REDESCRIPTION OF THE MONOZOIC CESTODE KHAWIA ARMENIACA (CHOLODKOVSKY, 1915) SHULMAN, 1958 (CESTODA: CARYOPHYLLIDEA)FROM CYPRINID FISH IN TANA LAKE, ETHIOPIA
2021-07-17KIBETCarolineJepkorirZHAOWenTingSARWARHudaandNIEPin
KIBET Caroline Jepkorir , ZHAO Wen-Ting SARWAR Huda and NIE Pin
(1. State Key Laboratory of Freshwater Ecology and Biotechnology, Key Laboratory of Aquaculture Disease Control, Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan 430072, China; 2. University of Chinese Academy of Sciences, Beijing 100049, China; 3. Zoology Department, Ichthyology Section, National Museums of Kenya, P.O Box 40568-00100, Nairobi,Kenya; 4. School of Marine Science and Engineering, Qingdao Agricultural University, Qingdao 266109, China)
Abstract: Khawia armeniaca (Cholodkovsky, 1915) (Cestoda: Lytocestidae) is described from cyprinids Labeobarbus intermedius and Labeobarbus tsanensis in Tana Lake in Ethiopia. It is characterised with its afossate scolex in a semi-bulbate shape and a smooth anterior margin. Testes are distributed at the median,with the testicular field reaching the anterior region of cirrus sac. Previtelline follicles are distributed at median and lateral body, extending to anterior or posterior region of the cirrus sac. Uterus is looped between the posterior region of the ovary and the posterior region of the cirrus sac, not reaching the anterior region of the cirrus sac. Few vitelline follicles are present along the uterus margin and few or rare along the ovarian margin. K. armeniaca possesses follicular and butterfly-shaped ovary, with gonopores separated but close to each other, and male gonopore is at the anterior region of the female gonopore, but form a common genital atrium at the surface. Postovarian vitelline follicles are less than 100, touching or far from the posterior ovarian arm.
Key words: Lytocestidae; Khawia armeniaca; Morphology; Morphometrics; Tana Lake; Ethiopia
Cestodes, which are obligate endoparasites, can be grouped into unsegmented or monozoic, and segmented or polyzoic tapeworms, the former of which are classified in the subclass Cestodaria, containing three orders, Amphilinidea, Caryophyllidea, Gyrocotylidea[1,2]. Species in the Caryophyllidea van Beneden in Carus, 1863, are normally intestinal parasites of teleost fish, and can be further classified into four families: Lytocestidae Hunter, 1927, Caryophyllaeidae Leuckart, 1878, Capingentidae Hunter, 1930 and Balanotaeniidae Mackiewicz & Blair, 1978,based on the arrangemnt of longitudinal musculature[3].
In the order Caryophyllidea,KhawiaHsü, 1935 in the Lytocestidae, which was erected for the new species,Khawia sinensis, in the intestine of common carpCyprinus carpioin China[4], was considered as probably the most specious genus[5]. However, in a revision with morphological and molecular data,Scholz,et al.[5]only recognized seven valid species from all seventeen nominal species in the genus, includingK. sinensisHsü, 1935,K. armeniaca(Cholodkovsky, 1915) Shulman, 1958,K. balticaSzidat,1941,K. japonensis(Yamaguti, 1934) Hsü, 1935,K.parva(Zmeev, 1936) Kulakovskaya, 1961,K. rossittensis(Szidat, 1937) Markevich, 1951,K. saurogobiiXi, Oros, Wang, Wu, Gao et Nie, 2009. Recently, Xi,et al.[6]reported a new species,K. abbottinaefrom the gudgeonAbbottina rivularis(Cyprinidae: Gobioninae) in Taihu Lake, Wuxi, and from the Yangtze River in Wuhan, China. However, as presented in the revision,K. balticaSzidat, 1941 was phylogenetically separated from other six species of theKhawiaon the basis of ssrDNA and D1-D3 lsrDNA, or mitochondrialnad3 andcox1 genes, or the combination of these markers[5]. Much recently, it has been reported thatK. balticaSzidat, 1941 should be replaced into the genusCaryophyllaeusGmelin, 1970, asC. baltica(Szidat, 1941) Barčák, Oros, Hanzelová et Scholz, 2017[7].
