Long-term survival after pancreaticoduodenectomy in patients with ductal adenocarcinoma of the pancreatic head
2021-07-24AndresMinhLuuChrisBrumnnOrlinBelyevMonikJnotMtushekHenrikRudolfMihelPrktiknjoWldemrUhl
Andres Minh Luu ,,Chris Brumnn ,Orlin Belyev ,Monik Jnot-Mtushek ,Henrik Rudolf ,Mihel Prktiknjo ,Wldemr Uhl
a Department of General and Visceral Surgery, St. Josef Hospital, Ruhr University Bochum, Gudrunstrasse 56, Bochum 44791, Germany
b Department of Medical Informatics, Biometry and Epidemiology, Ruhr University Bochum, Universitaetsstrasse 105, Bochum 44789, Germany
c Department of Internal Medicine, University of Bonn, Venusberg-Campus 1, Bonn 53127, Germany
Keywords:Pancreaticoduodenectomy Whipple’s procedure Pancreatic cancer survival Ductal adenocarcinoma Pancreas Pancreatic surgery
ABSTRACT Background:Pancreatic ductal adenocarcinoma (PDAC) has the worst prognosis of all malignant tumors due to unavailable screening methods,late diagnosis with a low proportion of resectable tumors and resistance to systemic treatment.Complete tumor resection remains the cornerstone of modern multimodal strategies aiming at long-term survival.This study was performed to investigate the overall rate of long-term survival (LTS) and its contributing factors.Methods:This was a retrospective single-center analysis of consecutive patients undergoing pancreaticoduodenectomy (PD) for PDAC between 2007 and 2014 at the St.Josef Hospital,Ruhr University Bochum,Germany.Clinical and laboratory parameters were assessed and evaluated for prediction of LTS with Cox regression analysis.Results:The overall rate of LTS after PD for PDAC was 20.4% (34/167).Median survival was 24 months regardless of adjuvant treatment.Carbohydrate antigen 19-9 levels,tumor grade,lymph vessel invasion,perineural invasion and reduced general condition were significantly associated with LTS in univariate analysis ( P < 0.05).Serum levels of carbohydrate antigen 19-9,American Joint Committee on Cancer stage,tumor grade,abdominal pain,male,exocrine pancreatic insufficiency and duration of postoperative hospital stay were independent predictors of cancer survival in multivariable analysis.Conclusions:Cancer related characteristics are associated with LTS in multimodally treated patients after curative PDAC surgery.
Introduction
Pancreatic cancer is a lethal disease most commonly diagnosed at an advanced stage.Despite advances in medical and surgical treatment options,patients with pancreatic cancer often remain asymptomatic in early stages,which result in rare cases of early detection [1–4].The most common histological type of pancreatic cancer is pancreatic ductal adenocarcinoma (PDAC) representing approximately 95% of all pancreatic cancer [5–7].PDAC is most commonly located in the pancreatic head (54.7%) [ 4,8 ].To date,no screening methods have been established for early diagnosis of pancreatic cancer.Thus,the window of opportunity for surgical resection as the only curative therapy is often missed.Unfortunately,less than 20% of the patients diagnosed with pancreatic cancer have a resectable tumor [ 3–5,8 ].One third of the patients suffer from a locally advanced tumor while the majority already have distant metastases [4].The 5-year overall survival for all stages of pancreatic cancer ranges from 2.5% to 5% [ 8,9 ].Several risk factors are associated with a poor prognosis such as a large tumor,a high tumor grade,lymph-node metastases,a high level of carbohydrate antigen 19-9 (CA19-9) and a positive resection margin [4].
Current strategies in diagnosis and therapy require a quick interdisciplinary workflow which often begins with the general practitioner.He/she should always be aware of symptoms such as painless jaundice,abdominal pain,or weight loss.In those cases,computed tomography or magnet resonance imaging of the abdomen should be initiated as soon as possible.Afterwards,interdisciplinary discussion of the case will result in three treatment strategies such as primary surgical resection,neoadjuvant intended chemotherapy in cases of locally advanced pancreatic cancer,or primary palliative chemotherapy in cases of metastatic tumor stage.Development of biomarkers for early detection,radiation therapy,primary neoadjuvant chemotherapy in resectable pancreatic cancer,immunotherapy or targeted therapy have not been established yet and are under investigation [10].
Long-term survival (LTS) in PDAC is commonly defined as a postoperative survival of at least 5 years [ 8,11 ].In this study,we performed a detailed analysis of all patients who underwent PD due to PDAC of the pancreatic head to investigate clinical or pathohistological factors contributing to LTS.
