of Agricultural Technology for Lowland Plain of Hebei,Wuqiao 061802,P.R.China
Abstract Plants must maintain a balance between their carbon (C) supply and utilization during the day–night cycle for continuous growth since C starvation often causes irreversible damage to crop production.It is not well known how C fixation and allocation in the leaves of crops such as maize adapt to sudden environmental changes.Here,to quantify primary C fixation and partitioning in photosynthetic maize leaves under extended darkness and to relate these factors to plant growth,maize seedlings were subjected to extended darkness (ED) for three successive days at the 6th leaf fully expanded stage (V6).ED reduced plant growth and leaf chlorophyll levels but not the rate of net CO2 exchange.As a result of the reduction in photoassimilates,the accumulation of starch and total soluble carbohydrates (TSC) in mature leaves also decreased under ED.However,the percentage of the daily C fixation reserved in mature leaves increased.These transient C pools were largely composed of TSC and were mainly used for consumption by increased nocturnal respiration rather than for transport.As the days went on,both the amount of C accumulated and the percentage of the daily fixed C that was reserved in leaves decreased,which could be largely accounted for by the attenuated starch synthesis in all treatments.The activities of ADPglucose pyrophosphorylase and soluble starch synthase decreased significantly over time.Therefore,this study concluded that both starch and TSC are involved in the coordination of the C supply and plant growth under a sudden C shortage but that they may be involved in different ways.While the ratio of reserved C to daily fixed C increased to maintain blade function under acute C starvation,both the amount and the proportion of C reserved in mature leaves decreased as plant growth continued in order to meet the growth demands of the plant.
Keywords:maize,starch,total soluble carbohydrates,carbon allocation,extended darkness,ongoing growth
1.Introduction
Plants must coordinate carbon (C) availability with their growth during the day–night cycle,as photosynthesis only occurs in the light while plants grow continuously (Smith and Stitt 2007).Hence,in addition to C that is directly transported as sucrose,considerable amounts of fixed C are retained in leaves,mainly as nonstructural carbohydrates(NSC),for nocturnal respiration and continued export to C sinks (Fig.1;Stitt and Zeeman 2012;Ruan 2014).These reserved sugars provide a buffer for plants,maintaining continuous growth and preventing acute “C starvation” in fluctuating environments.A temporary imbalance between the C supply and utilization could cause dramatic damage to plant growth and development.For example,inArabidopsisand tobacco,the starchlesspgmmutant is unable to survive under short light periods due to the rapid depletion of sugars early in the night and later growth inhibition (Fernieet al.2002;Gibonet al.2004).In maize,an imbalance between C supply and depletion from source to sink is supposed to directly induce kernel abortion in response to a sudden episode of heat or drought stress (McLaughlin and Boyer 2004;Shenet al.2019).
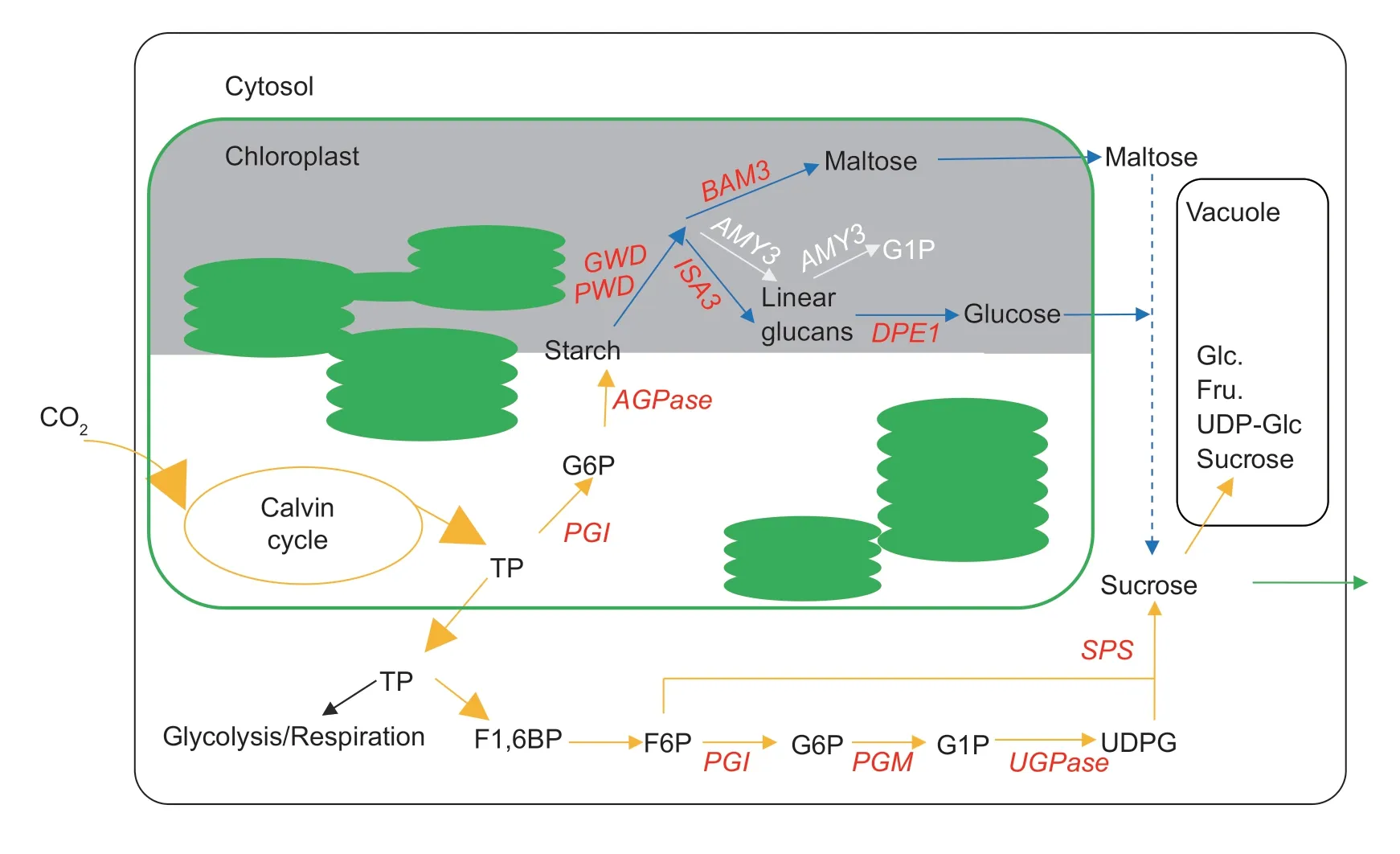
Fig.1 Schematic of primary carbon partitioning in plant leaves over 24 h.The white and grey fills represent light and dark periods,respectively.TP,triose phosphate;F1,6BP,fructose 1,6-bisphosphate;F6P,fructose 6-phosphate;G6P,glucose 6-phosphate;G1P,glucose 1-phosphate;UDPG,uridine diphosphate glucose.PGI,phosphoglucose isomerase;AGPase,adenosine diphosphate glucose pyrophosphorylase;PWD,phosphoglucan-water dikinase;GWD,glucan-water dikinase;BAM3,β-amylase 3;ISA3,isoamylase 3;AMY3,α-amylase 3;DPE1,disproportionating enzyme 1;PGM,phosphoglucomutase;UGPase,UDPG pyrophosphorylase;SPS,sucrose phosphate synthase.Fru.,fructose;Glc.,glucose.