Khawia armeniacawas originally described asCaryophyllaeus armeniacusCholodkovsky, 1915 in the family Caryophyllaeidae, fromCapoeta capoeta sevangi(Cyprinidae: Barbinae) from Sevan Lake in Armenia. But, this species was transferred to the Lytocestidae and theKhawia, because testes in the species are medullarilly located and vitellarium cortically located[8]. This cestode has been reported mainly from barbels in some middle Asian countries such as Armenia, Azerbaidjan, Georgia, Iran, Iraq, Israel, and in some African countries such as Egypt,Morocco, Tanzania, Uganda (for details see the revision by Scholz,et al.[5]In an investigation into the helminth parasite fauna in fish in Tana Lake of Ethiopia, Africa in 2017, a monozoic cestode was collected and it was indicated in an initial examination that this cesotde should belong to the genusKhawia.In consideration of the lack of morphological description of anyKhawiasp. in the lake, cestodes colleted from intestines ofLabeobarbus intermediusandL.tsanensis(Cyprinidae: Barbinae) in Tana Lake,Ethiopia were described in detail. The present study thus represents the first finding ofKhawia armeniacain the Caryophyllidea from fish in Tana Lake, although a previous report has indicated the occurrence ofKhawiasp. in the lake[9].
1 Materials and methods
1.1 Sample locality
Tana Lake is situated about 1800 m above sea level in the north-western highland of Ethiopia, and is the largest lake in the country, which is formed through volcanic activity, ca 5 million years ago to block the course of inflowing rivers. The lake has a surface area of 3673 km2, with a surrounding drainage area of 11650 km2, and a maximum depth of 14 m.The water level has been regulated recently since the construction of control weir, and the lake drains to its southern extremity and to its only outflowing river,the Blue Nile by about 40 m high spectacular falls at Tissisat. In 2015, the Tana Lake region was nominated as UNESCO Biosphere Reserve in recognition of its national and international natural and cultural importance. The geographical location of the lake is 11°25′07′′—12°29′18′′ N, and 36°54′01′′E—37°47′20′′E[10—14].
Being the largest lake in Ethiopia,Tana Lake accounts for 50% of the total inland water of the country, and the Biosphere Reserve is part of the Eastern Afromontane Biodiversity Hotspot with the distribution of many Palaearctic migrant water birds, indigenous trees and agricultural crops. Fish community in the lake is dominated with cyprinid fish, of which about twenty species are endemic. Cyprinids in the lake are represented by four genera,Varicorhinus,Garra,LabeobarbusandBarbus. A total of fifteen species of labeobarbs,Labeobarbusspp., which belong to a unique flock of endemic cyprinids, were supported by the examination of mitochondrial DNA markers[10], and it was reported that they occupied different habitats in relation with water depth and substratum[11]. The possible adaptive speciation hypothesis was proposed to explain the existence of labeobarb species in the lake, in which available new lacustrine habitats since the formation of Tana Lake through the volcanic blocking with the lower Blue Nile might have resulted in the speciation of labeobarbs[12,13], and in the presence of highly variable populations of riverineL. intermediusin the lake[14].
1.2 Cestode collection and examination
Investigations into the helminth parasites of fish in Tana Lake were conducted from the 19thto 25thin May, 2017. Fish were purchased from fishermen in a fishery dock, and fish were either trapped using trap net or caught using fyke net. All fish samples were brought back to a local research station, where they were dissected under a microscope. A total of eightysixLabeobarbus intermediusand eighteenL. tsanensis, with the mean body length of 23.70 cm (± 1.80 SD) and 24.65 cm (± 2.62 SD), respectively, were examined during the investigation.