Methods
Data acquisition
All patients who underwent pancreaticoduodenectomy (PD) due to PDAC of the pancreatic head from January 2007 to December 2014 at a high-volume pancreatic surgery center (St.Josef Hospital,Ruhr University Bochum,Germany) were included in this study.Patients suffering from other pancreatic cancer types like intraductal papillary mucinous neoplasia (IPMN)-associated carcinomas or acinar cell carcinomas were excluded.Survival was defined as the period from day of surgery until death.Primary endpoint was survival of at least 5 years.Secondary endpoints were postoperative complications.Follow-up to evaluate LTS in all patients was performed until August 2019.Patients’ data were gathered from our prospective database.
If the patients continued their oncologic treatment at another institution,discharge letters were obtained from that institution or from the patient’s general practitioner.Patients or their relatives were visited by phone to confirm survival.
Covariates
The following demographic,clinical and pathohistological variables were collected: age,sex,ASA score (American Society of Anesthesiologists,I-IV),diabetes mellitus,cardiovascular diseases,nicotine consumption,alcohol consumption,exocrine pancreatic insufficiency,body mass index (BMI),preoperative stenting of the common bile duct (CBD),protein level,CA19–9 and carcinoembryonic antigen (CEA).Further,we assessed clinical findings leading to the diagnosis of PDAC like obstructive jaundice,abdominal pain,pancreatitis,reduced general condition (including weight loss,nausea and night sweat),coincidental detection,diarrhea and deterioration of diabetes mellitus.Pathohistological data were analyzed in detail: tumor size (T),lymph node invasion (N),lymph node ratio (number of resected invaded lymph nodes/total number of resected lymph nodes),metastases,lymph vessel invasion,venous invasion,perineural invasion,AJCC stage (American Joint Committee on Cancer,I-IV) and resection margin (R0–2).Postoperative outcome was determined by investigation of total hospital stay,postoperative hospital stay,minor complications (Clavien-Dindo ≤2),major complications (Clavien-Dindo ≥3),overall complications (Clavien-Dindo 1-5 or non-surgical complications),readmission rate,neoadjuvant treatment and adjuvant chemotherapeutical treatment).
Surgical approach
At our institution,standard procedure in resectable pancreatic head cancer is a pylorus-preserving pancreaticoduodenectomy.Further,the standard type of pancreatic anastomosis is a doublelayered end-to-side duct-to-mucosa pancreaticojejunostomy,as described by Warren and Catell [12].
Statistical analysis
The characteristics of patient data were expressed as median(interquartile range,IQR) or numbers (percentages).A two-tailed Chi-square test,a Fisher’s exact test or a Mann-WhitneyUtest was performed to compare long-term survival patients with patients surviving less than 5 years after curative surgery.A two-sidedPvalue<0.05 indicated statistical significance.Kaplan-Meier curves were determined to univariably compare pancreatic cancer survival between groups.Hereby,death from other causes was excluded.Multivariable logistic regression was not feasible due to low absolute number of patients with long-term survival.Therefore,Cox proportional hazards regression was used.Stepwise selection based on complete cases was executed with limits for model entry and stay as 0.25 and 0.15.In case of obvious high collinearity between factors we excluded variables from the selection procedure.This was in the cases of lymph node invasion and LN ratio.Further,BMI,albumin and CEA were excluded due to the number of missings exceeding 5%.Hazard ratios (HR) were presented with 95% CI and Wald Chi-square test.Statistical analysis was performed with R (R Foundation for statistical computing,Vienna,Austria).
Results
Basic results
During the study period,203 patients underwent PD due to a malignant tumor of the pancreatic head,167 (82.3%) of whom had a PDAC.Patients with other malignant lesions like an IPMNassociated carcinoma or a neuroendocrine carcinoma were excluded (36 patients,17.7%).Lost to follow-up rate was 3.6% (6 patients).Death not related to the PDAC occurred in 5.6% (9 patients).The total cohort of patients with PDAC analyzed in this study comprised of 167 patients,including lost to follow-up and patients who suffered from not-cancer-related deaths.
Patient characteristics
The overall proportion of long-term survivors was 20.4%(34/167,Table 1 ).Median survival was 24 months ( Fig.1 ).Median age was 66 years at the time of diagnosis.The overall male to female ratio was 0.96.Most common ASA score was ASA II in 47.3%,followed by ASA III 35.3% and ASA I in 17.4%.Nearly half of the patients suffered from cardiovascular diseases (49.7%).Diabetes mellitus was present in a third of the patients (36.5%).Also,one third of the patients had exocrine pancreatic insufficiency (36.5%).Nicotine or alcohol consumption played only a minor role in the cohort.Median body mass index was 24.0 kg/m2.Two thirds of the patients underwent preoperative stenting of the common bile duct(65.7%).Levels of protein and CEA were not predictive factors in survival rate.Table 2 demonstrates the most common symptoms being present at the time of diagnosis.Obstructive jaundice was present in 52.7% of the patients.Abdominal discomfort or pain was reported by 39.5% of the patients.Pancreatitis,diarrhea or deterioration of diabetes mellitus were rare underlying clinical findings (<6%).Coincidental detection of pancreatic carcinoma e.g.in routine abdominal ultrasound or due to elevated laboratory values was also rare (5.4%).There were few significant differences between the LTS and non-LTS patients.Serum levels of CA19–9 of the non-LTS patients were significantly higher compared to those of LTS patients (126.0 vs.73.0 U/mL;P= 0.032).Serum levels of CA19-9 were not significantly different between AJCC stages IIa and IIb (P= 0.507).Symptoms like reduced general condition,weight loss,nausea or night sweats were significantly associated with LTS(P= 0.035; Table 2 ).
