Termite-killing components in Serratia marcescens (SM1)
2021-07-15RenjieFuJianLuoKaiFengXiaoyuLuFangTang
Renjie Fu·Jian Luo·Kai Feng·Xiaoyu Lu·Fang Tang
Abstract The bacteria,Serratia marcescens (SM1) was previously obtained from the black-winged termite,Odontotermes formosanus Shiraki.SM1 was highly toxic to O.formosanus,however,the mechanism of toxicity is unclear.In this study,toxicity test results showed that the main components that affected O.formosanus were in a supernatant and that the insecticidal protease in the supernatant resulted in the death of O.formosanus.In addition,zinc sulphate recovery experiments indicated that the metalloproteinases in the supernatant were more harmful.These results provide a theoretical foundation for the future biological control of termites,the basis for the development of pest control technology and the discovery of new pesticides.
Keywords Odontotermes formosanus Shiraki·Serratia marcescens strain SM1·Supernatant·Protease·Metalloproteinases
Introduction
Termites damage buildings reservoir dams and other structures made of wood,agricultural and forestry crops,and cable transportation facilities(Cosme et al.2020;Dahlsjo et al.2020;Djuideu et al.2020 ;Du et al.2020),and hazardous areas account for approximately 50% of the global area.Termites live in social groups and their resistance to natural enemies and adverse environmental factors is greatly enhanced due to their group defense function.In addition,the secluded nature of termites makes them more difficult to study and control.Therefore,the prevention and treatment of termite infestations has always been a challenge of pest management (Zhang et al.2015).At present,the most common and important way to control termites is to use chemical agents (Sapkota et al.2020).The advantage of this approach is that it is effective,but chemical pesticides pose considerable threat to the environment and to human health,including causing pesticide poisoning,cancer,deformities in children and gene mutation (Liu et al.2010).With increasing awareness of the need for safety and environmental protection,these chemical termite control agents will eventually be eliminated.Compared with chemical controls,biological control is environmentally safe and durable,and avoids the problems caused by chemicals.It is foreseen that biological control methods will become the mainstream of future control work.
Serratia marcescensis a rod-shaped,anaerobic,gramnegative bacterium of the family Enterobacteriaceae.It is generally smaller than other intestinal bacteria,without capsules and occasionally with long filaments (Hejazi and Falkner 1997).It is commonly found in water,plants,animals and soil,and produces a secondary metabolite–prodigiosin–in the process of growth (Montaner and Perez-Tomas 2003).S.marcescensis pathogenic to many insects,including the cotton bollworm,Helicoverpa armigeraHüber(Chen et al.2005),Myrmeleotettix palpalis(Jin et al.2005),Phyllotreta striolata(Yang et al.2014),the tobacco cutworm,Spodoptera lituraFabricius (Niu et al.2015;Aggarwal et al.2017),S.exigua(Niu et al.2015),and the larvae and eggs of the Asian palm weevil,Rhynchophorus ferrugineusOliver (Zhang et al.2011).Secretory product ofS.marcescens,prodigiosin,have also been found to be pathogenic to some organisms,such asBursaphelenchus xylophilus(Hu et al.2017),the yellow fever mosquito,Aedes aegyptiL.and the Indian malaria mosquito,Anopheles stephensiListon (Patil et al.2011).Niu et al.(2018) usedS.marcescensmixed with five insecticides to treatLaodelphax striatellusand found that the mortality rate ofL.striatellusto the insecticides could be improved.It is clear thatS.marcescensis pathogenic to many types of pests and has wide market prospects in biological control.
Our laboratory recently isolated a red pigment-producing bacterium from dead termites and identified it asSerratia marcescens(SM1) (Fu et al.2019).SM1 was significantly toxic toO.formosanus(Fu et al.2020),but the specific components and mechanisms of this toxicity need further examination.Research has shown that the pathogenesis ofS.marcescensis due to chitinase enzymes (Regev et al.1996;Zhang et al.2000;Xu and Peng 2004;Yin et al.2004;Jin et al.2005),and Tao 2006 has reported that the insecticidal protein ofS.marcescensis a metallic protein that exists in a supernatant of the bacteria.However,the active components ofS.marcescensthat are toxic to termites have not been identified.The purposes of this study are:(1) to clarify the termite-killing components ofS.marcescens;and,(2) to provide a basis for the biological control of termites.