The cestodes were collected from the intestines of freshly killedL. intermediusandL. tsanensisof the Ethiopian largest lake, Tana Lake, a UNESCO Biosphere reserve[15]. Parasites were washed in 0.9% saline, fixed in hot formalin and transferred to 70% alcohol. For light microscopy, the specimens were stained in iron acetocarmine solution, destained in 70% hydrochloric acid alcohol, dehydrated in a series of gradually increasing percentages of ethanol, 70%,80%, 95% and 100%, clarified in clove oil and mounted in Canada balsam. Measurements were taken under BH-2 Olympus Japan microscope and illustrations were made using a drawing attachment of Olympus MVX10 microscope with the use of Nomarski interference contrast. For histological sectioning, the specimens were embedded in wax, sectioned in 4—8 μm thick through cross and longitudinal sections, stained using Harris’ hematoxylin stain, destained in 1% hydrochloric acid, counterstained in 1% water soluble eosin, dehydrated in alcohol, cleared in Xylene, airdried and mounted in Canada balsams[5,16]. Specimens are deposited at the Institute of Hydrobiology,Chinese Academy of Sciences, Wuhan, Hubei Province, China.
2 Result
2.1 Morphology and morphometric analysis
Family:Lytocestidae Hunter, 1952
Species:Khawia armeniaca(Cholodkowsky,1916), Shulman, 1958
Synonyms:Caryophyllaeus armeniacusCholodkowsky, 1915;Khawia barbiRahemo et Mohammad, 2002;Khawia luteiAl-Kalak et Rahemo,2003,Khawia balticadescribed by Chubb,et al.,1997
Host:Labeobarbus intermediusandLabeobarbus tsanensis, with the prevalence of 16.28%, 16.67%,and the intensity ranging from 0—31, 0—10, respectively.
Site of infection:Intestine
Locality:Tana Lake, Ethiopia
Description based on 10 gravid samples, all measurements are in mm unless otherwise indicated.Body length of (n=8) 30—40, maximum width at 1/3 of the body from the anterior region, being 0.88—1.41, and narrows towards the posterior end. The scolex is afossate, with a semi-bulbate shape and a smooth anterior margin (Fig. 1A) with a width of 1.27—1.79, slightly wider than the neck. The neck width (n=3) is 0.74—1.21. The genital pores are seen on surface, but are separate, being close to each other.The body is covered with acicular fillitriches (Fig. 1B).Tetes are medullary (Fig. 2A), almost oval to spherical, being (95.76—247.38) μm×(99.75—247.38) μm(n=9) in size, and approximately>200 in number, distributed at the median and intermingled with vitelline follicles. Anteriormost testis begins at 2.26—4.38 from the anterior extremity and (n=8) 0.40—2.23 from the posterior of anteriormost vitelline follicle.The testicular field reaches almost the anterior margin of the cirrus sac, representing 47.51%—61.87%of the body length. Cirrus sac is thick and oval in shape (Fig. 2B, 2C, and 2D), with a size of(0.39—0.70)×(0.68—1.43), representing 44.63%—49.30% of the body width. Vas deferent looped at the anterior region of the cirrus sac (Figs. 2C and 3A, B,C, D, E), and the inner seminal receptacle is present.Ovary is follicular, and butterfly shaped with wide and short arms (Fig. 3C, 3D and 3E) at a total length of 0.76—1.80, representing 2.52%—4.48% of the body length. The total width at the isthmus is 0.68—1.24, representing 77.27%—88.03% of the body width. The anterior arm is longer than the posterior arm by 0.24—0.67. Seminal receptacle is present at the dorsal of ovarian isthmus (Fig. 2D).
Vitelline follicles are numerous, spherical to almost oval in shape, being 71.82—159.60 μm×71.82—175.56 μm, located at cortical parenchyma (Fig. 2A),the anteriormost vitelline follicle begins at 1.09—2.15 from anterior extremity. Preovarian vitelline follicle almost reaches the anterior margin of the cirrus sac(Fig. 3D), while in some specimens it reaches almost the posterior margin of the cirrus sac (Fig. 3C and 3E).Few vitelline follicles are present along the uterus margin and few or rare along the ovarian arm (Fig. 3C,3D, and 3E). Preovarian and postovarian vitelline follicles are separated. The postovarian vitelline follicles are approximately<100 in number and touching the ovarian arm (Fig. 3C), while in other species,it is far from the posterior ovarian arm. Uterus looped between posterior region of the ovary and posterior region of the cirrus sac, with a length of 2.23—4.92,representing 7.43%—12.29 % and 47.52%—61.87 %of the body and testicular field length respectively.The uterine gland is present (Fig. 2C). Vagina tubular,almost straight and joins with the uterus to form uterovaginal duct (Fig. 2C and 2D). Genital pores are separated but close to each other. The male gonopore is situated at the anterior region of the uterovaginal pore, but forms common genital atrium at surface. Intrauterine eggs are oval in shape, operculated and unembryonated, with the size of 35.91—55.86 μm×47.88—83.79 μm (Tab. 1).