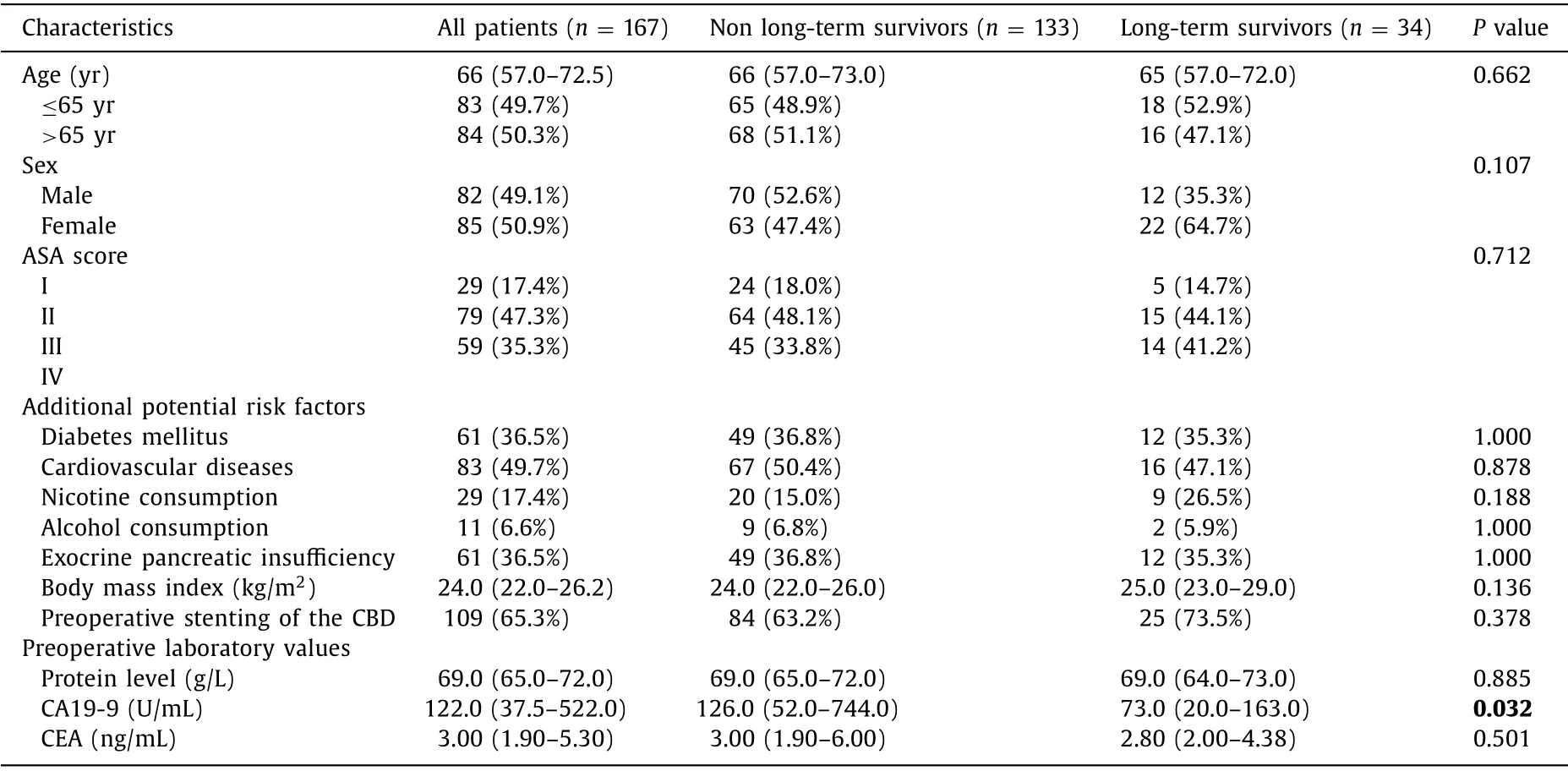
Table 1 Patient characteristics.

