ATP-citrate lyase regulates stemness and metastasis in hepatocellular carcinoma via the Wnt/ β-catenin signaling pathway
2021-07-24QinHnCiAnChenWenYngDongLingHongWeiLvGuiShuiLvQinNiZongHongYngWng
Qin Hn ,b,#,Ci-An Chen ,b,#,Wen Yng ,b,#,Dong Ling ,c,Hong-Wei Lv ,b,Gui-Shui Lv ,b,Qin-Ni Zong ,b,Hong-Yng Wng ,b,d,∗
a International Co-operation Laboratory on Signal Transduction, Eastern Hepatobiliary Surgery Hospital, Second Military Medical University, Shanghai 200438, China
b National Center for Liver Cancer Research, Shanghai 20 0 0 0 0, China
c Mengchao Hepatobiliary Hospital of Fujian Medical University, Fuzhou 350108, China
d State Key Laboratory of Oncogenes and Related Genes, Shanghai Cancer Institute, Renji Hospital, Shanghai Jiao Tong University, Shanghai 20 0 032, China
Keywords:Hepatocellular carcinoma ATP-citrate lyase Liver tumor-initiating cells Metastasis β-catenin
ABSTRACT Background:Hepatocellular carcinoma (HCC) is one of the most highly malignant tumors.Liver tumorinitiating cells (LTICs) have been considered to contribute to HCC progression and metastasis.ATP-citrate lyase (ACLY),as a key enzyme for de novo lipogenesis,has been reported to be upregulated in various tumors.However,its expression and role in HCC and LTICs remain unknown.Methods:The expressions of ACLY in HCC tissues were detected by quantitative real-time PCR (qRT-PCR),Western blotting and immunohistochemistry.Kaplan-Meier curves and Chi-square test were used to determine the clinical significance of ACLY expression in HCC patients.A series of assays were performed to determine the function of ACLY on stemness,migration and invasion of HCC cells.Luciferase reporter assay,Western blotting and immunoprecipitation were used to study the regulation of the Wnt/ β-catenin signaling by ACLY.Rescue experiments were performed to investigate whether β-catenin was the mediator of ACLY-regulated stemness and migration in HCC cells.Results:ACLY was highly expressed in HCC tissues and LTICs.Overexpression of ACLY was significantly correlated with poor prognosis,progression and metastasis of HCC patients.Knockdown of ACLY remarkably suppressed stemness properties,migration and invasion in HCC cells.Mechanistically,ACLY could regulate the canonical Wnt pathway by affecting the stability of β-catenin,and Lys49 acetylation of βcatenin might mediate ACLY-regulated β-catenin level in HCC cells.Conclusions:ACLY is a potent regulator of Wnt/ β-catenin signaling in modulating LTICs stemness and metastasis in HCC.ACLY may serve as a new target for the diagnosis and treatment of HCC.
Introduction
Liver cancer is the sixth most frequently diagnosed cancer and the fourth leading cause of cancer-related deaths worldwide [1].It is responsible for approximately 782000 deaths in 2018 [1].Hepatocellular carcinoma (HCC) is the most common histological type of liver cancer,accounting for 75% to 85% of all cases [1].The rapid progression and metastasis are the leading causes of treatment failure and poor prognosis in HCC patients [2].Thus,elucidation of the intricate molecular mechanisms that drive HCC progression and metastasis,which may provide clues for novel therapeutic strategies to improve the clinical outcomes of HCC patients,is urgently needed.
The concept of cancer stem cells (CSCs,also called tumorinitiating cells,TICs) is one of the main models of tumor development.It proposes that cancer cells differ in their tumorigenic abilities,and only a small subset of stem cell-like cancer cells,CSCs,harbor the ability to self-renew [ 3,4 ].Although this hypothesis is still being debated,the validity of the CSC model has been proven in various tumors including HCC [5–7].Accumulating evidences have revealed that liver tumor-initiating cells (LTICs) contribute to HCC progression and metastasis [8].Therefore,exploration of the molecular mechanisms involved in LTICs is important.
Unlike most normal cells and tissues,which satisfy their requirement for fatty acids by importing them from the circulation,tumor cells display high rates ofdenovolipogenesis [ 9,10 ].This type of metabolic reprogramming is now considered pivotal for membrane biogenesis,energy storage and the generation of signaling molecules in rapidly proliferating cancer cells and has been viewed as a hallmark of cancer cells [11].The elevateddenovofatty acid synthesis observed in tumors is reflected by the increased expression of lipogenic enzymes,including fatty acid synthase (FASN),acetyl-CoA carboxylase (ACC) and ATP-citrate lyase(ACLY),which has been described in multiple cancers [12].ACLY,which cleaves citrate to produce acetyl-CoA and oxaloacetate in the cytoplasm,is a key enzyme linking glucose/glutamine metabolism anddenovofatty acid synthesis [13].As the main catalytic product of ACLY,acetyl-CoA is not only the requisite building block for endogenous synthesis of lipids but also the donor substrate used by lysine acetyltransferase (KAT) for histone acetylation.Many studies have shown that ACLY is upregulated in tumors [14],and its overexpression is associated with tumor growth and poor prognosis in ovarian cancer [15],lung adenocarcinoma [16]and acute myeloid leukemia [17].However,to date,the expression and biological effects of ACLY in HCC have not yet been reported.The present study was to investigate whether ACLY is a new target for the diagnosis and treatment of HCC.