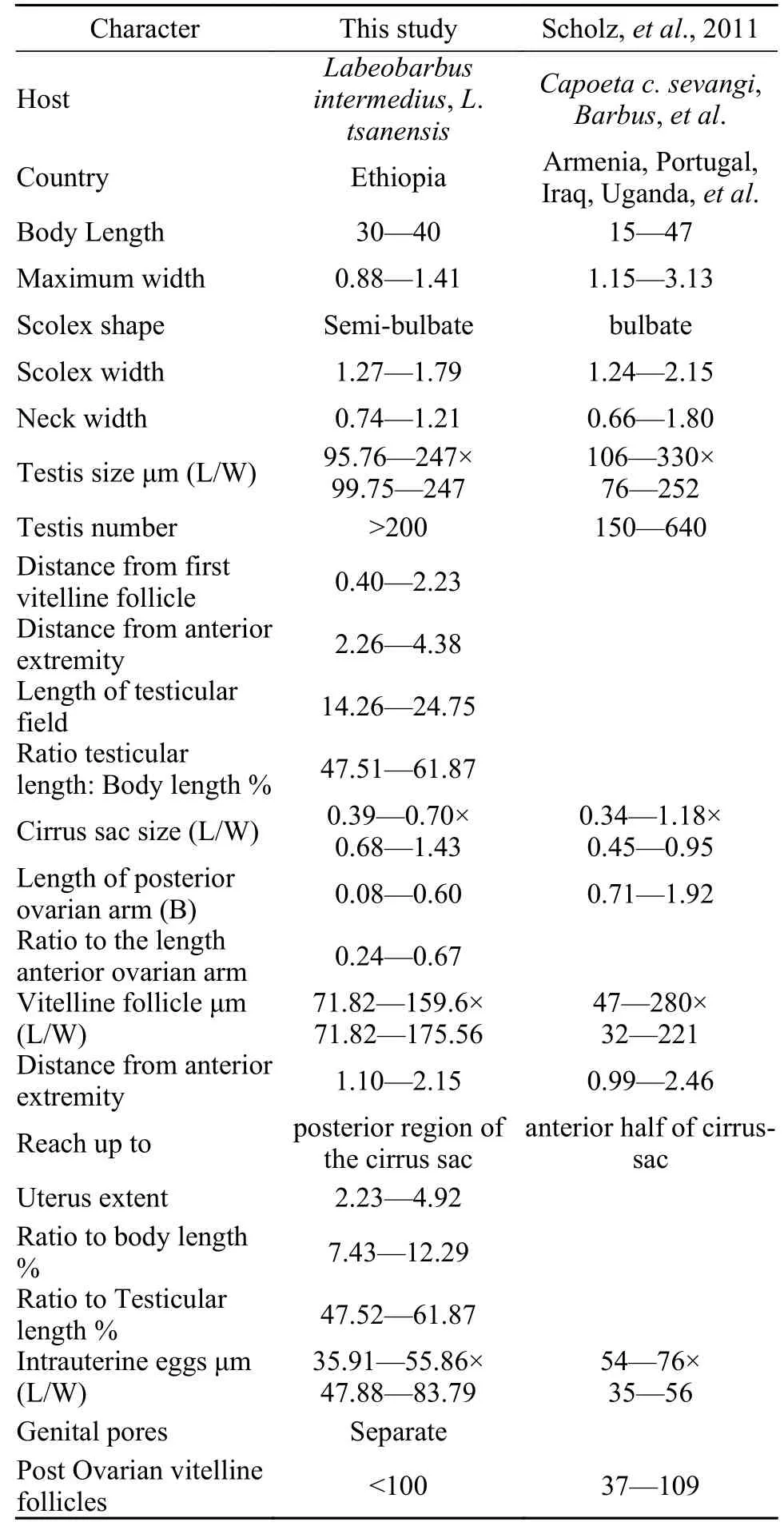
Tab. 1 Morphometrics analysis of Khawia armeniaca (Cholodkovsky, 1915) from different hosts and regions (in millimetres unless otherwise stated)
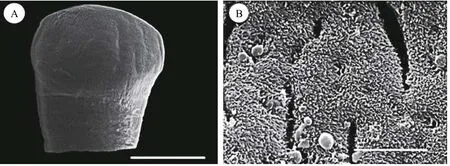
Fig. 1 Scanning electron micrograph (SEM) of Khawia armeniaca from Labeobarbus intermedius in Tana Lake, Ethiopia
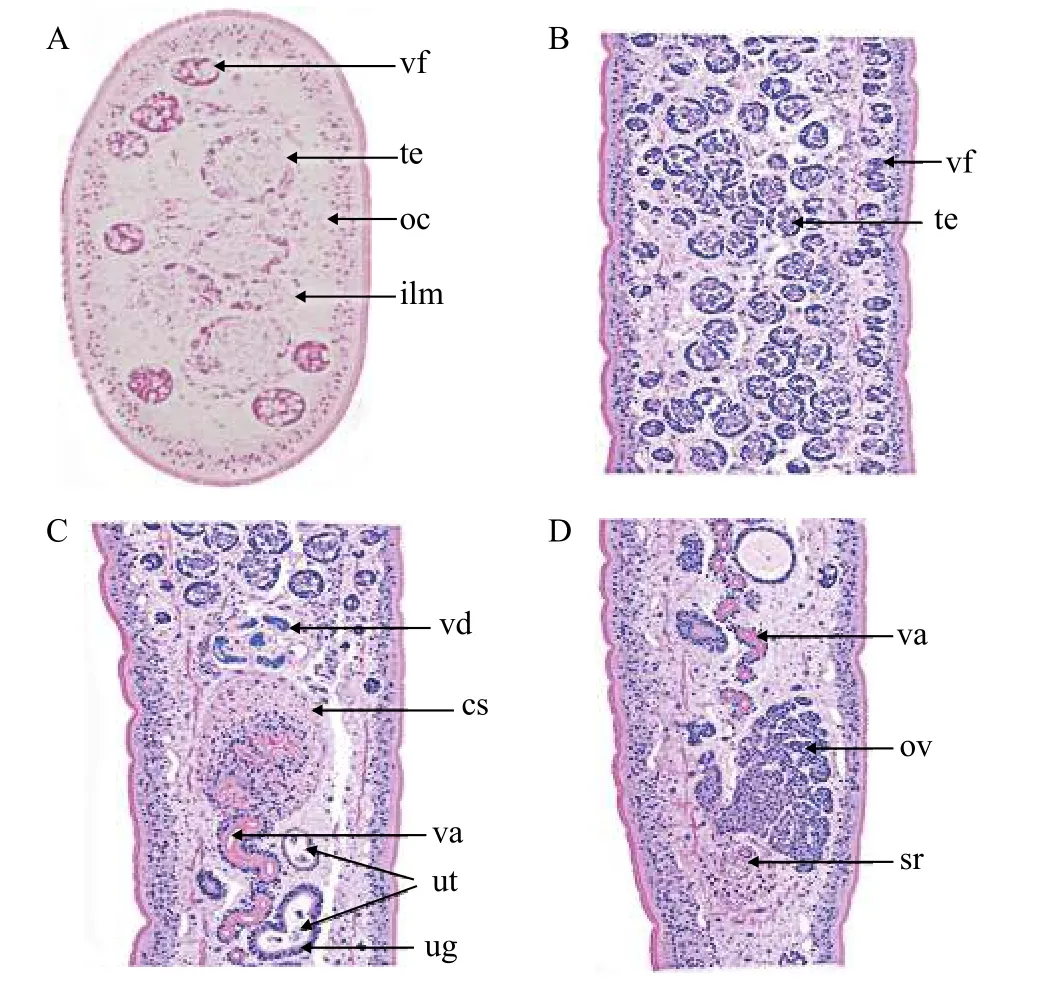
Fig. 2 Histological sectioning of Khawia armeniaca from Labeobarbus intermedius in Tana Lake, Ethiopia

Fig. 3 Khawia armeniaca from Labeobarbus intermedius in Tana Lake, Ethiopia
2.2 Remarks
The tapeworm in the present study is placed in the Lytocestidae family because the testes are situated at the medullary and vitellarium at the cortical parenchyma. It possesses diagnostic features ofKhawiaspp. proposed to be valid by Scholz,et al.[5],includingK. sinensisHsü 1935,K. armeniaca(Cholodkovsky, 1915),K. balticaSzidat 1941,K. parvaZmeev, 1936,K. rossittensisSzidat, 1937,K. saurogobiiXi, Oros, Wang, Wu, Gao et Nie 2009. The anteriormost testis starts at distance from the anterior extremity and testicular field extends to the anterior margin of the cirrus sac. Anteriormost vitelline follicle begins pretesticularly. Preovarian vitelline follicle extends backwards reaching the anterior or almost to the posterior margin of the cirrus sac. The preovoarian vitelline follicles are separated from postovarian vitelline follicles, and few vitelline follicles are present along the uterine loop, and few or absent along the ovarian arm. The uterine is looped between the posterior region of the ovarian isthmus and the posterior region of the cirrus sac, not extending to the anterior region of the cirrus sac with the presence of uterine gland. The seminal receptacle is present at the dorsal of the ovarian isthmus. Genital gonopores are separated, forming