Materials and methods
Insects
Six colonies ofOdontotermes formosanuswere collected from Jurong in Zhenjiang,Jiangsu Province,China.Termites
Culture and concentration determination of the S.marcescens strain,SM1
SM1 was isolated from infectedO.formosanusand deposited at Nanjing Forestry University,Nanjing.A solid bacterial medium (1 L) was prepared,consisting of 10 g peptone,20 g beef extract,2 g NaCl,2 g K2HPO4,18 g agar and 1 L H2O with pH 7.2–7.4.A seed medium (1 L) of 10 g peptone,20 g yeast extract,2 g NaCl,2 g K2HPO4in 1 L of H2O was also prepared.A zymotic medium (1 L) was prepared of 10 g peptone,30 g soybean oil,2 g NaCl,2 g K2HPO4in I L H2O.As the amount of SM1 increased,its optical density at 600 nm (OD600) also increased.The OD600value was linearly related to the number ofS.marcescensSM1 bacteria and used to determine the bacterial count of the liquid culture.To obtain the concentration of theS.marcescensSM1 suspension,the OD600value was measured on a spectrophotometer.
Bioassay of components of S.marcescens SM1 fermentation
The concentration of the fermentation medium ofS.marcescensSM1 was 1.17 × 1010cells/ml,and the fermentation medium ofS.marcescensSM1 was centrifuged at 5000 r for 20 min at 4 °C.After centrifugation,a surface oil liquid(pigment layer),a middle layer supernatant and a lower layer of bacterial precipitate were obtained.The lower layer of the bacteria precipitate was dissolved by medium.According to the transfer toxicity method (Tang et al.2007),quality filter paper was first wetted,placed in a 9-cm diameter glass Petri dish,and fiveO.formosanusworker termites were dipped in each treatment solution.Five soaked termites marked in red on the abdomen and twenty untreated worker termites were placed in the culture dish.The five marked worker termites dipped in the medium and the 20 untreated worker termites were the controls.The termites were observed and recorded in darkness at 25 °C.The experiment group and the control groups were repeated three times in the same manner.

were kept in sealed plastic containers in total darkness at 27 ± 1 °C and 75 ± 1% relative humidity.All colonies were maintained under laboratory conditions without soil and with moist filter paper for one day before the subsequent experiments.
Effect of protease inhibitors on the bioassay of the SM1 fermentation
Bioassay of components ofS.marcescensSM1 fermentation showed that the culture supernatant of the bacterial fermentation had a strong toxicity toO.formosanus.Therefore,the supernatant was treated separately with proteinase K at 50 μg/ml,ethylenediaminetetraacetic acid (EDTA)at 10 mmol/L,1,10-phenanthroline at 10 mmol/L,and phenylmethanesulfonyl fluoride (PMSF) at 1 m mol/L.There was also a treatment without reagents added and was only heated at 60 °C for 15 min.The toxicity assay was then performed using the transfer toxicity method.The five marked worker termites dipped in the medium and the 20 untreated worker termites were the controls.The experimental groups and the control groups were assayed three times.
Recovery experiment of ZnSO4
After the bioassays using the supernatant treated with different protease inhibitors,the supernatants treated with the two types of protease inhibitors,EDTA and 1,10-phenanthroline,were subjected to zinc sulphate (ZnSO4) recovery treatments.The two supernatants were incubated with ZnSO4at a concentration of 0.5 mmol/L for 1 h at 22 °C,and the same toxicity assay performed again.The medium incubated at 22 °C for 1 h with a concentration of 0.5 m mol/L ZnSO4was the control.The concentration of the SM1 fermentation was 1.69 × 1010cells/ml and the toxicity assay was then performed using the transfer toxicity method.The experimental group and the control group were independently assayed three times.
Statistical analysis
The results were subjected to an analysis of variance(ANOVA) using InStat software (GraphPad,San Diego,CA,USA) with a level of significance atP< 0.05.Tukey’s test was used for multiple comparisons.
Results
Bioassay of components of the SM1 fermentation
The transfer toxicity bioassay againstO.formosanuswas carried out using the components of the SM1 fermentation.The results show that the toxicity of the components was not different from that of the supernatant (P> 0.05).After 36 h,the corrected mortality rate of the supernatant was approximately 50%,and at 54 h,the rate was close to 80%.After 54 h,toxicity of the lower bacterial layer was relatively poor at only 28.5%.Among the three components,the uppermost oil pigment layer had the poorest toxicity (Table 1).