For many plants such asArabidopsisand soybean,starch is considered as a major integrator in the regulation of plant growth and maintenance (Chatterton and Silvius 1979;Sulpiceet al.2009;Stitt and Zeeman 2012).Up to 50% or more of starch accumulates in theArabidopsisrosette during the day,and this starch is almost completely consumed during the night (Gibonet al.2004;Stitt and Smith 2007).In addition,the shorter the light period is,the faster starch is synthesized in the light and the slower it degrades at night (Mugfordet al.2014;Sulpiceet al.2014).This highlights the precisely controlled turnover of starch as a diurnal transport buffer and a nocturnal energy supplier.By using differentArabidopsisaccessions,researchers found that starch accumulation is negatively correlated with biomass accumulation (Crosset al.2006;Sulpiceet al.2009).Lianget al.(2019) also reported a similar but not identical relationship for maize hybrids,i.e.,the ratio of the starch reserved to the daily fixed C,rather than the starch amount,in leaves correlated well with maize yield and final biomass.Crops have evolved to increase sink demands due to selection pressure.In addition,the form of carbohydrate storage can be used to classify leaves into three types:(i)sugar leaves,such as wheat and rice,(ii) starch leaves,such asArabidopsisand soybean,and (iii) intermediate leaves,such as maize and sorghum (Xiaet al.1989;Smith and Stitt 2007).These differences could possibly lead to differing strategies for C balance coordination under changing environments among different species,as mentioned above.Currently,little is known about the diurnal C turnover and growth responses to environmental changes in crops such as maize.
As a major C4crop,maize is one of the world’s most important cereal crops and has the largest yield in China;it provides a staple food,animal feed and industrial material(FAO 2017).The character of carbohydrates in maize leaves is intermediate,i.e.,amounts of both starch and total soluble carbohydrate (TSC) turnover occurs during the day–night cycle (Kalt-Torres and Huber 1987;Henryet al.2014;Lianget al.2019).Significant works on the spatial analyses of C availability and growth have been carried out by using specific unexpanded leaves classified into four zones,i.e.,cell division,transition,expansion,and maturity (Wanget al.2014;Czedik-Eysenberget al.2016).Considerable differences in C turnover among the different zones were found under control and extended darkness treatments,mainly due to developmental diversity.However,the quantification of primary C fixation and the ratios of C allocation into transient starch,TSC and transport are not well studied in maize crops,particularly under environmental stress,such as extended darkness.Lianget al.(2019)established a C-balance model for photosynthetic maize leaves and examined the differences in NSC storage among hybrids under optimal field conditions.The model has a good quantitative ability and could be used to further explore how maize crops coordinate the C balance and plant growth under different environments.
To investigate the gradual responses of primary C fixation and partitioning in photosynthetic leaves to sudden C starvation and to relate C turnover to successive ontogeny and ongoing growth,maize seedlings were treated with extended darkness,and growth,photosynthesis and NSC storage traits in the leaves were investigated over three days.Based on the C-balance model,the daily C fixation and primary partitioning were calculated for specific leaves.It was assumed that while the percentage of the daily fixed C reserved in leaves might change with prolonged darkness and ongoing growth,both TSC and starch could play important roles during these processes.