Methods
Patient samples and cell lines
Tissue microarrays (TMAs) and frozen biopsies were obtained from HCC patients who underwent liver resection at Eastern Hepatobiliary Surgery Hospital (Shanghai,China).The TMAs contained 102 primary HCC tissues,of which 100 were paired samples with the corresponding nontumorous liver tissues.Pathologic diagnosis was performed by independent pathologists.Informed consent was obtained from all patients before collection of the resected tissues.
The human HCC cell lines Huh7,HCC-LM3,and PLC were purchased from the Shanghai Cell Bank of the Chinese Academy of Sciences (Shanghai,China).All cells were routinely cultured in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen,Carlsbad,CA,USA) containing 10% fetal bovine serum (FBS; Life Technologies,Grand Island,NY,USA) in a 5% CO 2 incubator at 37 °C.
Antibodies and reagents
Antibodies for ACLY (#4332,1:1000),β-actin (#3700,1:1000),β-catenin (#8480,1:1000),c-Myc (#13987,1:1000),cyclin D1(#2978,1:1000),acetyl-β-catenin (Lys49) (#9030,1:100 for immunoprecipitation (IP),1:1000 for Western blotting) and CREBbinding protein (CBP) (#7389,1:100 for IP,1:1000 for Western blotting) were purchased from Cell Signaling Technology (Beverly,MA,USA).Antibodies for Lamin B1 (ab16048,1:1000) were purchased from Abcam (Cambridge,MA,USA).Cycloheximide (CHX)was purchased from Sigma-Aldrich (St.Louis,MO,USA).MG-132 and EX527 were purchased from Selleck (Shanghai,China).Xenolight D-Luciferin-K + Salt Bioluminescent Substrate was purchased from PerkinElmer (Waltham,MA,USA).
Western blotting and IP
The cells were lysed in RIPA buffer supplemented with protease inhibitor (Roche,Basel,Switzerland) and centrifuged at 12000 rpm,at 4 °C,for 15 min.A PierceTMBCA Protein Assay kit(Thermo Fisher,Waltham) was used to measure the protein concentrations.After electrophoresis on 8%–10% Bis-Tris protein gels,the proteins were transferred to nitrocellulose membranes (GE Healthcare,Chicago,IL,USA),which were then incubated with the specific primary antibodies overnight at 4 °C,followed by a fluorescein-conjugated secondary antibody.Lastly,the membranes were scanned with an Odyssey scanner (Li-Cor,Lincoln,NE,USA).The relative protein levels were quantified and normalized to theβ-actin or GAPDH levels.Each graph is representative of at least three independent experiments.
For IP,cell lysates were incubated with the indicated antibodies overnight at 4 °C followed by incubation with protein A/G beads(Pharmacia,Piscataway,NJ,USA) for 2 h at 4 °C.The immunoprecipitates were washed five times and then subjected to Western blotting analysis.
RNA extraction and quantitative real-time PCR (qRT-PCR)
Total RNA of the frozen HCC tissues and the cultured cells was extracted using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions.Then,the total RNA samples were reversetranscribed to cDNA using Superscript III RT (Invitrogen) and random primers.qRT-PCR was conducted with the SYBR Green PCR kit on a LightCycler® 96 Real-Time PCR System (both from Roche,Mannheim,Germany).The fold change in the mRNA expression was determined using theΔΔCt method,with the housekeeping gene actin beta (ACTB) or 18S for normalization.The PCR primer sequences are shown in Supplementary Table S1.
Magnetic sorting of OV6 + HCC cells
The cells were labeled with anti-Human/Rat OV6 monoclonal antibody (mouse IgG1) (R&D Systems,Minneapolis,MN,USA),followed by the incubation with rat anti-mouse IgG1 microbeads(Miltenyi Biotec,Bergisch Gladbach,Germany).Then,microbeadslabeled cells were separated by a MACS LS column (Miltenyi Biotec) and collected separately.
RNA interference and plasmids
Small interfering RNA (siRNA) targeting ACLY and a negative control (designated siACLY and siCtrl,respectively) were designed and synthesized by RiboBio Co.,Ltd.(Guangzhou,China).ACLY knockdown and control lentiviruses (designated shACLY and shCtrl,respectively) were constructed by GeneChem Co.,Ltd.(Shanghai,China).The RNA interference (RNAi) target sequence was as follows: GAC CAA AGA TGG AGT CTA T.Huh7 and HCC-LM3 cell lines were infected with lentivirus at a multiplicity of infection (MOI) of 20 in the presence of polybrene (8μg/mL) for 12 h.The infected cells were selected with 5μg/mL puromycin (Sigma,St.Louis,MO,USA).The ACLY plasmid and empty vector were a gift from Professor Qun-Ying Lei of Fudan University.The recombinantβ-catenin(S37Y) expression plasmid and control vector were cloned as described previously [18].
Luciferase reporter assay
ACLY knockdown or overexpression cells and the corresponding control Huh7 and HCC-LM3 cells grown in 48 well plates were transiently transfected with the TOP-Flash luciferase reporter constructs (a gift of B.Vogelstein) [19]using Lipofectamine 2000 (Invitrogen,Carlsbad,CA,USA).The Renilla luciferase reporter vector(pRL-TK) (Promega,WI,USA) was cotransfected for normalization.Luciferase activities were measured using the Dual Luciferase kit(Promega) according to the manufacturer’s instructions 48 h post transfection.All experiments were performed in triplicate.
