Topology impacts TRAIL therapy: Differences in primary cancer growth and liver metastasis between orthotopic and subcutaneous xenotransplants of pancreatic ductal adenocarcinoma cells
2021-07-24BstinKettlerAnnTruzoldChristinderJnHendrikEgertsHolgerKlthoff
Bstin Kettler ,Ann Truzold ,Christin Röder ,Jn-Hendrik Egerts ,Holger Klthoff,
a Clinic for General-, Abdominal- and Transplant-Surgery, Medical School Hannover, Carl-Neuberg-Str. 1, 30625 Hannover, Germany
b Institute for Experimental Cancer Research, University of Kiel and University Clinic of Schleswig-Holstein, Campus Kiel, Hs. U30, Arnold-Heller-Str. 3, 24105 Kiel, Germany
c Clinic for General, Visceral, Thoracic, Transplantation- and Pediatric Surgery, University Clinic of Schleswig-Holstein, Campus Kiel, Hs. C, Arnold-Heller-Str.3,24105 Kiel, Germany
Keywords:Pancreatic cancer Animal models Immunohistochemistry Subcutaneous metastasis
ABSTRACT Background:To study novel treatment modalities for pancreatic ductal adenocarcinoma (PDAC),we need to transfer the knowledge from in vitro to in vivo.It is important to mirror the clinical characteristics of the typically local invasive growth of pancreatic cancer and the distant spread resulting in liver metastasis.Notably,for xenotransplant studies using human specimen,two models,i.e.subcutaneous (s.c.) and orthotopic (o.t.) transplantation are widely used.Methods:The subcutaneously and orthotopically inoculated Colo357 Bcl-x L cell-derived tumors were directly compared with and without TNF-related apoptosis inducing ligand (TRAIL) treatment.The size of primary tumors,number of liver metastasis and the histologic markers Ki67,M30,TNF- α and CD31 were assessed.Results:Upon TRAIL treatment,the primary tumors did not change their size,neither in the s.c.nor in the o.t.approaches.But when s.c.was compared to o.t.,the size of the s.c.tumors was more than twofold bigger than that of the o.t.tumors ( P < 0.01).However,mice with orthotopically inoculated PDAC cells developed liver metastasis upon TRAIL treatment much more frequently ( n = 13/17) than mice with subcutaneously inoculated PDAC cells ( n = 1/11) ( P < 0.01).As a likely driving force for this increased metastasis,a higher TNF- α staining intensity in the o.t.tumors was observed by immunohistochemistry.Conclusions:These data from a direct side-by-side comparison underline the importance of the proper inoculation site of the PDAC cells.Local invasion and liver metastases are a hallmark of PDAC in the clinic; the o.t.model is clearly superior in reflecting this setting.Moreover,a serious side-effect of a possible new therapeutic compound became obvious only in the o.t.model.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a cancer with a poor survival rate.Its surgical resection with curative intention is possible in only 20% of all patients [ 1,2 ].The prognosis in an advanced state without any therapy is limited; the average survival time is three months after the diagnosis [2].A surgical treatment alone can cure the disease in less than 10%; therefore,adjuvant chemotherapy is the standard recommendation for treatment [3].Despite chemotherapy,up to 75% of these patients have a relapse within two years [3].
A better understanding of this devastating malignancy and the investigation of novel clinical treatment modalities aiming at a translation frombenchtobedsideneed intensive experimental efforts in the establishment of appropriate model systems,which reflect the aspects of PDAC development,progression and metastasis and mutual effects of cancer,surgery and adjuvant therapy.Despite advances in complexinvitrosystems using co-culture or organoid models [4],a more realistic complexity of the disease pattern compared to the clinical setting can only be mirrored byinvivoanimal models [5].There is a striking discrepancy among the many experimental treatment achievements reported in the past,based on bothinvitroandinvivostudies,and the (mostly unsuccessful) clinical translation,in particular on the basis of subcutaneous(s.c.) xenotransplantation models [6].Such effects were also observed earlier in our own research group comparinginvitroand subsequentinvivoexperiments [ 7,8 ].Moreover,the general reproducibility of animal experiments has been shown to be critically low [9].The s.c.inoculation of PDAC cells is still frequently used since it is easy to perform and does not require specialized surgical experience.However,the tumor growth is mostly confined to the inoculation site only and the clinical challenge of the development of metastases is a rare event [10].In effort to mimic aninvivosituation as close as possible,we have refined an orthotopic (o.t.) murine xenotransplantation model with an o.t.inoculation of PDAC cells in the pancreatic body/tail [7].Using this model,we have extensively described the effects of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in PDAC [11–13].In the present study,we set out to compare in a direct side-by-side setting the growth and TRAIL-responsiveness of orthotopically and subcutaneously inoculated PDAC cells and report on striking differences both in the control groups (saline-treated) and particularly in the treatment groups (TRAIL-treated).