a common genital atrium at the surface. The present caryophyllidean highly resemblesK. armeniacaCholodkovsky, 1915 in the scolex afossate, semi-bulbate with smooth anterior margin, butterfly shaped and follicular ovary,few postovarian vitelline follicle, < 100 in number,and postovarian vitelline follicle touching the posterior region of the ovarian arm, while in other species not reaching at the ovarian arm[5].
Khawia armeniacais distinguished fromK.parvaZmeev, 1936 in the shape of ovarian arm which is H-shaped with posterior arm bent slightly inward. It is separated fromK. sinensiswhich possesses a festoon-like scolex with deep folds, long H-shaped ovary and extensive postovarian vitelline follicles. It differs fromK. rossittensisSzidat, 1937 which has a long ovarian arm and posterior arm bent inwards forming an inverted A-shaped and extensive postovarian vitelline follicles[5]. It is distinguished fromK. saurogobiiXi, Oros, Wang, Wu, Gao et Nie, 2009 which possesses a truncated cuneiform-flabellate scolex with superficial groove, long ovarian arm which is bent towards each other almost forming inverted A shaped,while in other species the posterior arm joins[17]. It is also different fromK. abbottinaewhich possesses two longitudinal bands formed by testes and equal ovary arm length, posterior arm bent slightly inwards[6,18].The tapeworm described in the present study from Tana Lake is considered asK. armeniaca(Cholodkovsky, 1915).
3 Discussion
Khawia armeniacain the present study highly resemblesK. balticastudied by Chubb,et al.[19]in important taxonomical features, such as in having few vitelline follicles along the margin of uterine loop and few or absent along the ovarian arm. The postovarian vitelline follicles touch the posterior region of the ovarian arm, while in other species it is far from the ovarian arm with the ovary being a butterfly-shaped not an H-shaped. However,K. balticaChubbet al.1997 possesses fan-shaped scolex with incision, whileK. armeniacapossesses a semi-bulbate scolex with smooth anterior margin, as indicated by Scholzet al.