Sphere-forming and colony formation assays
For the sphere-forming assay,the cells were seeded in lowadhesion 6-well plates (3000 cells/well) and cultured in 2 mL DMEM without FBS for 7 days.For the colony formation assay,the cells were seeded in 6-well plates (1000 cells/well) with 2 mL DMEM containing 10% FBS.After growing for 2 weeks,the cells were fixed and stained with crystal violet (Sigma-Aldrich) and counted.
Flow cytometry analysis
Single-cell suspensions were harvested at the indicated time points,washed,and stained with PE-conjugated anti-CD44 (Biolegend,San Diego,CA,USA) or PE-conjugated anti-CD133 (Miltenyi Biotec,Bergisch Gladbach,Germany) for 30 min at room temperature followed by flow cytometry analysis using a Moflo XDP flow cytometer (Beckman Coulter,Brea,CA,USA).
Cell migration and invasion assays
For the cell migration assay,equal numbers of cells (1 × 105cells) resuspended in 200 μL of serum-free DMEM were added to the upper transwell chamber (Corning Incorporated Costar,NY,USA),and 500 μL DMEM containing 10% FBS was placed in the lower chamber of a 24-well plate.After 24 h,the cells that had migrated through the membrane were fixed in 4% paraformaldehyde and stained with 0.1% crystal violet solution.The numbers of cells were counted using an IX70 microscope (Olympus,Tokyo,Japan)and then imaged.Experiments were repeated independently three times.
The cell invasion assay was performed in a similar manner to the migration assay,except that the cells (2 × 105cells) were placed in the upper invasion chamber (BD Biosciences,Franklin Lakes,NJ,USA).The cells that had invaded the lower surface of the membrane through the matrix layer after 48 h were calculated.
Statistical analysis
All statistical analyses were carried out with SPSS Statistics 19.0(IBM SPSS,19.0,Armonk,NY,USA).Data were presented as the mean ± standard deviation (SD).Comparison of two mean values was performed using the two-tailed unpaired Student’st-test.For the analyses of paired samples for qRT-PCR,pairedt-test was used;for the analyses of paired samples in TMA,Wilcoxon rank-sum tests were applied.Survival analysis was performed using Kaplan–Meier curves.Chi-square test was used to analyze the association between expression of ACLY and expression of stemness markers in HCC tissues,and expression of ACLY and disease progression of HCC patients.The statistical significance was set atP<0.05.
Results
ACLY is upregulated in HCC tissues and correlated with HCC prognosis
We first evaluated the expression of ACLY in human HCC tissue samples.The mRNA expression of ACLY in HCC samples from the HCCDB ( http://lifeome.net/database/hccdb ) was analyzed.ACLY was significantly increased in the HCC tissues compared with that in the paired adjacent normal tissues ( Fig.1 A).Then qRT-PCR and Western blotting were used to verify these observations.Consistent with these findings,the ACLY mRNA and protein expressions were dramatically enhanced in primary HCC tissues compared with their adjacent normal tissues ( Fig.1 B,C).To further confirm our findings,we employed TMAs to examine the ACLY protein levels by immunohistochemistry.As shown in Fig.1 D,in 100 matchedpairs of HCC and adjacent normal tissues,the protein levels of ACLY in HCC tissues were significantly higher than those of adjacent normal tissues.Moreover,Kaplan-Meier survival analysis and log-rank tests using clinical data from the HCCDB showed that the overall survival of the patients with high ACLY expression was significantly poorer than that of the patients with low ACLY expression ( Fig.1 E).Furthermore,when we examined the protein levels of the important LTICs markers CD133 and Oct-4 using the above TMAs,we found that high ACLY group more frequently expressed high CD133 and Oct-4 levels in HCC tissues ( Table 1 ).These results demonstrated that ACLY is a significant prognostic factor and may have a regulatory role in LTICs.
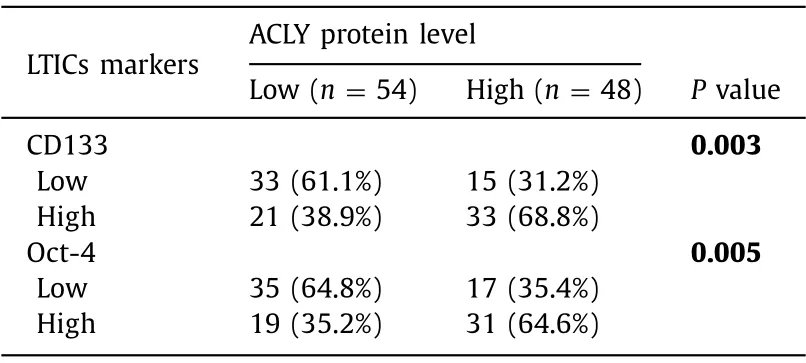
Table 1 Association between expression of ACLY and LTICs markers in HCC TMAs.