Methods
Cell culture
The human pancreatic adenocarcinoma cell line Colo357 [14]was cultured in Roswell Park Memorial Institute (RPMI)-1640 medium,supplemented with 10% fetal calf serum,2 mmol/L glutamine and 1 mmol/L sodium pyruvate.The cells had been transfected with a retroviral vector carrying the coding sequence for human Bcl-xL,which resulted in a constitutive overexpression of Bcl-x L [11].
Animal models and treatments
The animal experiments were performed in accordance with the institutional guidelines and approved by the competent local authorities [Ref.no.: V362-72241.121 (16-1/06)].Four-week-old female mice with the “severe combined immunodeficiency/beige”phenotype (SCID/bg) were bought from Harlan-Winkelmann(Borchen,Germany).These mice had a deficiency in their NK-,B- and T-cells and were unable to reject xenogenic cells [15].The husbandry of these mice including bedding,food and water was maintained under sterile conditions.The inoculation of the PDAC cells was performed under a general anesthesia [fentanyl,midazoloam and medetomidine (0.05/5/0.5 mg/kg,respectively)].Colo357 Bcl-x L cells were trypsinized,washed and resuspended in MatrigelTM(BD Bioscience,Heidelberg,Germany) at a concentration of 106cells/mL.Cell suspensions were drawn into 1-mL syringes and were stored on ice until injection.The inoculations(40 μL of tumor cell suspension) were either placed in the pancreatic body and tail (o.t.inoculation) (Fig.S1) or s.c.under the skin in the flank region [5].The mice were separated into two treatment groups; the control group received saline (0.9% NaCl,200 μL)intraoperitoneally (i.p.) and the TRAIL group received 15 μg TRAIL i.p.(Axxora/Alexis,Grünberg,Germany; in 200 μL saline).In both cases the i.p.application was performed at day 10,13 and 16.The experiment was stopped at day 41 and the animals were sacrificed.Most of the tissue was stored in liquid nitrogen (vapour phase)until histological analysis.Some of the tissue samples were fixed in formalin.Therefore,the number of possible histological slides was lower than the number of animals.Some tissue slides exhibited an unspecific background preventing any immunohistochemical evaluations.Consequently,immunohistochemical analyses were not available for all tissue samples.The frozen tissue was sliced into 5 μm sections onto microscopic slides,air-dried overnight and stored at -20 °C after vacuum sealing.For immunohistological staining,the slides were warmed up to room temperature,fixed in acetone(5 min at room temperature) and stained with the EnVision® Doublestain Kit or DAKO REALTMKit (DAKO Cytomation,Hamburg,Germany).The applied antibodies were described below.After staining,slides were covered with cover slips under Aquatex® (Merck,Darmstadt,Germany) and stored in dark boxes.All the reagents were used according to the guidelines of the manufacturers.
Antibodies
To detect apoptotic cells,M30 antibody (Cat No.12140322001,monoclonal,anti-human specific,Roche,Mannheim,Germany),recognizes caspase-cleaved cytokeratin 18 (amino acids 387-396),was used.Necrotic cells were negative [16].Dividing cells in G1-,S-,G2- or M-phase were detected by the Ki67 S2 antibody (gift of the Institute of Pathology,anti-human,University of Kiel,Germany).G0 resting cells were negative with Ki67 S2 [ 17,18 ].The differentiation between human (positive) and murine (negative)epithelial tissue was ensured by staining with the human pancytokeratin antibody KL1 (monoclonal,anti-human specific,Immunotech,Marseille,France [19]).Endothelial cells were detected using an anti-CD31 antibody (Cat No.SM027P,anti-human,Clone 390,Arcis,Heidenheim,Germany) and TNF-αwas stained with an anti-human TNF-αantibody (clone NYRhTNFa-E2,monoclonal,anti-human specific,Biologo,Kronshagen,Germany [20]).