[5]and observed in the present study. However, the incision inK. balticacould be resulted from the contraction during fixation[19].K. armeniacaidentified in the present study resemblesK. armeniacacollected inCopeata copeataandC. busheifrom River Zayandeh in Iran[20]. However,K. armeniacafrom Iran has a
long posterior arm than the anterior arm, but in the present study, the anterior arm is slightly longer than the posterior arm.K. barbiRahemo et Mohammad,2002 was distinguished fromK. armeniacain the presence of constriction on the cirrus sac region,which is proposed to be an artifact by Scholzet al.[5],andK. barbialso has frilled scolex, which is different fromK. armeniacawith smooth anterior margin.K. luteiAl-Kalak et Rahemo, 2003 was separated fromK. armeniacabased on the size of cirrus sac,separate genital opening and the lack of seminal vesicle in which these are partial morphological features used in the classification. Other characteristic features provided by Al-Kalak and Rahemo[21]have some discrepancies: vitelline follicle situated both in cortical and medullary is a classification feature of the Caryophyllaeidae Leuckart, 1878 (Mackiewicz 1982),formation of an oncosphere in the uterus, in which no oncosphere is formed in the uterus as observed inK.sinensis[23], but formed after the eggs being released into water[24—26]. However,K. barbiRahemo and Mohammad, 2002 andK. luteiAl-Kalak and Rahemo,2003 possess the important morphological features as observed inK. armeniaca, such as follicular butterfly shaped ovary and few postovarian vitelline follicles.