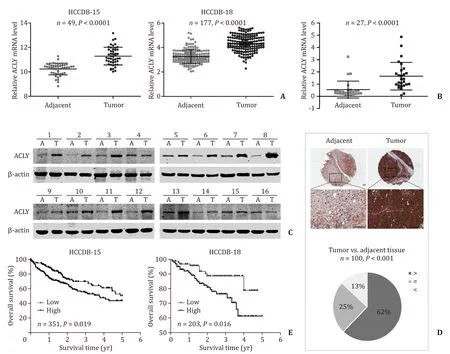
Fig.1.ACLY was overexpressed in primary HCC tissues and correlated with poor prognosis.A : The mRNA expression of ACLY in HCC tissues compared with their paired adjacent non-tumor tissues from HCCDB; B and C : the mRNA and protein expression levels of ACLY in HCC tissues compared with their paired adjacent non-tumor tissues;D : immunohistochemistry detection of ACLY protein levels in HCC tissues and the adjacent non-tumor tissues in tissue microarrays (TMAs).Upper panel: representative immunohistochemistry images; lower panel: pie chart of the statistical comparison between the two groups.Scale bar,100 μm; E : overall survival in relation to ACLY expression from HCCDB.ACLY: ATP-citrate lyase; HCC: hepatocellular carcinoma.
ACLY is highly expressed in LTICs and crucial for the maintenance of LTICs
Based on the above findings,we investigated the effect of ACLY on LTICs.We have previously found that OV6 + HCC cells exhibit highly invasive and tumorigenic LTIC properties [8].First,OV6 +LTIC-enriched cells were isolated from PLC and Huh7 HCC cell lines by magnetic sorting.qRT-PCR were performed to test ACLY expression in OV6 + and OV6- PLC and Huh7 cells.We found that ACLY was upregulated in OV6 + LTIC-enriched cells compared with the control OV6- HCC cells,accompanied by the upregulation of CSC markers ( Fig.2 A).To further confirm this observation,sphereforming cultures were employed to enrich LTICs in Huh7 and HCCLM3 cell lines based on their self-renewal ability [20].The same results for ACLY expression were found in the sphere cells compared with the corresponding parental HCC cells ( Fig.2 B),suggesting that ACLY was enriched in LTICs.To further investigate the effect of ACLY on LTICs,we knocked down ACLY in the Huh7 and HCC-LM3 cells.The efficiency of ACLY knockdown was verified by qRT-PCR( Fig.2 C) and Western blotting analyses ( Fig.2 D).ACLY silencing substantially decreased the sphere formation ( Fig.2 E) and colony formation ( Fig.2 F) in HCC cells.Knockdown of ACLY significantly reduced the proportion of CD133 + cells and CD44 + cells ( Fig.2 G,H).Additionally,the expressions of stemness-related markers were dramatically decreased in the shACLY cells compared to those in the shCtrl cells ( Fig.2 I).Taken together,these data suggest that ACLY is preferentially expressed in LTICs and plays a crucial role in the maintenance of LTICs.

Fig.2.ACLY is required for the maintenance of LTICs.A : ACLY is preferentially expressed in OV6 + LTIC-enriched cells compared with controlled OV6- HCC cells,accompanied by the upregulation of CSC markers; B : Expression of ACLY and CSC markers in LTICs-enriched tumor spheres and control cells; C : ACLY-siRNA decreased ACLY expression; D :The efficiency of ACLY knockdown in HCC cell lines; E and F : The effect of ACLY silencing on sphere formation and colony formation capacities of LTICs.Scale bar,150 μm;G and H : The proportions of CD133 + and CD44 + cells in shACLY and shCtrl cells; I : Stemness-related markers in shACLY and shCtrl cells.∗: P < 0.05; ∗∗: P < 0.01; ∗∗∗:P < 0.001.ACLY: ATP-citrate lyase; LTIC: liver tumor-initiating cell; HCC: hepatocellular carcinoma.
ACLY silencing suppresses migration and invasion in HCC cells
Given the importance of CSCs in tumor progression and metastasis,we investigated the effect of ACLY on metastasis in transwell migration and invasion assaysinvitro.ACLY silencing significantly reduced the cell migration ( Fig.3 A) and invasion ( Fig.3 B) in Huh7 and HCC-LM3 cells compared with the control cells.Further analysis of clinical parameters related to disease progression revealed that HCC patients with high ACLY mRNA levels exhibited more advanced TNM stages and more frequently portal vein invasion( Table 2 ),where the latter is a critically important step for liver cancer metastasis.Taken together,these data indicate that ACLY is an important regulator of HCC metastasis.
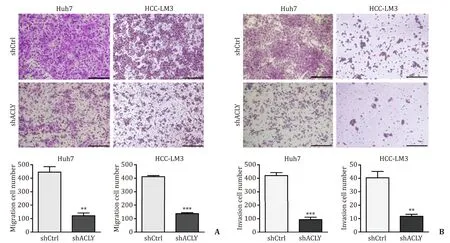
Fig.3.ACLY silencing suppresses migration and invasion of HCC cells.A and B : ACLY knockdown inhibited cell migration and invasion.Scale bar,150 μm.∗∗: P < 0.01; ∗∗∗:P < 0.001.ACLY: ATP-citrate lyase.

Table 2 Association between ACLY expression and HCC progression from HCCDB-18.