Histological analysis
The analysis of the histological staining with the antibodies Ki67 and M30 was performed quantitatively.For this purpose,cells of three different,randomly selected regions of the slide were analyzed by counting positive cells.The histological staining was done with Envision® Double stain Kit (Ki67 and KL1)and DAKO REALTMKit (M30,CD31); both kits were from DAKO Cytomation [21].The analyses of CD31- and TNF-α-stainings were performed semi-quantitatively according to the Chalkley counting procedure [ 22,23 ].For both markers (vascular density and TNF-αdensity),the interpretation was based on a three-score classification: low (1),moderate (2) and high (3) expression of the markers(supplementary materials).In case of marker negativity,the score was set to zero.Furthermore,the murine stromal tissue and the human tumor tissue were interpreted separately.
Statistical analysis
Statistical analysis was done with the GraphPad Prism Software 4.0 (GraphPad.Com,San Diego,CA,USA).For the statistical analysis of phenotypic differences between the series of experimental animals the nonparametric Mann-WhitneyUtest was used and differences were considered significant ifPvalues were below 0.05.
Results
In the group of mice with subcutaneously inoculated PDAC cells(s.c.group),the mean tumor volume was more than twice as large as in the group of mice with orthotopically inoculated PDAC cells(o.t.group) (P<0.01,Fig.1 ).No significant difference in the tumor size was observed comparing the saline- and TRAIL-treated mice,neither in the s.c.group nor in the o.t.group.

Fig.1.Tumor volume (mm ³) in orthotopically and subcutaneously inoculated pancreatic ductal adenocarcinoma cells.o.t.: orthotopic; s.c.: subcutaneous; TRAIL: TNFrelated apoptosis inducing ligand.∗∗: P < 0.01.
Strikingly,the number of liver metastases differed significantly between the s.c.and o.t.groups (P<0.01,Fig.2 ).In the o.t.group,both treatment groups (saline- and TRAIL-treated) developed liver metastases.The o.t.saline-treated mice revealed a mean number of 0.7 liver metastases per animal.Remarkably,after TRAIL treatment,the mice showed a more than 4-fold higher mean number of liver metastases (2.9 metastases per animal,P<0.01,Fig.2,left panel).Conversely,the mice with s.c.primary tumors showed either no metastasis in the liver (saline treatment) or only one liver metastasis in a single animal in the TRAIL-treated group ( Fig.2,right panel).

Fig.2.Number of liver metastases in orthotopically and subcutaneously inoculated pancreatic ductal adenocarcinoma cells.o.t.: orthotopic; s.c.: subcutaneous; TRAIL:TNF-related apoptosis inducing ligand.∗∗: P < 0.01.
To investigate the proliferative capacity and malignant potential of the tumor cells in both models and treatment schemes,the percentages of dividing,Ki67-positive tumor cells were analyzed.Interestingly,after o.t.transplantation,tumors exhibited a roughly 3-fold higher Ki67 proliferation index than those in the s.c.tumor model (saline-treated: 5.5% vs.2.0%,TRAIL-treated: 6.9% vs.1.9%;P<0.01,Fig.3 ).TRAIL treatment showed no significant influence on the proliferation index in both transplantation models ( Fig.3 ).

Fig.3.Number of Ki67 positive/dividing cells in orthotopically and subcutaneously inoculated pancreatic ductal adenocarcinoma cells.o.t.: orthotopic; s.c.: subcutaneous; TRAIL: TNF-related apoptosis inducing ligand.∗∗: P < 0.01.