2.Materials and methods
2.1.Plant cultivation and sampling
A commercial maize hybrid,Zhengdan 958,which is widely used in China,was grown in a phytotron at China Agricultural University.Sand-cultivated seedlings were screened at the second leaf fully expanded stage.Plants were transferred into 1/2 concentrated Hoagland solution (pH 6.0) for 3 d(Yanget al.2018).Then,the solution was changed to full strength and refreshed every 3–4 d to maintain a sufficient nutrient supply.Hydroponic plants were grown at (350±50)µmol m−2s−1light intensity,with 14 h light/10 h dark cycles(14/10),25°C/20°C.Air humidity was maintained at (70±5)%by a moistener,and the carbon dioxide concentration was kept at atmospheric level by ventilation.An air pump was used for oxygen support for maize roots.These conditions provided similar rates of leaf expansion to those of plants grown in the field (approximately 3–4 d per fully expanded leaf).
To achieve effective C starvation in a typical growth period,extended darkness (ED) was applied just after the 6th leaf fully expanded for 4 and 8 h at the end of the dark period,i.e.,10-h/14-h (10/14) and 6-h/18-h (6/18) light/dark cycles,respectively.The 14/10 ratio was set as the control.With four biological replicates,samples of the 6th expanded leaves were collected as a representative source every 4 h and when the lights turned on and off for 3 d (Fig.2-A).Whole plants were sampled to determine shoot dry weight(DW) at the beginning (b) and end (e) of the ED treatments.Leaf area was measured manually using the same plants as for the DW determination.The plant relative growth rate(RGR) was calculated based on DW as follows:
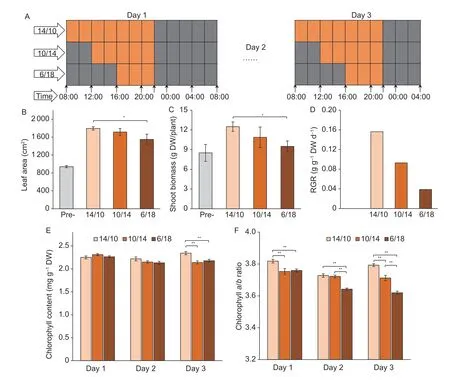
Fig.2 Illustrations showing the treatments (A) and the effects on maize growth (B–F) at the 6th leaf fully expanded stage (V6).A,3 d of extended darkness for 4 h (10/14) and 8 h (6/18);14/10 was the control.Each grid represents 2 h,and the orange and grey fills represent light and darkness,respectively.B,leaf area.C,shoot biomass accumulation,calculated on a dry weight (DW)basis.D,relative growth rate (RGR),calculated by the increase in shoot biomass.E,dynamic change in chlorophyll content.F,dynamic change in chlorophyll a/b ratio.Pre-,data before extended darkness.The light,medium and deep orange fills indicate 14-h/10-h (14/10),10-h/14-h (10/14) and 6-h/18-h (6/18) light/dark cycles,respectively.* and ** indicate significant differences at P<0.05 and P<0.01,respectively.Bars mean SE (n=4).
2.2.Gas exchange,chlorophyll and fast fluorescence
Diurnal gas exchange,i.e.,net photosynthesis during the light period and leaf respiration during the dark period was measured every 4 h using a Li-Cor 6400 (Li-Cor Biosciences,Lincoln,Nebraska,USA) with the settings of flow,250 µmol s–1;Ref CO2,400 µmol mol–1;Tblock,20°C;relative humidity,set as default;300 µmol m–2s–1photosynthetic photon flux density (PPFD) in the light and zero in the dark according to previous studies (Lianget al.2019;Shenet al.2020).The chlorophyll content and chlorophylla/bratio for the uppermost expanded leaves were determined each day using the ethanol extraction method (Lichtenthaler and Wellburn 1983;Lianget al.2018).For fast fluorescence,according to Sayed (2003),leaves were incubated in darkness using clips for 30 min.Then,fast chlorophyll fluorescence was measured by a Hansatech fluorimeter(Handy-PEA,Hansatech Instruments Ltd.,Kings’ Lynn,Norfolk,USA) with saturating pulses set as the default(650 nm,up to 3 500 µmol m–2s–1) during the daytime.The minimum fluorescence intensity (Fo),maximal fluorescence intensity (Fm) and maximal dark-adapted PSII yield (Fv/Fm,Fv=Fm–Fo) were obtained.For measurements of both gas exchange and fast fluorescence,no significant differences were found within time points (the theoretical morning and afternoon) because of the relatively constant environmental light and temperature in the phytotron.Hence,the data were averaged for each light and/or dark period and are shown in the results.