ACLY regulates the Wnt/ β-catenin pathway
Since Wnt/β-catenin signaling is one of the major pathways regulating normal adult stem cells and CSCs [21],and this axis is involved in HCC invasion and metastasis [22],we hypothesized that Wnt/β-catenin signaling might play a role in mediating the above phenotypic changes caused by ACLY downregulation.To test this conjecture,we performed a TOP-Flash luciferase reporter assay to detect the transcriptional activity of theβ-catenin/T-cell factor 4 (TCF4) complex,which activates the transcription of Wnt/βcatenin target genes.Knockdown of ACLY dramatically attenuated the activity of the Wnt signaling reporter ( Fig.4 A),while overexpression of ACLY significantly enhanced the TOP-Flash activity( Fig.4 B).Validation of the expression of WNT/β-catenin downstream targets by qRT-PCR and Western blotting analyses of the ACLY knockdown and overexpression HCC cells further confirmed this finding.The mRNA and protein levels of two key WNT/βcatenin pathway targets,c-MycandCCND1,were evidently reduced in the ACLY knockdown HCC cells compared to those in the control cells ( Fig.4 C,D),while overexpression of ACLY in these cells resulted in enhanced expression of the target gene ( Fig.4 E).However,the expression changes ofCTNNB1were occurring at the protein level but not at the mRNA level ( Fig.4 C-E).β-catenin,as a transcriptional coactivator,is the core component of the transcriptional complex that activates downstream genes of Wnt/β-catenin signaling [23].Its accumulation in the cytoplasm and subsequent translocation into the nucleus to complex with TCF4 are the key steps for the modulation of Wnt/β-catenin pathway.We evaluated the nuclear level ofβ-catenin by Western blotting.As shown in Fig.4 F,the protein level ofβ-catenin in the nucleus was remarkably reduced in Huh7 shACLY cells compared with that in the shCtrl cells,which further confirmed that ACLY regulated Wnt/βcatenin pathway by modulating theβ-catenin protein level.
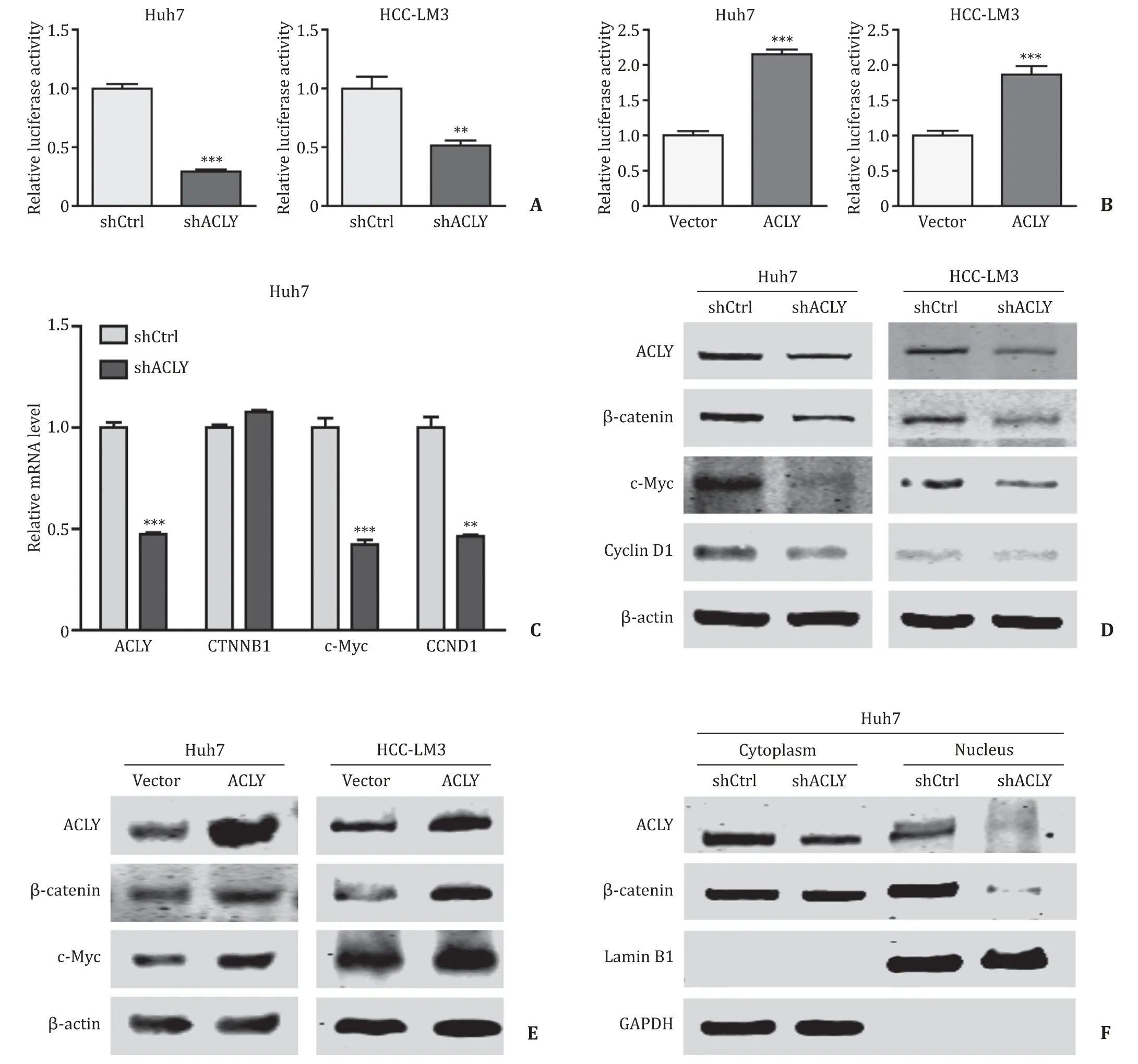
Fig.4.ACLY regulates Wnt/ β-catenin pathway.A and B : The effects of ACLY knockdown/overexpression on the TOP-Flash luciferase reporter activity; C and D : ACLY knockdown decreased the mRNA ( C ) and protein ( D ) expression of WNT/ β-catenin pathway target genes c-Myc and CCND1; E : ACLY overexpression upregulated β-catenin and c-Myc expressions; F : ACLY silencing reduced the nuclear level of β-catenin.∗∗: P < 0.01; ∗∗∗: P < 0.001.ACLY: ATP-citrate lyase.