Since TRAIL is capable of inducing programmed cell death,we investigated whether the rate of apoptosis was influenced by TRAIL treatment in both transplantation models.With regard to the apoptotic index measured as caspase-mediated fragmentation of keratin 18 (M30-assay),the TRAIL treatment did not reveal any striking influence on the number of M30-positive cells in the o.t.system (saline-treated 4.9%,TRAIL-treated 5.5%,Fig.4,left panel).However,in the s.c.group,the mice showed a significantly lower apoptosis rate (lower number of M30-positive cells) after TRAIL treatment compared to that of the control group (saline-treated 5.8%,TRAIL-treated 2.6%,P<0.01,Fig.4,right panel).Consequently and remarkably,the rate of apoptotic cells in TRAIL-treated mice was significantly lower in the s.c.group than in the o.t.group (P<0.01).

Fig.4.Number of M30 positive/apoptotic cells in orthotopically and subcutaneously inoculated pancreatic ductal adenocarcinoma cells.o.t.: orthotopic; s.c.: subcutaneous; TRAIL: TNF-related apoptosis inducing ligand.∗∗: P < 0.01.
CD31 staining was used to determine the vessel density according to Chalkley counting method [23](Fig.S2),in order to investigate whether tumor vascularization reveals obvious differences in dependence on the inoculation site.The vascularization showed differences between the o.t.and s.c.groups.In the stromal tissue areas,the density of CD31-positive structures was higher than that in the tumor areas in both transplantation models (P<0.01,Fig.5 ).Within their epithelial tumor tissue areas,the saline-treated orthotopically inoculated mice exhibited a significantly lower vessel density than the subcutaneously inoculated mice (P<0.01,Fig.5 ).
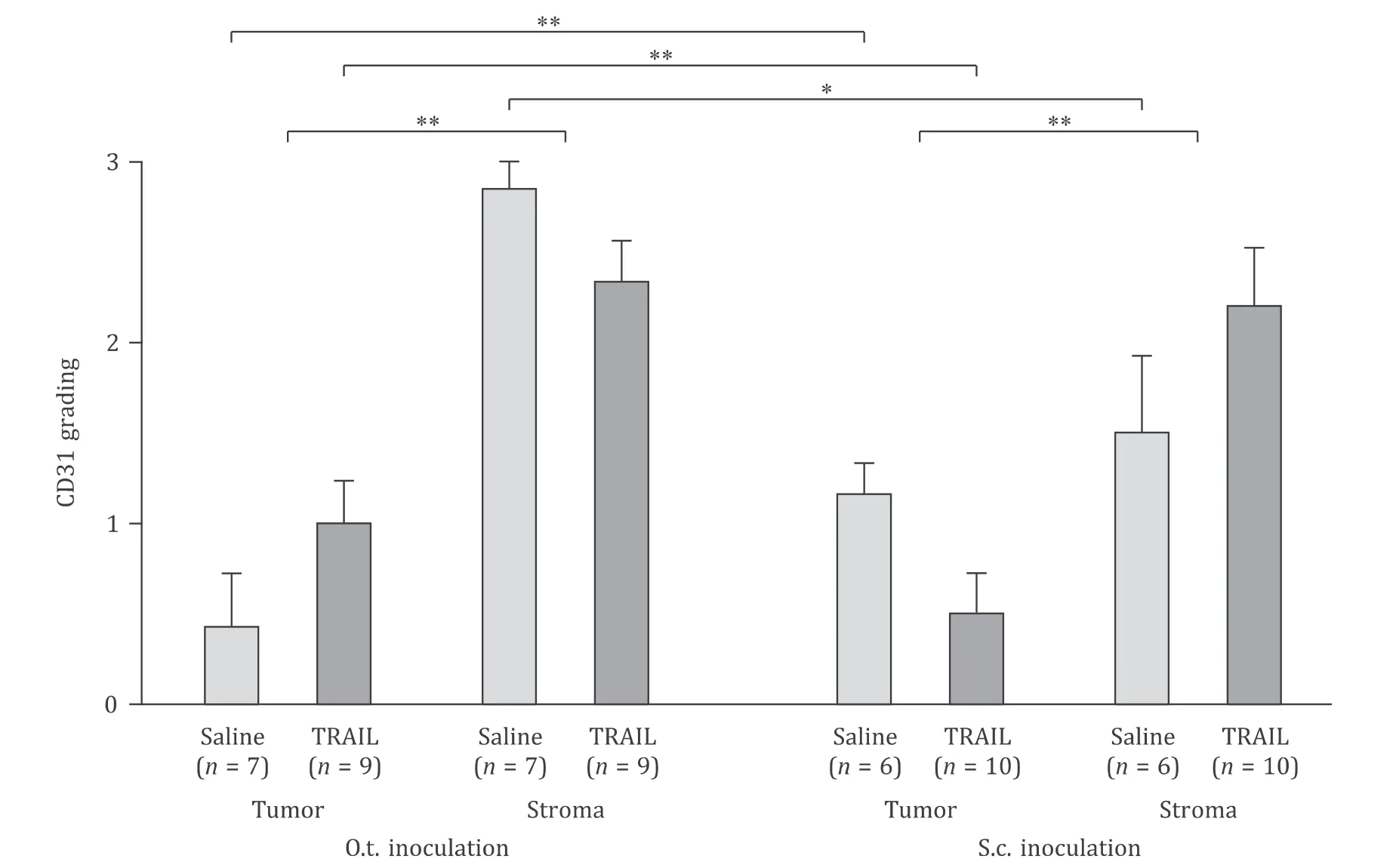
Fig.5.Vascular density (Chalkley counting).o.t.: orthotopic; s.c.: subcutaneous; TRAIL: TNF-related apoptosis inducing ligand.∗: P < 0.05; ∗∗: P < 0.01.
Immunohistological analysis of TNF-αrevealed a significantly stronger TNF-αexpression in the o.t.tumors than in the s.c.tumor tissues ( Fig.6,S3).In both model systems,the stroma area showed a higher TNF-αimmunoreactivity compared to the epithelial tumor area in the tissue samples (P<0.01,Fig.6 ).Moreover,TNF-αwas not detected in the tumor area in the s.c.model.

Fig.6.TNF- α density (Chalkley counting).o.t.: orthotopic; s.c.: subcutaneous; TRAIL: TNF-related apoptosis inducing ligand.∗: P < 0.05; ∗∗: P < 0.01.
Discussion