2.3.Nonstructural carbohydrate and enzyme activities
Starch and TSC were determined by the anthrone-sulfuric acid method with several modifications (Hansen and Møller 1975;Zhanget al.2017;Wanget al.2019).The sampled leaves were immediately dried at 75°C for 48 h to constant weight and were well powdered and weighed to 50 mg in a centrifuge tube.The tube with distilled water was incubated in boiling water three times,and the supernatant,i.e.,the TSC sample,was collected into a volumetric flask to a constant volume.The starch in the pellet was digested into glucose by a diluted hydrochloric acid solution incubated in boiling water and neutralized with an equal amount of sodium hydroxide.Starch and TSC were measured as glucose equivalents using a spectrophotometer (TU-1901,PRESEE,Beijing,China) at 625 nm.ADP-glucose pyrophosphorylase (AGPase) and soluble starch synthase(SSS) activities were determined according to Nakamuraet al.(1989) with several modifications.Fresh leaf blades(~0.5 g) were homogenized with a pestle in an ice-cold mortar containing 5 mL of 50 mmol L–1HEPES-NaOH (pH 7.5),5 mmol L–1MgCl2,1 mmol L–1EDTANa2,4 mmol L–1DTT,3% (v/v) glycol,and 1% BSA.The homogenate was centrifuged at 12 000 r min–1for 8 min,and the supernatant was used for crude enzyme extraction.For AGPase,200 µL of sample was conducted in 450 µL of solution I (50 mmol L–1HEPES-NaOH,5 mmol L–1MgCl2,4 mmol L–1DTT,and 3 mmol L–1ADPG).The reaction was incubated at 30°C for 30 min and terminated by placing in boiling water for 1 min.The resulting solution was cooled and mixed with 100 µL of 12 mmol L–1NADPNa2,and the AGPase activity was assayed by the increase in absorbance at 340 nm after adding 1 µL each of phosphoglucomutase (0.4 U) and G6P dehydrogenase (0.4 U) for 30 min.For SSS,the enzyme sample was incubated with solution II (50 mmol L–1HEPESNaOH,1 mg amylopectin,15 mmol L–1DTT and 3 mmol L–1ADPG) at 30°C for 15 min and inactivated by boiling water for 1 min.Then,300 µL of solution III (50 mmol L–1HEPESNaOH,8 mmol L–1PEP,200 mmol L–1KC1,10 mmol L–1MgCl2,pyruvate kinase (1.5 U)) was added to the mixture,incubated at 30°C for 20 min and heated in boiling water for 1 min.The reaction solution was then mixed with solution IV(50 mmol L–1HEPES-NaOH,20 mmol L–1glucose,15 mmol L–1MgCl2,and 3 mmol L–1NADPNa2).The SSS activity was measured in the same way as AGPase after adding 1 µL of hexokinase (2.8 U) and G6P dehydrogenase (0.7 U).
2.4.Primary carbon allocation and sugar turnover
According to previous reports inArabidopsis(Sulpiceet al.2014;Pilkingtonet al.2015),a C-balance model was established to quantify the primary C fixation and partitioning for photosynthetic maize leaves (Lianget al.2019).In this model,photoassimilates,respiration and sugars are translated into moles of C for uniform calculation.This model could apply to both C3and C4crops due to the law of conservation of C mass.The formulas are:
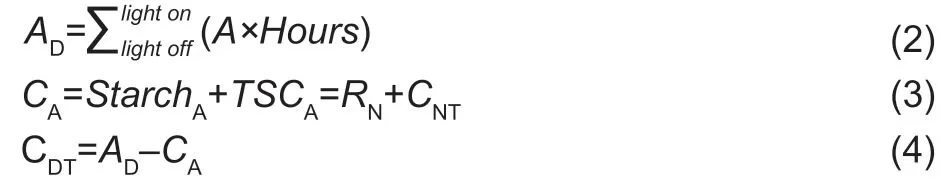
whereAD,the amount of net daily carbon fixation;A,the measured photosynthesis rate;CA,the C accumulation in leaves estimated by the diurnal NSC pool,i.e.,the sum of starch and TSC accumulation (STAA,TSCA) due to the relatively stable protein and organic acid contents in mature leaves (Sulpiceet al.2014);CA,the C metabolized for nocturnal respiration (RN) and nocturnal C transport (CNT);CDT,the daily C transport from the measured leaves.
2.5.Statistical analysis
All calculations were conducted using Microsoft Excel 2016 and IBM SPSS Statistics 20.
3.Results
3.1.Biomass,leaf area and chlorophyll
Extended darkness (ED) resulted in decreased leaf expansion and biomass accumulation (Fig.2-B and C).The relative growth rate (RGR),as calculated on a dry weight basis,also decreased linearly (Fig.2-D).Compared to that under 14/10,the RGR decreased by 40 and 75% under 10/14 and 6/18,respectively.During the 3-d prolonged darkness,the chlorophyll content in the mature leaves gradually decreased and showed significant differences on the 3rd d,while the ratios of chlorophylla/bsignificantly decreased starting on the first day of the ED treatment(Fig.2-E and F).
3.2.Gas exchange and fast chlorophyll fluorescence
Both net photosynthesis and nocturnal respiration were similar among the 14/10,10/14 and 6/18 treatments(Fig.3-A).No significant differences (P<0.05) were found.Nocturnal respiration ranged from–0.88 to–1.20 µmol CO2m–2s–1,which was 7.5–10.3% of the rate of net photosynthesis.Diurnal C fixation and nocturnal costs in the newly expanded leaves were calculated by multiplying the CO2exchanges with the corresponding time span(Fig.3-B).TheADdecreased while theRNincreased under ED.There was an approximately 2.5-fold reduction inADand a 2-fold increase inRNfor leaves under 6/18 compared with those under 14/10.There was no evidence of differences among treatments inFo,FmandFv/Fm,in the 3 d observation(Fig.3-C–E).