Table 2 Causes leading to diagnosis
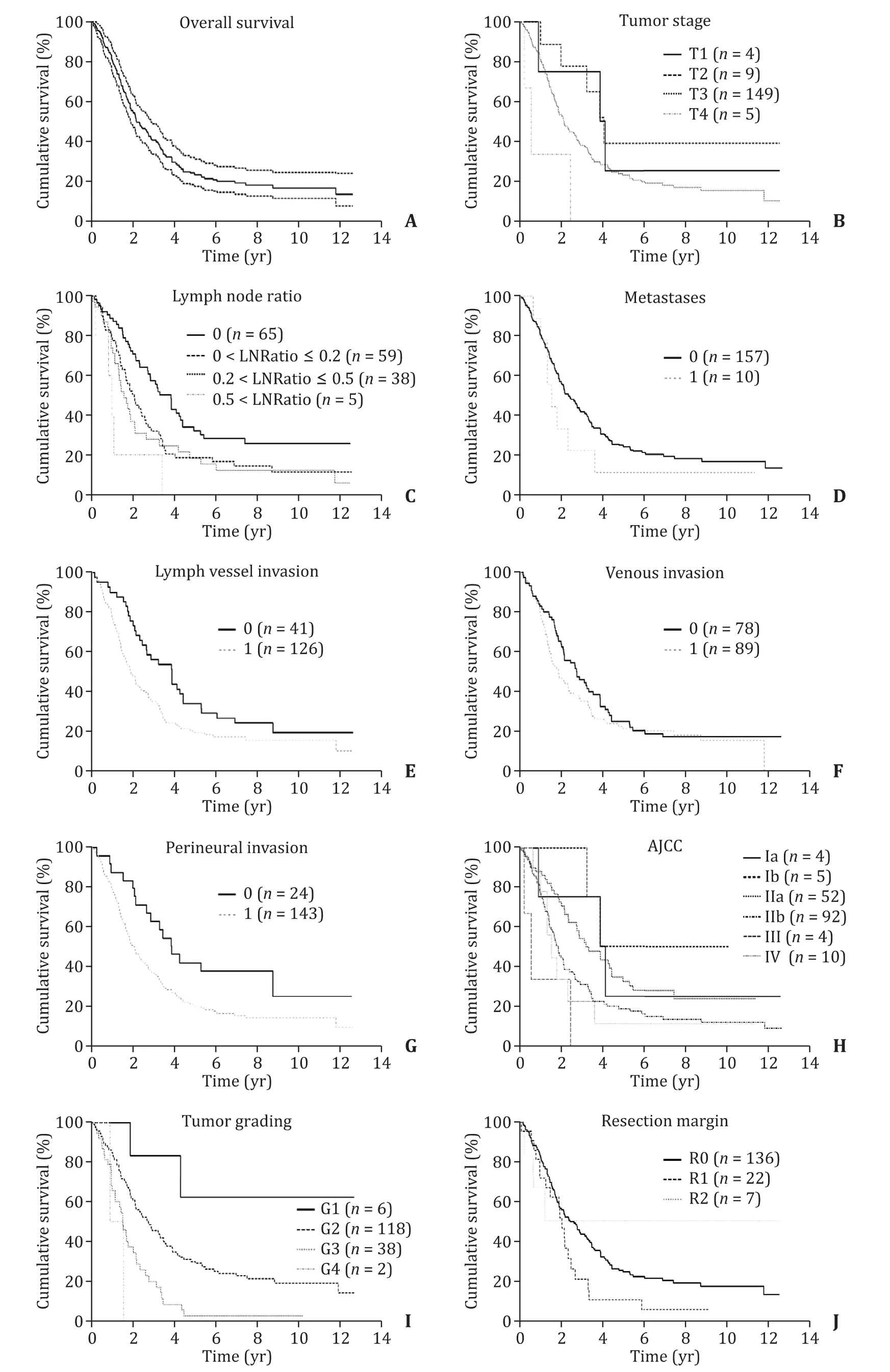
Fig.1.Kaplan-Meier curves in regard to pathohistological findings of the patients.Kaplan-Meier curves indicating cumulative survival of patients with pancreatic cancer (PC)( A ) depending on tumor size (T) ( B ),lymph node ratio ( C ),metastases ( D ),lymph vessel infiltration ( E ),venous infiltration ( F),perineural infiltration ( G ),AJCC (American Joint Committee on Cancer) stage ( H ),tumor grade ( I ) and resection margin ( J ).
Histopathological features
T3 (89.2%) was the most frequent tumor size.T1,T2 and T4 tumors were rare and present in only 2.4%–5.4% ( Table 3 ).There were no patients with T4 tumors in the LTS group.More than half the patients in the LTS group had N0 stage (52.9%).We could not demonstrate a significant difference in LTS depending on lymph node invasion,although more patients with LTS were free of lymph node invasion (P= 0.093).Over 93% of the patients in both groups were free of metastases.Lymph vessel invasion was present in 79.7% of the non-LTS group and in 58.8% of the LTS group (P=0.021).Approximately half of the patients in both groups had venous invasion.Perineural invasion was associated with a lower chance for LTS (P= 0.011).