ACLY regulated Wnt/ β-catenin pathway by modulating the stability of β-catenin
To further test whether ACLY regulates the stability ofβcatenin,we measured the protein half-life ofβ-catenin after treatment with CHX to block protein synthesis.The results showed that knockdown of ACLY significantly accelerated the degradation rate ofβ-catenin ( Fig.5 A).In the presence of MG132,an inhibitor of proteasome-dependent protein degradation,reduction inβ-catenin and its downstream target induced by ACLY silencing was reversed ( Fig.5 B).Together,these results showed that ACLY regulated Wnt/β-catenin pathway by modulating the stability ofβ-catenin.Given previous reports showing that acetylation ofβ-catenin can improve its stability,thereby leading to its accumulation [24],we used EX527,an inhibitor ofβ-catenin deacetylase SIRT1 [25],to examine the effect of deacetylation on the protein level ofβ-catenin in Huh7 cell line.We found that inhibition of the deacetylation ofβ-catenin significantly increased the protein level ofβ-catenin( Fig.5 C).Moreover,EX527 treatment dramatically reversed theβcatenin reduction induced by ACLY knockdown without influencing the ACLY level in HCC-LM3 cell line ( Fig.5 D),suggesting that acetylation ofβ-catenin may mediate the effect of ACLY expression on Wnt/β-catenin signaling.It has been reported that the K49 mutant ofβ-catenin decreased its protein levels compared to the wild-typeβ-catenin [26].Combining the fact that ACLY inhibition or silencing suppresses the synthesis of acetyl-CoA,which is the acetyl donor for protein acetylation [27],we hypothesized that acetylation of the Lys49 residue may be affected by ACLY and play some role in these processes.As shown in Fig.5 E and 5 F,depletion of ACLY dramatically downregulated acetyl-β-catenin (Lys49)and attenuated the interaction between CBP and acetyl-β-catenin(Lys49); the former is responsible forβ-catenin acetylation at the Lys49 site [28].These results suggest that ACLY regulatesβ-catenin stability by modulating Lys49 acetylation.
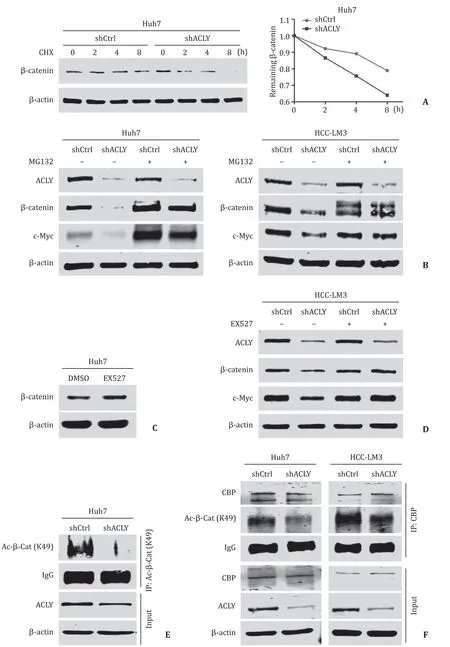
Fig.5.ACLY regulated Wnt/ β-catenin pathway by modulating the stability of β-catenin.A : ACLY knockdown significantly accelerated degradation rate of β-catenin after the treatment of cycloheximide (CHX); B : MG132,a proteasome inhibitor,significantly reversed the expressions of β-catenin and c-Myc reduced by ACLY silencing; C : inhibition of the deacetylation of β-catenin by EX527 enhanced the protein level of β-catenin in Huh7 cell line; D : reduction of β-catenin and its downstream target c-Myc induced by ACLY silencing was reversed in the presence of EX527; E and F : depletion of ACLY expression downregulated acetyl- β-catenin (Lys49) ( E ) and diminished the interaction between CBP and acetyl- β-catenin (Lys49) ( F ).ACLY: ATP-citrate lyase; CBP: CREB-binding protein.
Wnt/ β-catenin pathway mediates the ACLY-regulated stemness and migration of HCC cells
To further investigate whetherβ-catenin is the mediator by which ACLY regulates stemness and migration in HCC cells,we used a mutant activeβ-catenin (S37Y) expression plasmid [pCMVβ-catenin (S37Y)]and the empty vector as a control.We transiently transfected these plasmids to the established Huh7 and HCC-LM3 shCtrl and shACLY cell lines.The effects of the mutantβ-catenin (S37Y) plasmid on the activation of Wnt/β-catenin signaling and the expression ofβ-catenin and target gene were validated by luciferase reporter assay and Western blotting,respectively ( Fig.6 A,B).Then,sphere-forming assays and flow cytometry analysis were performed to test the effect of reactivation of Wnt/β-catenin signaling on the self-renewal ability and proportion of CD44 + LTICs suppressed by ACLY knockdown.Exogenous activeβ-catenin expression significantly reversed the self-renewal and proportion of LTICs ( Fig.6 C,D).Results from transwell migration assays showed that the reduced migration of HCC cells in the ACLY-deficient HCC cells was only partially rescued by expression of activeβ-catenin compared to the control cells ( Fig.6 E).These data indicated that the Wnt/β-catenin pathway mediates the ACLYregulated stemness and migration in HCC cells,and some other factors may play a role in ACLY-regulated migration.