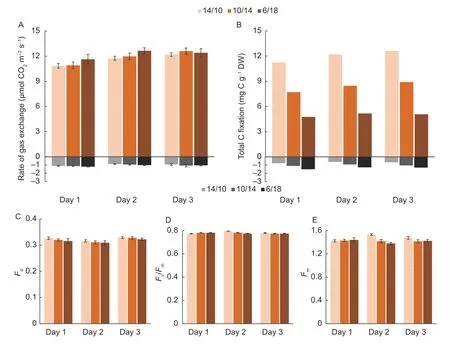
Fig.3 Dynamic changes in fast fluorescence and CO2 exchange in the newly expanded leaves.A,the rates of net photosynthesis in the daytime (orange) and nocturnal respiration (black).B,the amounts of net daily carbon fixation (orange) and respirationbased nocturnal consumption (black).C,the minimum fluorescence intensity (Fo).D,the maximal dark-adapted PSII yield (Fv/Fm).E,the maximal fluorescence intensity (Fm).14/10,10/14 and 6/18 indicate 14-h/10-h,10-h/14-h and 6-h/18-h light/dark cycles,respectively.Bars mean SE (n=12–20).
3.3.Turnover of starch and total soluble carbohydrates
Diurnal changes in starch and total soluble carbohydrates(TSC) in the 6th leaves were investigated.ED decreased the accumulation levels of both starch and TSC in the newly expanded leaves (Fig.4).The turnover of both starch and TSC was nearly linear for each treatment.However,there seemed to be a delay in starch synthesis and boosted TSC accumulation at the beginning of the light period for plants under ED (Fig.4).TSC degraded very fast initially in the dark period,and both starch and TSC metabolized at constant low levels at the predicted morning time (8:00) for all plants.To further characterize the turnover of starch and TSC,the metabolic rates of starch and TSC were caculated in the first 2 h and for all times of the light/dark periods.The rates of starch synthesis were constantly lower under ED during the first 2 h of light but were similar to those under the control when averaged over the light period (Fig.5-A).The rates of TSC synthesis in ED plants were generally higher than those in the control plants,especially in the first 2 h on day 1 and for the data over the light period (Fig.5-B).In darkness,the rates of starch degradation and,to a lesser extent,of TSC consumption were generally lower in 10/14 and 6/18 than in 14/10 (Fig.5-C and D).Sugar degradation was generally faster in the first 2 h of darkness than throughout the dark periods,especially for TSC.
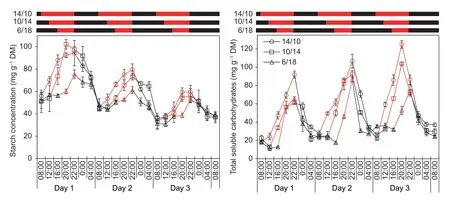
Fig.4 Diurnal patterns of starch and total soluble carbohydrates in the newly expanded leaves over 3 successive days.14/10,10/14 and 6/18 indicate 14-h/10-h,10-h/14-h,and 6-h/18-h light/dark cycles,respectively.The red and black indicate light and darkness,respectively.Bars mean SE (n=4).
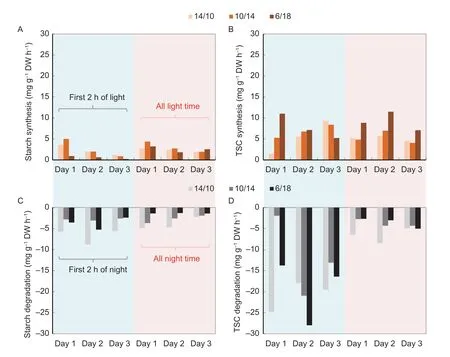
Fig.5 Turnover of starch and total soluble carbohydrates (TSC) for the first 2 h and all of the light/dark periods.A,starch synthesis.B,TSC synthesis;C,starch degradation.D,TSC degradation.14/10,10/14 and 6/18 indicate 14-h/10-h,10-h/14-h,and 6-h/18-h light/dark cycles,respectively.
While ED obviously reduced the TSC concentration,daily accumulation and nocturnal consumption,the starch content at the end of the predicted and actual night was not significantly affected (Fig.4;Table 1).Interestingly,the starch accumulation for ongoing growth at the end of the light period gradually decreased significantly over the 3 d (Fig.4;Table 1).The activities of AGPase and SSS,two critical enzymes in starch synthesis,significantly and consistently decreased over the 3 d in the uppermost expanded leaves(Fig.6;Table 1).There was little difference in enzyme activity among treatments for each day,except for the SSS between 14/10 and 6/18 on day 3 (Fig.6;Table 1).