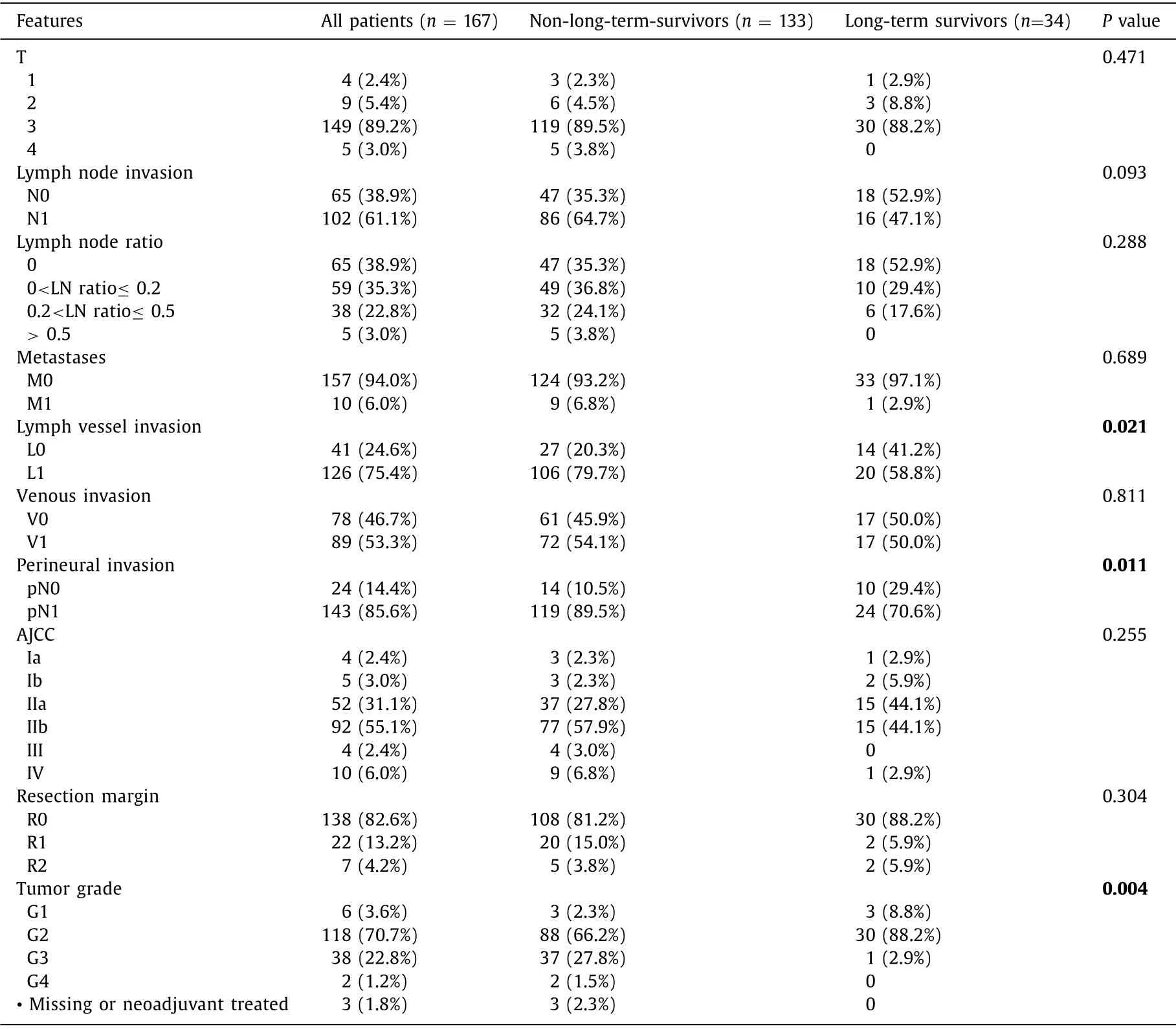
Table 3 Histopathological features according to the American Joint Committee on Cancer (AJCC).
Most patients in the whole cohort undergoing surgery were classified AJCC stage II a and b (86.2%).Stage I (5.4%),stage III(2.4%) and stage IV (6.0%) played only a minor role.82.6% of the patients had negative resection margins (R0).There was no significant difference in resection margin between the groups (P=0.304).Tumor grading was significantly associated with LTS (P=0.004).While most PDACs were classified G2,only 1 patient (2.9%)in the LTS group was classified G3 compared to 37 patients (27.8%)in the non-LTS group.No patient in the LTS group had a G4-staged PDAC.