Khawia armeniacawas originally recorded by Cholodkowsky, 1915 asCaryophyllaeus armeniacusfromVaricorhinussp. in Sevan Lake in Armenian SSR. Even though it was placed in the family Caryophyllaeidae, theCaryophyllaeusGmelin, 1970, the description was very brief without any diagrammatic illustration. Later, extensive studies have been done on the same tapeworm in the same locality[27—30].Shulman[31]redescribed the species and transferred it to the family Lytocestidae due to the position of testes and vitellarium in relation to inner longitudinal musculature.
Khawia armeniacahas been reported in different geographical regions and in different fish species.It has been recorded inBarbus longicepsfrom Galilee Lake, Israel[32],Capoeta capoetasevangiin Mingechaursk and Barbarinsk resevoivor[33],C. capoetaandC. busheifrom River Zayandeh, Iran[20],Barbus bynnifrom River Nile, Cairo,Silurus triostegusandB. grypusin Iraq,B. callensisfrom Altas Mountain, Morocco. It has also been recorded inB. altianalisfrom Victoria Lake, Uganda and fromB.tropidolepisin Tanganyika Lake[5]. Some of the publications are in Russian, such as Cholodkovsky[34],Popov[27], Kulakovskaya[34], Paperna[32], Mikailov[33],Dinnik[28], Rahemo and Al-Kalal[35], Poddubnaya and Karen[30]references are cited from William and Gibson[20]and Scholz,et al.[5].
K. armeniacaidentified in the present study exhibits some morphological variability in the shape of scolex, such as start of anteriormost testes, extent of vitelline follicle at cirrus sac, number of vitelline follicles along the uterine loop and ovarian arm margin and the extent of postvitelline follicle at the posterior region of ovarian arm. The intraspecific variation inK. armeniacain the present study shows the existence of morphological plasticity not only in different geographical regions[5], but also in a same locality as in the present study. However, this requires further molecular verification. The classification in the Caryophyllidea based on the shape of scolex, start of anteriormost testes and vitelline follicle, posterior extent of testes and vitelline follicle, presence or absence of vitelline follicle along the uterine loop and ovarian arm are incongruent with circumscription stability of taxon[36]. This is because of the features shared by species in different families[5,7,37]. The position of inner longitudinal musculature in relation to internal genital organ is a key feature in the family classification of caryophyllideans[3,22]. In addition,the shape of scolex combined with the shape of ovary should be considered. This is because they are features rarely shared at the species level[5,7]. The extend of postvitelline follicles in relation to the ovarian arm should be considered as a preliminary feature inK. armeniaca, as proposed by Scholz,et al.[5], as number and extent of postovarian vitelline follicle to the ovary, and the shape it forms at posterior region are taxonomical features inPseudoglaridacrissp.[37]and presence or absence of post-ovarian vitelline follicle inPromonobothriumsp.[37].
Acknowledgements:
Thanks are due to Ms. Hui Liang at Wuhan University for preparing scanning electron microscope photomicrographs. The samples were obtained with the assistance from Professor E Zhang and Dr. Liang Cao at the Institute of Hydrobiology, Chinese Academy of Sciences, and Ms. Wang He at Hunan Fisheries Science Institute in Changsha, Hunan Province,and also local fishery administration in Ethiopia. Caroline J. Kibet obtained a scholarship from the University of Chinese Academy of Sciences, and the State Key Laboratory of Freshwater Ecology and Biotechnology, through the Ichthyology Section, National Museums of Kenya.