Pancreatic cancer is still a disease with a poor prognosis,particularly in the most prevalent advanced stages [ 2,24 ].In principle,the transfer “frombenchtobedside” of experimental results is only possible with the help of animal experiments.Unfortunately,invitrofindings may differ frominvivoresults,because of the much higher complexity of a malignant diseaseinvivo[25].Therefore,it is at least a good compromise to use a reliable and reproducible animal model,which mirrors the clinical characteristics of that malignancy as close as possible.Many research groups utilize pre-clinical model system with s.c.inoculation of tumor cells.This method does not require high level surgical skills and is fast to perform.The disadvantage of the technique is that it does not map any prognosis-relevant features of tumor progression such as metastasis,which is the most challenging characteristic of PDAC in the clinical routine.Our results clearly revealed that s.c.tumors can grow to a very huge size in its ectopic location compared to orthotopically inoculated tumors ( Fig.1 ).The tumor size in this particular model system can be attributed to the overexpression of Bcl-xLby the tumor cells,leading to apoptosis inhibition,induction of invasiveness and increased NF-κB activity [12].
For the o.t.inoculation of PDAC cells into the pancreas,a higher surgical skill level is necessary.With an experienced surgeon,this procedure can be performed without an elevated mortality or morbidity of the animals [7].The orthotopically inoculated PDAC cells grew in the body/tail of the pancreas and further enabled the establishment of a resection model by our group [ 2,7 ],which allowed appropriate adjuvant therapeutic regimens to be studied.Importantly,most of the mice developed liver metastases and thus,this model reflects the clinical situation much better than the s.c.model [10].This holds also true if o.t.inoculation is performed into the head of murine pancreas as performed by other groups [ 25,26 ].A variation of these models was suggested using tissue fragments orthotopically implanted after prior s.c.inoculation of PDAC cells [ 26,27 ].Besides the higher expenditure of time of this procedure,the question remains,whether the s.c.step specifically selects cell variants,which are subsequently seeded into the “appropriate” environment.Besides the size and number of metastases,we observed a difference in the number of actively dividing cells between both tumor models.The orthotopically growing PDAC showed an about 3-times higher proliferation rate presumably because of a better nutrient supply and growth rate compared to the post-exponential growth arrest of the very large s.c.tumors.Obviously,the endpoint situations as analyzed by immunohistochemical techniques of post-mortem tissues have their limitations since they do not reflect the dynamic changes during the entire treatment period.Clearly,non-invasive imaging modalities as ultrasound,magnetic resonance imaging,PET,CT,or others techniques are mandatory for the future despite their high costs and additional efforts [27].