Postoperative course
The postoperative courses listed in Table 4 were not associated with LTS.Median total hospital stay was 21.0 days and postoperative hospital stay was 15.0 days in both groups.Overall complication rate was 28.1%.Minor complications were present in 21.6%.Major complications occurred in 6.0%.Only one patient in the LTS group suffering from a major complication required revision surgery due to a grade C postoperative pancreatic fistula.The other two patients in the LTS group underwent angiographic coiling of a bleeding right hepatic artery branch and a computedtomography-guided drainage placement.The readmission rate was approximately 8.4%.
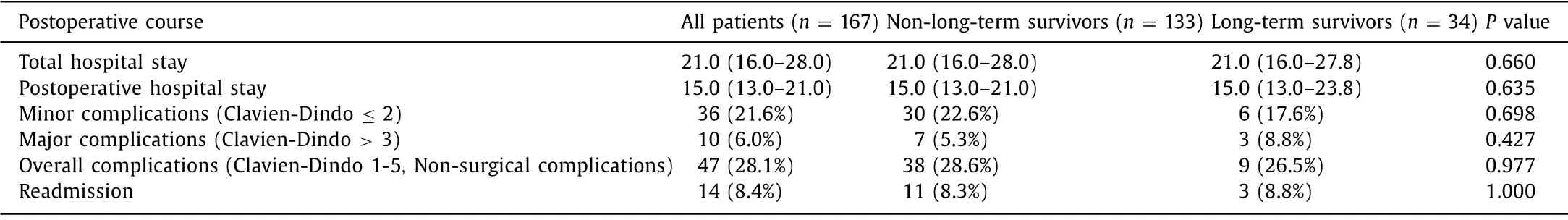
Table 4 Postoperative course.
Postoperative oncologic cours e
Neoadjuvant treatment played a minor role in the whole cohort(n= 7,4.2%).Standard adjuvant chemotherapeutical agent was gemcitabine (70.1%,Table 5 ).Second most common treatment form was combination of gemcitabine with oxaliplatin/cisplatin (n= 11,6.6%),cetuximab (n= 4,2.4%),or erlotinib (n= 1,0.6%) (total:9.6%) followed by gemcitabine + radiation therapy (7.2%).
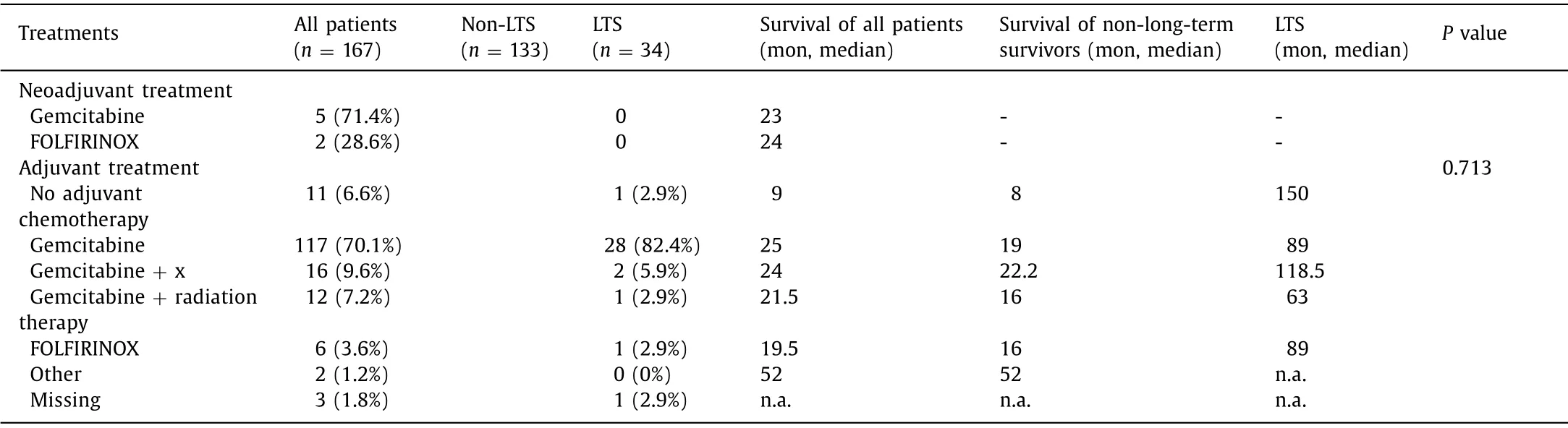
Table 5 Postoperative oncologic course.
Most patients (82.4%,n= 28) in the LTS group received an adjuvant treatment with gemcitabine.Other treatments like administration of a platin based chemotherapy,erlotinib or radiation therapy played a minor role.FOLFIRINOX treatment was associated with LTS in one patient.One patient in the LTS group did not receive adjuvant chemotherapy.The type of chemotherapy was not associated with LTS (P= 0.713).