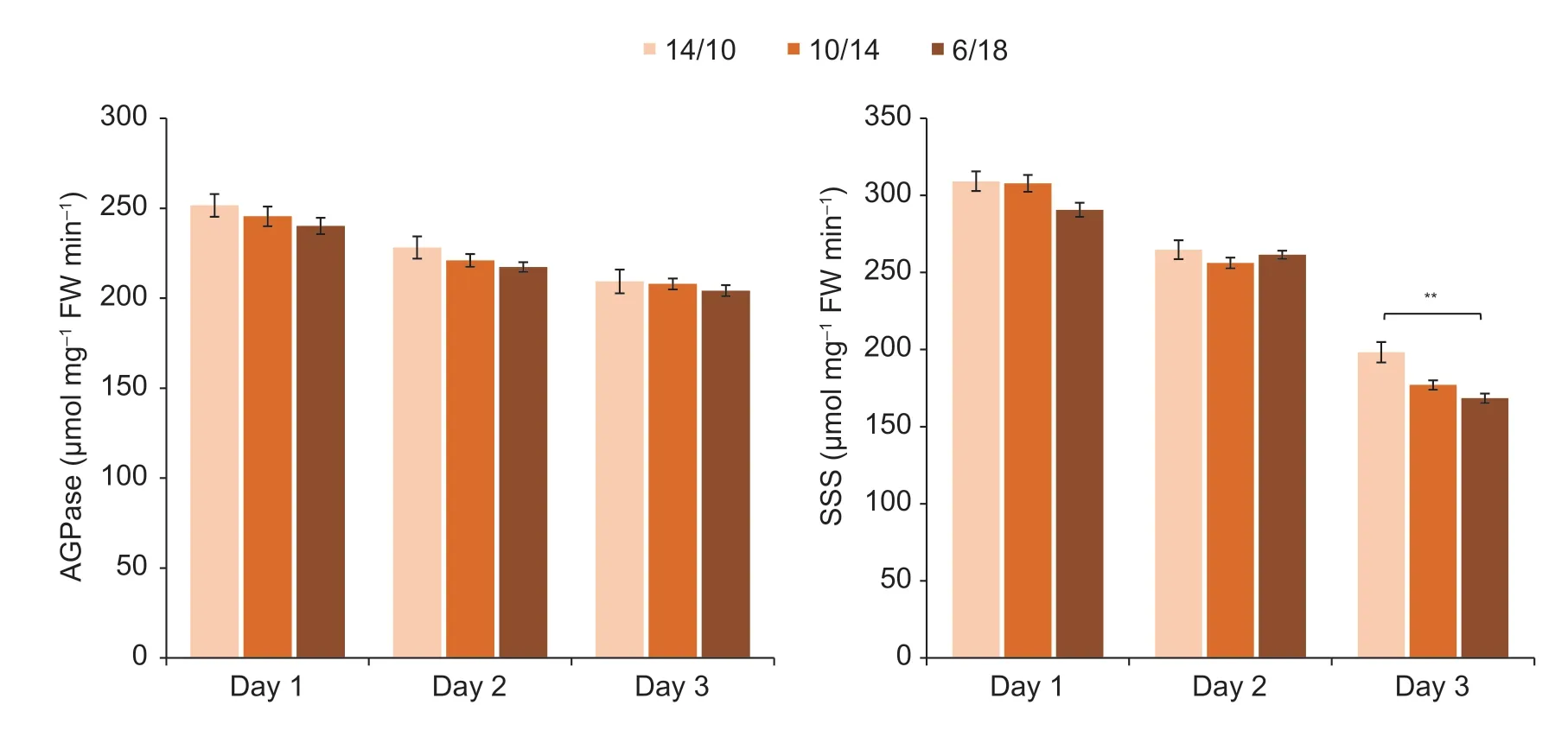
Fig.6 Enzyme activities of AGPase and soluble starch synthase (SSS) for the uppermost expanded leaves.14/10,10/14 and 6/18 indicate 14-h/10-h,10-h/14-h and 6-h/18-h light/dark cycles,respectively.Bars mean SE (n=4).**,significant difference at P<0.01.
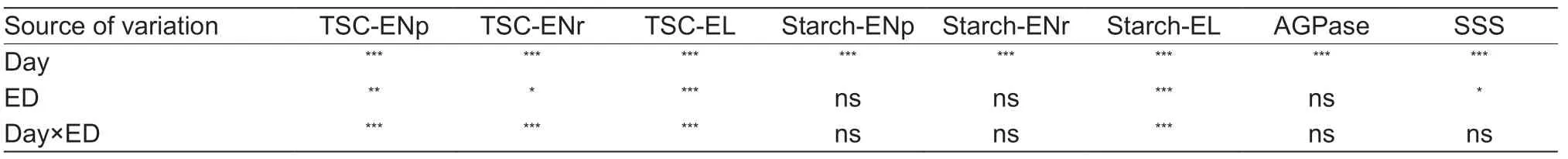
Table 1 Summary of multiway ANOVAs for the effects of the days and extended darkness (ED) treatment on leaf carbohydrates(starch and TSC) and the activity of starch synthesis-related enzymes (AGPase and SSS)1)
3.4.C partitioning in the three successive days
Based on the previously created C-balance model for photosynthetic maize leaves (Lianget al.2019),this study quantified diurnal C partitioning in the newly expanded leaves for 3 successive days.The amounts of NSC synthesized in the light and degraded during the dark period were equivalent and were used to calculate the ratio of each to the daily C fixation (Fig.4).In addition to a decrease inAD(Fig.3-A) under ED,STAAandTSCAandCDTduring the light period were decreased by up to approximately 3-,1.5-and 3-fold,respectively,compared with those under control conditions (Table 2).TheCAin the leaves was estimated as the sum ofSTAAandTSCA,and was used forRNandCNT(eq.(3)).As a trade-off under prolonged darkness,the partitioning ofADtoCA,i.e.,CA/ADincreased,which is used for nocturnal consumption in respiration (RN;RN/AD) rather thanCNTto sinks (Table 2).