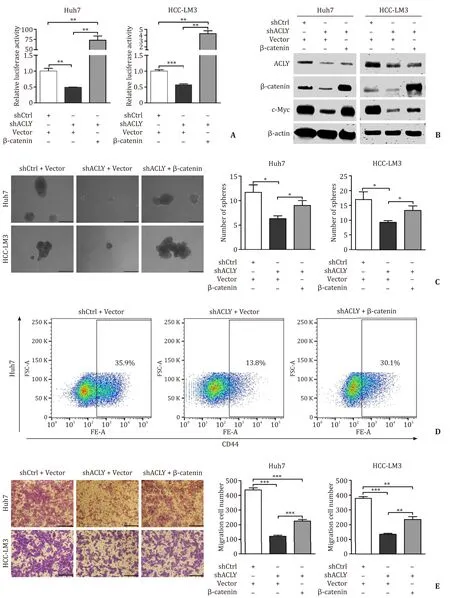
Fig.6.Wnt/ β-catenin signaling mediated ACLY-regulated stemness and migration in HCC cells.A and B : Efficiency of the mutant β-catenin (S37Y) plasmid on the activation of TOP-Flash luciferase reporter and expression of β-catenin and c-Myc; C : Overexpression of β-catenin completely reversed the self-renewal of LTICs in ACLY deficient HCC cells; D : Overexpression of active β-catenin completely reversed the CD44 + LTICs reduced in ACLY deficient HCC cells; E : Overexpression of β-catenin partially rescued the cell migration ability inhibited by ACLY silencing in HCC cell lines.Scale bar,150 μm.∗: P < 0.05; ∗∗: P < 0.01; ∗∗∗: P < 0.001.ACLY: ATP-citrate lyase; LTICs: liver tumor-initiating cells; HCC: hepatocellular carcinoma.
Discussion
In this study,we found that ACLY expression was frequently upregulated in primary HCC tissues.A high level of ACLY was associated with poor overall survival of HCC patients.HCC patients vary greatly in clinical outcome depending on the progression status and aggressiveness of the tumors.Currently,the TNM staging system is the most commonly used clinical prognostic indicator of cancer progression to determine the disease outcome.Metastasis is a specific stage of tumor progression,and distant metastasis always results in poor prognosis in HCC patients.Portal vein invasion is one of the most important steps for intrahepatic and distant metastasis in HCC.Our results showed that ACLY expression was not only correlated with disease progression but also correlated with the clinical metastasis in HCC patients.
On the basis of the discovery of the correlation between ACLY expression and the clinical prognosis,we investigated the effect of ACLY on the stemness of LTICs and metastasis of HCC.We found that ACLY displayed enhanced expression in LTICs enriched by different methods.Knockdown of ACLY dramatically reduced the expression levels of stemness-related genes,self-renewal,clonogenic ability and the percentages of LTICs,indicating that ACLY plays important role in the modulation of stemness of LTICs.Moreover,we proved that ACLY silencing inhibited the migration and invasion of HCC cells.These data combined with the association we found between expression of ACLY and LTICs markers in HCC tissues,and expression of ACLY and clinical progression in HCC samples,indicated that targeting ACLY may be an effective strategy on improving the outcomes of HCC patients.To our knowledge,this study is the first to report these prognostic and biological effects of ACLY in HCC.
Wnt signaling is an evolutionarily highly conserved signaling pathway that regulates normal embryonic development,somatic stem cell maintenance and tissue homeostasis in various tissues,including the liver [ 29,30 ].Moreover,this signaling pathway is involved in tissue repair after damage,cell migration,and genetic stability and instability [31].Aberrant regulation of Wnt signaling has been found to be intensively associated with the carcinogenesis and progression of many cancers,including HCC [ 30,31 ].However,the exact function of Wnt signaling within the different contexts of tumor tissues and cell types is extremely complex and requires further investigation.Our study demonstrated that ACLY knockdown dramatically inhibited the transcriptional activity ofβ-catenin/TCF and decreased the expression of Wnt-responsive genes,suggesting that ACLY could regulate the Wnt/β-catenin cascade in HCC.This link between ACLY,a pivotal molecule in lipid metabolism,and a key signaling pathway involved in HCC,has never been reported before.