Multivariable analysis
After exclusion of 4 patients with missing data (1 missing information on stenting of the CBD,3 missing informations on tumor grade),163 patients comprised the analysis set for the parameter selected.All significant parameters from univariable analysis were included.The factors in the model were perineural invasion,lymph vessel invasion,tumor grade,and logarithm of CA19-9.
As shown in Table 6,lower levels of CA19–9,a lower AJCC stage,lower tumor grades,absence of abdominal pain,female gender and a shorter postoperative hospital stay were independent factors associated with a longer survival.Patients with AJCC stage IIb had higher chance of cancer mortality compared to patients with AJCC stage IIa (HR = 1.68,95% CI: 1.08–2.61;P= 0.021).Patients with tumor grade G3 (HR = 2.77; 95% CI: 1.79–4.31;P<0.001) and tumor grade G4 (HR = 15.36,95% CI: 3.03–77.90;P= 0.001) had higher chance for cancer mortality than patients with tumor grade G2.

Table 6 Univariable and multivariable analysis
Moreover,male gender showed an increased likelihood for cancer mortality (HR = 1.48; 95% CI: 1.01–2.18;P= 0.044).Exocrine pancreatic insufficiency was defined as a fecal elastase concentration ≤200 μg/g,which was positively associated with longer survival (HR = 0.61; 95% CI: 0.40–0.93;P= 0.002),whereas abdominal pain was negatively associated with longer survival (HR = 1.55;95% CI: 1.02–2.36;P= 0.040).Furthermore,higher CA19-9 values were associated with lower chance for survival (HR = 1.18; 95% CI:1.05–1.33;P= 0.005).For calculation of the HR,we used the logarithm of CA19-9.
Discussion
This study confirms low rates of LTS in resectable PDAC in a high-volume tertiary center.Moreover,we identified characteristics associated with LTS in a large single center cohort.
PDAC still remains a lethal disease with the lowest 5-year survival rate of all cancer forms ranging between 2.5% and 8% [ 2,8,9 ].While complete surgical resection is still the only curative therapy,even in cases of surgical resectable LTS remains at 20.4% in our cohort.This is in congruent with the current literature and underlines the robustness of our data [ 11,13,14 ].Although 5-year survival is a good result,there is no guarantee for cure,since recurrence can occur in up to 16% of patients with LTS [ 11,13–15 ].A nationwide cohort study by Paniccia et al.with 11081 patients revealed a 10-year survival rate of 3.9% only.However,these data relate to patients who underwent surgery between 1998 and 2002.Better outcomes with improved adjuvant therapy are expected within the following years [16].
Since the rate of LTS is low in PDAC,early identification of patients at risk is an unmet need in postoperative management and follow-up.Importantly,our data identified deterioration of general condition as a potential negative prognostic factor for LTS.Future studies should evaluate whether those patients might benefit from closer follow-up.Among other factors,our study suggests preoperative serum level of CA19-9,tumor stage,tumor grading,lymph node involvement,lymph node ratio,perineural invasion as predictors of LTS,confirming previous literature findings and further highlighting the quality of our data [ 8,11,17–19 ].
Interestingly,our cohort displayed a relatively high rate of exocrine pancreatic insufficiency (36.5%).This might be explained by the high rate of T3 tumor stage (89%) in our cohort.At first sight,exocrine pancreatic insufficiency might appear as detrimental.Nevertheless,our data suggest that exocrine pancreatic insufficiency may be associated with longer survival.Current literature just showed a reduced risk for postoperative pancreatic fistula with consecutive lower rate of morbidity [20].Interestingly,we observed tumor stage,but not resection margin as a predictor of LTS.This seems paradox but can be explained by the high rate of R0 resection (82.6%) in our cohort.Interestingly,two patients with LTS were identified each in the R1- and R2-resected groups.This finding raises the question about the importance of the resection margin on survival.Perhaps in certain cases other determinants of survival may play a leading role,as studies have shown that adjuvant chemotherapy plays an important role additionally to complete surgical resection [ 13,21 ].It remains to be elucidated in which cases the resection margin loses its key role.