Over time,theCA/ADratio generally decreased due to the reduction in starch accumulation and,to a lesser extent,TSC reserves in leaves in both the control and ED treatments.In all treatments,the amount of daily C transport and its ratio toADgradually increased over time (Table 2).
4.Discussion
Starch has been proven to be crucial in the adaptation of C allocation strategies to the environment in plants such asArabidopsis;in other crops,this may not be the case(Crosset al.2006;Sulpiceet al.2009).Previously,Lianget al.(2019) found changes in TSC and starch turnover in leaves depending on the growth period in field maize without obvious stress.However,little is known about the adaption and quantification of C turnover to sudden starvation and its role in coordinating maintenance and growth in maize crops.Here,by applying prolonged darkness to maize seedlings for three successive days,this study has shown that TSC and starch turnover responded differently to acute C starvation.Most importantly,increased partitioning of daily fixed C to reserves in ED-treated maize leaves was found,mainly caused by an increase inRNbut notCNT;a gradual but obvious decrease in starch accumulation in mature leaves was found in all treatments over time.These results help to understand how crops coordinate their growth in changing environments.
4.1.The inducing effect of ED on the partitioning of fixed C to reserves due to TSC in mature maize leaves used for nocturnal sustenance
C turnover in the starch-storing leaves of plants such asArabidopsishas been extensively studied,but little is known about fructan- and sucrose-storing leaves such as wheat leaves or intermediate leaves such as maize leaves.Specifically,maize is a C4monocot crop that could have different C turnover strategies than C3and/or dicotyledon crops.As shown in Figs.4 and 5,it is further revealed that considerable amounts of both starch and TSC were reserved in maize leaves,and both were used up during the prolonged darkness (Lianget al.2019).Unlike starch turnover,which provides a stable sugar flux during the transition of the daynight cycle and under extended darkness inArabidopsisrosettes (Gibonet al.2009;Pylet al.2012),TSC turnover appeared to be even higher during the normal diurnal cycle and responded more “positively” to extended darkness in the maize seedling leaves (Figs.4 and 5).A similar phenomenon which increased sucrose synthesis rather than starch synthesis after 24 h of extended darkness for maize seedlings was previously reported (Henryet al.2014).In the present study,the TSC accumulation in mature leaves during the day was approximately twice that of starch under the control when normalized as mol of C,and this gap increased to three times more for the 6/18 treatment(Table 2).Hence,the present study proposed that the adaptation of primary C allocation in maize leaves under acute C starvation differs to some extent from that in previous reports on plants such asArabidopsis.The different patterns of C accumulation between maize leaves and leaves of plants such asArabidopsismay be related to the different methods of C utilization.Treatments such as ED and a short light period lead to a reduced proportion of daytime growth but increased nocturnal growth,which is mainly supported by the altered starch turnover observed inArabidopsis(Gibonet al.2004,2009;Pilkingtonet al.2015).However,for the maize seedlings in this study,the ratio ofCNTtoADdecreased slightly under ED treatments (Table 2).In contrast,nocturnal respiration consumption may largely explain the increase inCAin mature leaves (Fig.3;Table 2).It is possible that the mature leaves allocate more C to nocturnal maintenance and repair to ensure the efficiency of their photosynthetic production (Fig.3)and to maintain other functions under such a sudden stress.The underlying mechanisms may involve regulation processes by sugar signals such as trehalose-6-phosphate and circadian rhythms that need further exploration (e.g.,Grafet al.2010;Poiréet al.2010;Wingleret al.2012;Henryet al.2015).
RN/CA(%)–19.9–15.7–20.8–33.9–27.5–50.2–60.4–46.8–65.4–18.82–37.21–57.56 RN/AD(%)–6.9–5.0–5.2–14.2–10.8–11.7–31.5–24.5–25.6–5.68–12.23–27.17 CNT/AD(%)27.5 27.0 19.6 27.7 28.5 11.6 20.6 27.8 13.5 24.7 22.6 20.6 CDT/AD(%)65.6 68.0 75.2 58.1 60.7 76.8 47.9 47.8 60.9 69.61 65.17 52.20 CA/AD(%)34.4 32.0 24.8 41.9 39.3 23.2 52.1 52.2 39.1 30.39 34.83 47.80 Table 2Quantificationof diurnal carbon turnover andtheratios of each to thenetdailycarbon fixation1)CNT(mg Cg–1DW)3.10 3.29 2.48 2.14 2.41 1.03 0.98 1.44 0.69 2.95 1.86 1.03 CDT(mg Cg–1DW)7.38 8.28 9.50 4.48 5.14 6.84 2.28 2.47 3.09 8.39 5.48 2.61 CA(mg Cg–1DW)3.87 3.90 3.13 3.23 3.33 2.07 2.48 2.70 1.98 3.63 2.88 2.39 TSCA(mg Cg–1DW)2.45 2.65 2.15 1.61 2.32 1.35 1.77 2.30 1.42 2.41 1.76 1.83 STAA(mg Cg–1DW)1.42 1.25 0.98 1.62 1.01 0.72 0.71 0.41 0.56 1.22 1.12 0.56 Day 123123123 14/10 10/14 6/18 Treatment2)14/10 10/14 6/18 Average 1) STAA,dailystarch accumulation; TSCA,dailytotalsolublecarbohydrate accumulation; CA,dailycarbon accumulation in leaves,estimatedas thesumof STAA and TSCA;CDT,daytimecarbon transport; CNT,nocturnalcarbon transport;AD,dailynetcarbon fixation;RN,respirationduring thedark period.Theamountsof carbon turnover areexpressedon adryweight (DW) basis.2) 14/10,10/14and6/18 indicate14-h/10-h,10-h/14-hand6-h/18-h light/dark cycles,respectively.