The analysis of the apoptotic cells showed that the numbers of M30-positive cells differ significantly in dependence on TRAIL treatment of the mice.The subcutaneously inoculated tumors showed significantly less apoptotic cells than the o.t.tumors after TRAIL administration; even the tumor was bigger at time of sacrifice.However,no difference emerged between the control groups without TRAIL treatment.The reasons for these differences in the apoptotic cells remain to be elucidated.
Determination of vessel density is a part of the diagnosis in some cancers.In our results the vessel density was inhomogeneous in comparison of the o.t.and s.c.models and furthermore between the treatment groups.The limitation of the experiment is that the observation can only be done at the end of the experiment.As written above,imaging like ultrasound or magnetic resonance imaging can give us a better understanding of the vessel density during the experiment than the immunohistochemical analysis.Therefore,we are not able to determine differences during cancer growth.In an independent experimental PDAC model of o.t.versus s.c.inoculation (of another research group),in which the functional blood flow was assessed by power-doppler ultrasound,the results were strikingly different (Dr.C.Heneweer,Institute for Diagnostic and Interventional Radiology,University Clinic,Cologne,Germany,unpublished personal communication).This might become the superior analytical system for future analyses.The cytokine TNF-αis an important player in the immune system and the serum levels had been shown a huge impact of the PDAC cells [ 2,8 ].There is a significantly higher rate of detection in all groups in the mice with o.t.PDAC,in the epithelial tumor tissue parts as well as in the stroma compartment,in the saline controls and particularly,upon TRAIL treatment.Since the antibody used here for immunostaining was specifically raised against human TNF-α,the stroma staining must be interpreted as PDAC-cellderived secreted cytokine.Due to the impact of TNF-αon PDAC cells causing elevated invasiveness and growth stimulation,the higher rate of metastasis in the o.t.system can be explained [ 2,8 ].
In conclusion,the present study demonstrated a striking influence on the biological behavior of the PDAC cells upon their site of inoculation in a direct comparative setting.The typical characteristics of PDAC with the local invasiveness and liver metastasis are only represented in the o.t.group.In case of the s.c.murine xenotransplant model,the PDAC grows only at the site of inoculation,expresses much less TNF-αand spreads very modest towards the liver even after challenged with TRAIL.
Acknowledgments
The authors greatly acknowledge the technical support of Doris Emme,Bianca Zinke,Bianca Körtge and Birgit Fricke.
CRediTauthorshipcontributionstatement
BastianKettler:Conceptualization,Data curtion,Formal analysis,Methodology,Project administration,Writing – original draft.AnnaTrauzold:Formal analysis,Methodology,Supervision,Validation.ChristianRöder:Formal analysis,Methodology,Supervision,Validation.Jan-HendrikEgberts:Data curtion,Formal analysis.HolgerKalthoff:Conceptualization,Methodology,Funding acquisition,Writing – review & editing.
Funding
This study was supported by an intramural grant from UKSH Kiel to H.Kalthoff.
Ethicalapproval
This study was performed in accordance with the institutional guidelines and approved by the competent local authorities [Ref.no.: V362-72241.121 (16-1/06)].
Competinginterest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.hbpd.2021.04.005.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Cross-talk between hepatic stellate cells and T lymphocytes in liver fibrosis
- Diabetes mellitus is a risk factor of acute kidney injury in liver transplantation patients✩
- Hepatobiliary&Pancreatic Diseases International
- Application of machine learning models for predicting acute kidney injury following donation after cardiac death liver transplantation
- Postoperative adjuvant transcatheter arterial chemoembolization improves the prognosis of patients with huge hepatocellular carcinoma
- The effects of stereotactic body radiotherapy on peripheral natural killer and CD3 + CD56 + NKT-like cells in patients with hepatocellular carcinoma