Surprisingly,despite the duration of postoperative hospital stay being a significant factor for LTS,our data suggest that postoperative complications do not have a significant impact on LTS at similar postoperative hospital stay.The CONKO-001 study demonstrated a significant longer survival with gemcitabine (5-year overall survival 20.7%) compared to patients being observed only (10.4%).Since the ESPAC-3(V2) study demonstrated a significantly lower rate of chemotherapy-related adverse events (7.5% vs.14%) most of our patients (86.9%) received a gemcitabine-based chemotherapy instead of 5-FU [19].As White et al.have proposed,chemotherapy should be initiated within a 66-day window postoperatively to increase the chance for LTS [17].We did not observe any significant difference in the overall survival when using the adjuvant treatments,probably due to the limited number of patients receiving a different regimen than single gemcitabine therapy.This study analyzed patients in the era before mFOLFIRINOX and gemcitabine/nab-paclitaxel became common regimes.Nevertheless,because of the observational character of this study,efficacy of regimes cannot be evaluated without high risk of bias due to selection.Promising survival results regarding different regimen have been published and proposed recently.Conroy et al.observed a median postoperative survival of 54.4 months with adjuvant treatment of modified FOLFIRINOX (gem.35 months) [22].As neoadjuvant therapy is not suggested in the German guidelines for pancreatic cancer,it has been performed only in rare experimental cases or in cases of locally advanced carcinomas in this study.
As reported in other studies with 4.5%–5.9% only,we observed complete pathological response with neoadjuvant FOLFIRINOX treatment two times [23–27].One of the two patients reported even suffered from liver metastases before chemotherapy and was free of metastases and any tumor load afterwards [ 1,28 ].Neoptolemos et al.reported a significant increase of survival in patients with gemcitabine combined with capecitabine (median survival 28 months) compared to patients treated with gemcitabine only (median survival 25.5 months) [29].
Although it takes nearly 20 years from initiation of the malignant clone to metastatic tumor state,diagnosis is often made at advanced stages because PDAC is most commonly asymptomatic.As our study showed,abdominal pain is associated with a worse prognosis in terms of survival.Furthermore,abdominal pain might just be a surrogate for perineural invasion by larger tumors.Routine CT-scan or MRI of the abdomen is not indicated for screening and further cannot detect the early stages or precursor lesions such as pancreatic intraepithelial neoplasias.Liquid biopsy enabling detection of circulating tumor cells,cell-free nucleic acid or exosomes which can become an important assay in the future to detect early cancer [ 2,30,31 ].Operative technique is crucial for postoperative survival.Early tumor resection,a R0 resection and a low postoperative morbidity allowing a timely initiation of adjuvant chemotherapy play a major role.Several techniques like the modified singleloop reconstruction have been established to reduce the incidence and severity of postoperative pancreatic fistula,which is the most feared complication in pancreatic surgery [ 20,32–34 ].Last,an effective adjuvant chemotherapy significantly prolongs the postoperative survival [ 13,19,22 ].Sadly,even in cases of complete resection more than 80% of the patients ultimately die due to tumor recurrence and/or distant metastases [35].Requirements for effective chemotherapy include longer median survival,increased time to tumor recurrence,good tolerance and a low rate of side effects.Eventually neoadjuvant therapy will become standard,following the fate of esophageal and rectal cancer treatment.As Versteijne et al.demonstrated,the R0 rate seems higher with a neoadjuvant approach.Further,neoadjuvant treatment can turn a primary unresectable tumor into a secondary resectable tumor in up to 40%,with similar survival rates of approximately 24.6 months compared to that of tumors being resectable initially [ 36,37 ].
In conclusion,a multimodal approach including precise,preoperative diagnostic studies,perfect surgical approach,low morbidity as well as an effective perioperative chemotherapy is required to increase the chance for LTS.AJCC stage,tumor grading,the level of CA19–9,abdominal pain,male,exocrine pancreatic insufficiency and duration of postoperative hospital stay are independent factors affecting survival in patients with PDAC of the pancreatic head.
Acknowledgments
We thank Jimmy Phan for language editing of the manuscript.
CRediTauthorshipcontributionstatement
AndreasMinhLuu:Conceptualization,Methodology,Software,Analysis,Data curation,Writing – original draft.WaldemarUhl:Data curation,Writing – review & editing,Supervision.ChrisBraumann:Conceptualization,Formal analysis,Writing – review & editing.OrlinBelyaev:Writing – review & editing.MonikaJanot-Matuschek:Data curation,Writing – review & editing.Michael Praktiknjo:Analysis,Data curation,Writing – review & editing.HenrikRudolf:Data curation,Analysis.
Funding
None.
Ethicalapproval
This study was approved by the Institutional Review Board of the Ruhr-University of Bochum (trial number: 19-6771-BR).
CompetinginterestNo benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Cross-talk between hepatic stellate cells and T lymphocytes in liver fibrosis
- Diabetes mellitus is a risk factor of acute kidney injury in liver transplantation patients✩
- Hepatobiliary&Pancreatic Diseases International
- Application of machine learning models for predicting acute kidney injury following donation after cardiac death liver transplantation
- Postoperative adjuvant transcatheter arterial chemoembolization improves the prognosis of patients with huge hepatocellular carcinoma
- The effects of stereotactic body radiotherapy on peripheral natural killer and CD3 + CD56 + NKT-like cells in patients with hepatocellular carcinoma