4.2.Gradually decreased starch pool over time related to the ongoing growth demand
Interestingly,decreased starch accumulation and,to a lesser extent,TSC accumulation was observed in the mature leaves as the day progressed in all treatments (Fig.4;Table 2).Consistently,the activities of AGPase and SSS in the mature leaves gradually decreased significantly (Fig.6;Table 1).As a result,the amounts and the ratios of total C reserved in leaves showed a decreasing trend,while the daytime transported C increased obviously over time.These temporal changes in daily C turnover in mature maize leaves have not been reported previously and may reflect the flexibility of starch turnover in coordinating the balance between maize plant growth and maintenance.Expanding young leaves were reported to contain higher levels of starch than mature leaves,and starch reserves were maintained at the end of the night (Czedik-Eysenberget al.2016).Hence,it is conceivable that as the leaves develop to maturity,these starch buffers are gradually moved to newly developing sinks.On the other hand,in newly expanded leaves with a stable C skeleton,there is less need for growth and maintenance,which could lead to gradually decreased C reserves.Last,the ratio of leaf mass to whole plant mass decreases with ongoing growth in plants,including maize (Poorteret al.2012).The gradually larger sink demand requires higher immediate C transport rather than storage in source leaves.The source and sink combination could lead to gradually decreased baseline and daily accumulation of starch,as observed in this study (Table 2;Figs.4 and 6).Overall,the adjustment of primary C allocation to create a balance between self-maintenance and continuous growth was modelled in mature leaves of maize seedlings under ED (Fig.7).As ED significantly reduced maize growth and leaf photoassimilation (Figs.2 and 3),the plants partitioned more fixed C to source leaves to maintain their function under sudden C starvation.Meanwhile,the sugar pools in mature leaves gradually decreased (largely as starch) as the day progressed,which may be related to the ongoing adaptive growth demand (Fig.7).This study proposes that this could be a critical feature of maize crops to cope with plant growth and adjust to a changing environment.
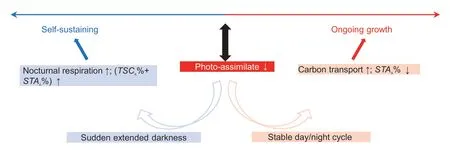
Fig.7 Schematic of adjustment of primary carbon allocation to ongoing growth under extended darkness in mature maize leaves.↑,increase;↓,decrease.%,percentage of each to the daily net carbon fixation;TSCA,daily total soluble carbohydrates accumulation;STAA,daily starch accumulation.
5.Conclusion
In a 3 d treatment with ED,this study successively investigated the CO2exchange and carbohydrate turnover in newly expanded maize leaves.Based on a C-balance model,the daily net C fixation and its primary allocation were quantified.First,as a consequence of decreased photoassimilation under ED,C reserves in the mature leaves,especially reserves of TSC,increased rather than being transported to growing tissues;this trend could be accounted for by the increased consumption of nocturnal respiration.Second,as the days continued,the ratio of reserved C to the daily C fixation in mature leaves decreased successively under both the control and ED treatments.In conclusion,while maize plants retain more C (largely as TSC) in leaves for self-sustenance under acute C starvation,the gradually decreased C accumulation in mature leaves over time may be related to ongoing plant growth and a dynamic source-sink relationship in which starch may play an important role.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2016YFD0300301),the earmarked fund for China Agriculture Research System of MOF and MARA (CARS-02-13) and the Education Department of Jiangxi Province,China (190233).
Declaration of competing interest
The authors declare that they have no conflict of interest.
杂志排行
Journal of Integrative Agriculture的其它文章
- Molecular characteristics and structure–activity relationships of food-derived bioactive peptides
- lntegrating the physical and genetic map of bread wheat facilitates the detection of chromosomal rearrangements
- Physiological response of flag leaf and yield formation of winter wheat under different spring restrictive irrigation regimes in the Haihe Plain,China
- Effects of plant density and mepiquat chloride application on cotton boll setting in wheat–cotton double cropping system
- Interactive effect of shade and PEG-induced osmotic stress on physiological responses of soybean seedlings
- Does heat accumulation alter crop phenology,fibre yield and fibre properties of sunnhemp (Crotalaria juncea L.) genotypes with changing seasons?