Effect of protease inhibitors on the bioassay of SM1 fermentation
After the middle layer supernatant was identified as the main active component,it was treated with protease inhibitors.It was first heated at 60 °C for 15 min to denature the protease.The results show that the toxicity effect of the supernatant decreased significantly (P< 0.05) after heating;the toxicity became stronger after 48 h and the corrected mortality reached 60% (P> 0.05).Four different protease inhibitors,PMSF,EDTA,proteinase K and 1,10-phenanthroline,were added separately to the supernatant.The results show that the toxicity decreased when protease inhibitors were used.When proteinase K was used,the toxicity of the supernatant decreased most,and the corrected mortality rate was 10.4% at 54 h;when EDTA was used,the toxicity of the supernatant was greatly reduced,and the corrected mortality rate was 22.4% at 54 h;when PMSF or 1,10-phenanthroline was used,the toxicity of the supernatant was reduced,and the corrected mortality rate failed to reach 50% by 54 h(Table 2).
Recovery experiment of ZnSO4
After protease inhibitors suppressed the toxicity of the SM1 fermentation supernatant,it was blocked by two inhibitors of metalloprotease and was subjected to a ZnSO4recovery experiment.This could confirm that the protease in the supernatant was toxic toO.formosanusand could initially indicate what the active protease was.There was no difference in toxicity between the medium and the medium treated with 0.5 m mol/L ZnSO4,neither of which can caused the death of termites.The EDTA-treated supernatant had an increase in insecticidal activity after the ZnSO4recovery test(P< 0.05).At 54 h,the corrected mortality increased from 23.6 to 44.4%.In addition,the insecticidal activity of the 1,10-phenanthroline-treated supernatant was improved after the ZnSO4recovery experiment,and the corrected mortalityat 48 h was significantly different from that before the recovery (P< 0.05).These results further indicate that particular metalloproteases were present in the supernatant,and these metalloproteases were also one of the effective components responsible for the death ofO.formosanus(Table 3).

Table 1 Toxicity test results of different components of SM1 fermentation against O.formosanus (transfer toxicity method)

Table 2 Toxicity test results of the supernatants treated with different protease inhibitors against O.formosanus (transfer toxicity method)
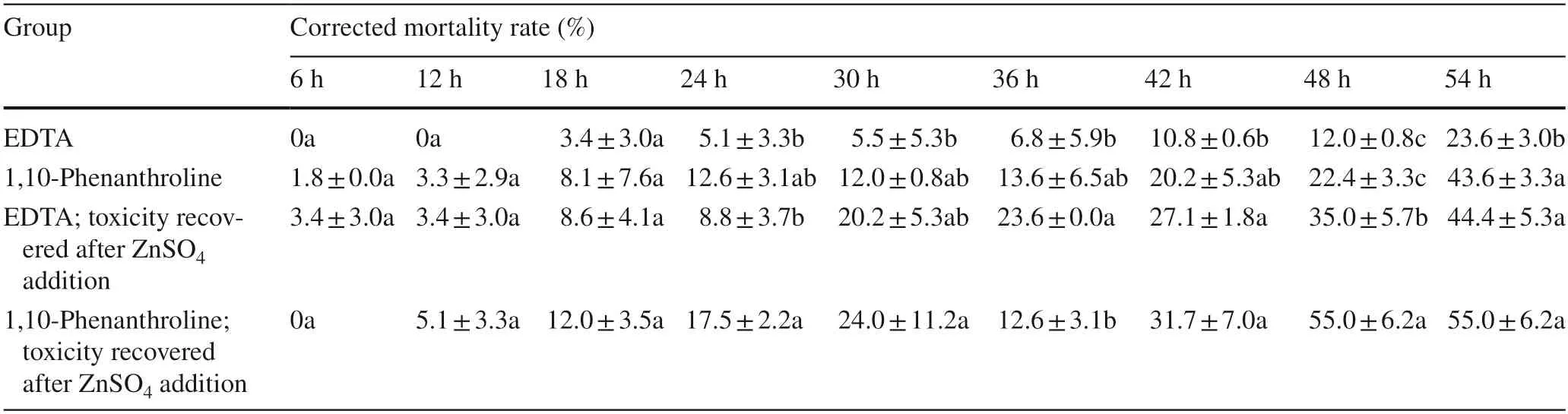
Table 3 The ZnSO4 recovery experiment (transfer toxicity method)
Discussion
Research on biopesticide proteins has been important in the development of biopesticides.Over the past few years,several studies ofBacillus thuringiensisinsecticidal protein(Schnepf et al.1998) have shown that protein crystals produced during sporulation are toxic to many organisms.The expression and structural functions of the protein genes have been studied by McGaughey et al.(1998) and Rajamohan et al.(1998).There are four gene clusters encoding the toxin protein complex in the luminescent bacillus,which makes these bacteria that are symbiotic with entomopathogenic nematodes a member of the microbial insecticide protein family (Bowen et al.1998).Reports of nematophagous pathogenic bacilli also earlier suggested this symbiosis with entomopathogenic nematodes,and their insecticidal components have a lethal effect on a variety of pests (Forst et al.1997).Studies have shown that active ingredients that play a major insecticidal role include small molecules (Volgyi et al.1998),lipopolysaccharides (Smigielski and Akhurst 1995) and proteins.A 1.2 kb gene was obtained from the nematophagous pathogenic bacterium A24,which encodes a 30 kDa insecticidal protein.A few microliters of the fermentation containing the toxic protein can cause the death of insect larvae (Smigielski and Akhurst 1995).Moreover,Clostridium difficile(Barloy et al.1998) andBacillus sphaericus(Li et al.2008) also produce insecticidal proteins which are toxic.Studies have shown thatS.marcescenssecretes several well-known extracellular proteins,including nuclease,phosphatase,hemolysin,iron-containing proteins,chitinase,protease,and lipase (Tao 2006).One or several of these components may become insecticide factors.Therefore,studying the specific components ofS.marcescensSM1 can help to further clarify its mechanism of action on termites.