Although some doubts remain regarding the exact function of Wnt/β-catenin signaling in HCC,the vital role of Wnt signaling in the maintenance of CSCs has already been commonly accepted.In colon cancer cells,high Wnt signaling activity can define colon CSCs because of its preponderant role in preserving the fate of colon CSCs [32].In HCC,although a defined marker to identify LTICs is still lacking,the activation of Wnt/β-catenin was found in almost all putative marker-positive LTICs,including EpCAM + [6],CD133 + [33],OV6 + [34]and Lgr5 + [35].Aberrant activation ofβcatenin-dependent Wnt signaling was reported to play an essential role in the stemness property of LTICs [ 34,35 ].In our study,we found that ACLY knockdown dramatically inhibited the stemness of LTICs,and exogenous expression of activatedβ-catenin reversed the Wnt/β-catenin signaling attenuation and stemness suppression induced by ACLY silencing,suggesting that ACLY regulated stemness of LTICs by Wnt/β-catenin signaling.These results further confirmed the central role of Wnt/β-catenin pathway in stemness maintenance in LTICs.However,the results of the cell migration assay were slightly different: reactivation of Wnt/β-catenin signaling partially,but not completely,reversed the ACLY depletioninhibited migration,suggesting that some other mechanisms may be involved in this process.A previous study reported that the production of ACLY-dependent acetyl-CoA promotes glioblastoma cell migration and adhesion by regulating H3K27 acetylation sitespecifically and nuclear translocation of the transcription factor NFAT1 [36].Whether these mechanisms also play a role in the effect of ACLY on the migration,invasion and metastasis of HCC cells is of interest.
β-catenin is a central effector of the canonical Wnt signaling pathway.When Wnt signals are absent,cytosolicβ-catenin is sequentially phosphorylated by casein kinase Iα(CK1α) and glycogen synthase kinase 3 (GSK3β),resulting in constant ubiquitination and the subsequent degradation ofβ-catenin by the ubiquitinproteasome system.Upon Wnt activation,phosphorylation ofβcatenin is inhibited,leading to its accumulation in the cytosol and translocation into the nucleus to form transcriptional complexes with T-cell factor/lymphoid enhancer-binding factor (TCF/LEF) to active target genes [31].Thus,the stabilization ofβ-catenin is one of the major mechanisms by which the canonical Wnt signaling pathway is regulated.Our results suggested that ACLY knockdown decreased the stability and protein level ofβ-catenin,whereas ACLY overexpression increased theβ-catenin level.Further analysis showed that ACLY modulated the proteasome-dependent degradation ofβ-catenin,which explained how ACLY regulated the Wnt/β-catenin signaling cascade.Acetylation ofβ-catenin was also shown to have an important role in the regulation ofβ-catenin stability [ 24,37 ].Three lysine residues (K49,K19 and K354) were shown to be acetylated atβ-catenin,and acetylation of K354 was identified critical forβ-catenin interaction with TCF4 [38]and LEF-1 [39].K19 is a major ubiquitination target [37],and its mutation leads to upregulation ofβ-catenin level [26].Only the K49 mutant ofβ-catenin showed substantially decreased protein levels in both the nucleus and cytoplasm compared to the wild-typeβcatenin [26].Lys49 is a highly conserved residue from Drosophila to humans,located near the destruction motif in the N-terminal region ofβ-catenin [26].This amino acid is one of the few residues that are frequently mutated in anaplastic thyroid carcinoma [40].However,its exact function inβ-catenin is unknown.Our results revealed that ACLY knockdown decreased Lys49 acetylation ofβcatenin,which may be the mechanism through which ACLY regulatesβ-catenin stability and participates in the Wnt/β-catenin signaling pathway,and this may also help explain how K49 mutant ofβ-catenin regulated its protein level.However,we did not explore the mechanism by which Lys49 acetylation affectsβ-catenin stability,and further studies are needed to clarify this issue in the future.
In conclusion,ACLY plays an important oncogenic role in the malignant phenotypes of HCC by regulating the Wnt/β-catenin pathway through acetylation ofβ-catenin.ACLY may be used as both a prognostic biomarker and a promising therapeutic target for HCC.
Acknowledgments
We thank Professor Qun-Ying Lei (Fudan University,Shanghai,China) for providing us with the ACLY plasmid.CRediTauthorshipcontributionstatement
QinHan:Data curation,Formal analysis,Validation,Writing -original draft.Ci-AnChen:Investigation,Data curation,Validation,Writing - original draft.WenYang:Conceptualization,Methodology,Supervision,Writing - review & editing,Project administration.DongLiang:Visualization,Software,Data curation,Investigation.Hong-WeiLv:Software,Visualization.Gui-ShuaiLv:Software,Visualization.Qian-NiZong:Software.Hong-YangWang:Funding acquisition,Project administration,Resources,Writing -review & editing,Supervision.
Funding
This work was supported by grants from the National Natural Science Foundation of China ( 81972779 ),Ministry of Education(MOE) Key Laboratory on signaling Regulation and Targeting Therapy of Liver Cancer,and Shanghai Key Laboratory of Hepato-biliary Tumor Biology,Chinese National Key Project ( 2018ZX10723204-006-003 ).
Ethicalapproval
Use of human samples was approved by the Ethical Review Committee of the Eastern Hepatobiliary Surgery Hospital.All animal handling procedures and experimental protocols were approved by the Institutional Animal Care and Use Committee of the Second Military Medical University,Shanghai,China.
Competinginterest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Supplementarymaterials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.hbpd.2020.05.010.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Cross-talk between hepatic stellate cells and T lymphocytes in liver fibrosis
- Diabetes mellitus is a risk factor of acute kidney injury in liver transplantation patients✩
- Hepatobiliary&Pancreatic Diseases International
- Application of machine learning models for predicting acute kidney injury following donation after cardiac death liver transplantation
- Postoperative adjuvant transcatheter arterial chemoembolization improves the prognosis of patients with huge hepatocellular carcinoma
- The effects of stereotactic body radiotherapy on peripheral natural killer and CD3 + CD56 + NKT-like cells in patients with hepatocellular carcinoma