The uppermost layer of the pigment oil layer,the middle layer supernatant and the bacterial sediment layer were obtained by centrifugation ofS.marcescensSM1 fermentation.The results show that the toxicity effect of the supernatant was the best and closest to that of the original fermentation.The toxicity of the bacterial sediment layer was poor and differed from that of the original fermentation.In addition,the pigment oil layer had the least effect,and themortality rate ofO.formosanustreated with the pigment oil layer was very close to that of the controls.Therefore,it was confirmed that the main active component ofS.marcescensSM1 fermentation was in the supernatant,and that the pigment oil layer had no insecticidal properties.Since the bacterial precipitate was added to the medium during dissolution,the undestroyed cells might continue to ferment to produceS.marcescensSM1 fermentation and induce a poisoning effect in the subsequent toxicity test experiments.
After identifying that the main active component was the supernatant,it was necessary to further clarify whether the active component was a protease by heating or adding different protease inhibitors.From the results,the toxicity effect of the heated supernatant was significantly reduced,and the corrected mortality rate of the treatment was only 30%.But as time increased,its toxicity gradually increased,which may be caused by the recovery of certain denatured proteases.Since protease K can inhibit most of the proteases,after protease inhibitor K was added,the toxicity effect of the supernatant was greatly reduced,and the mortality of treatedO.formosanuswas almost the same as that of the control.At the same time,the toxicity effects of the supernatants treated with the three types of protease inhibitors,PMSF,EDTA and 1,10-phenanthroline,were also reduced.At 48 h,the corrected mortality rate of each treatment group was less than 32%.These results indicate that there was more than one insecticidal protease in the supernatant and that these insecticidal proteins were one of the important causes of termite death.
Since both protease inhibitors EDTA and 1,10-phenanthroline inhibit metalloprotease,the supernatants treated with these inhibitors were incubated with 0.5 mmol/L ZnSO4for 1 h at 22 °C to confirm whether or not the metalloprotease was active.The results show that the insecticidal activity of the supernatant was restored after the ZnSO4recovery experiment,which indicated that the metalloprotease was present in the supernatant and the insecticidal protease had a good toxicity effect onO.formosanus.However,this experiment only preliminarily confirmed that the active components ofS.marcescensSM1 fermentation were metalloproteases and other proteins.More precise active components are not known and further investigation is necessary.
Open AccessThis article is licensed under a Creative Commons Attribution 4.0 International License,which permits use,sharing,adaptation,distribution and reproduction in any medium or format,as long as you give appropriate credit to the original author(s) and the source,provide a link to the Creative Commons licence,and indicate if changes were made.The images or other third party material in this article are included in the article’s Creative Commons licence,unless indicated otherwise in a credit line to the material.If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use,you will need to obtain permission directly from the copyright holder.To view a copy of this licence,visit http://creat iveco mmons.org/licen ses/by/4.0/.
杂志排行
Journal of Forestry Research的其它文章
- Flexible transparent wood enabled by epoxy resin and ethylene glycol diglycidyl ether
- Diversity and surge in abundance of native parasitoid communities prior to the onset of Torymus sinensis on the Asian chestnut gall wasp (Dryocosmus kuriphilus) in Slovenia,Croatia and Hungary
- Ozone disrupts the communication between plants and insects in urban and suburban areas:an updated insight on plant volatiles
- Testing visible ozone injury within aLight Exposed Sampling Site as aproxy for ozone risk assessment for European forests
- Logging and topographic effects on tree community structure and habitat associations in a tropical upland evergreen forest,Ghana
- Spatial pattern dynamics among co-dominant populations in early secondary forests in Southwest China
