Driving force of soil microbial community structure in aburned area of Daxing’anling,China
2021-07-15MengMengBingWangQiuliangZhangYuanTian
Meng Meng·Bing Wang·Qiuliang Zhang·Yuan Tian
Abstract Fires are an important factor impacting forest ecosystems of Daxing’anling and have a significant effect on soil microbial community structure.In this study,highthroughput sequencing for 16S rDNA and ITS rDNA were applied to analyze the changing characteristics and driving factors of bacterial and fungal community structures in burned areas with different fire severity.PICRUSt2 software was used to predict the functional characteristics of burned areas with different fire severity.The purpose was to unveil the responsive relationships among the structure and function of bacterial and fungal communities,fire severity,and post-disturbance restoration times.After high severity fires,the destruction of surface vegetation and loss of soil nutrients reduced the diversity and abundance of soil bacteria and fungi.The soil bacteria community structure,which was dominated by Acidobacteria,Proteobacteria,and Actinobacteria, changed to be dominated by Proteobacteria and Chlorofl exi.As well,soil fungal community changed from domination by Helotiales,Eurotiales and Russulales to domination by Archaeorhizomycetales and Helotiales.Over time,soil bacterial community was gradually restored to prefire levels 30 years after the fire.Soil fungal community changed and failed to restore to pre-fire levels after 30 years.After low/intermediate severity fires,environmental factors were relatively unchanged so that soil bacteria diversity and abundance increased,optimizing community composition.The diversity and abundance of soil fungi decreased and the community structure changed slightly.Over time,both bacterial and fungal communities were gradually restored to pre-fire levels 30 years after the fire.After fire disturbance,with increasing severity,soil carbon fixation,lignin degradation,mineralization of organic nitrogen and hydrolysis of organic phosphorus are enhanced.Denitrification is weakened.Therefore,forest fires have certain positive effects on carbon,nitrogen and phosphorus cycles where soil bacteria and fungi are involved.
Keywords Forest fire·Soil bacteria·Soil fungi·Highthroughput sequencing·Driving force
Introduction
Soil microbes have great complexity and diversity and are important for soil composition and nutrient cycling,and drivers of energy flow (Elsas and Boersma 2011) microbes play a critical role in plant growth and development,and in maintaining the productivity,functionality and stability of ecosystems (Felske et al.2000;Kuramae et al.2010).Since soil microbes are sensitive to changes in environment,they may be taken as indicators of soil restoration (Luo et al.2020).
Significant changes could result from either natural or anthropgenic disturbances to ecosystems,for which different types of soil microbes display divergent coping mechanism due to their contrasting survival characteristics and environments,thereby further changing the microbial community structure (Luo et al.2020).Coal mining activities on the Loess Plateau have caused soil sedimentation which reduced soil bacterial diversity and resulted in significant changes in community structure (Luo et al.2020).The removal of undergrowth vegetation caused chemical property changes in forest soils,including water contents,total nitrogen and phosphorus,hydrolyzed nitrogen,and available phosphorus,which in turn caused the reduction of relative abundance of soil bacteria ofProteobacteria,Planctomycetes,andFirmicutes,as well as a reduction in bacterial community diversity(Wu et al.2020).Appropriate applications of nitrogen fertilizer increased the fertility of rice field soils but did not significantly change bacterial community structure (Liu et al.2019).Fertilization in a short period increased available nutrients and soil water of a Eucalyptus forest,and the soil fungal community structure,which was dominated by Eurotiales,Archaeorhizomycetales,and Tremellales,changed to be dominated by Boletus and Eurotiales (Chen et al.2020).When forest soils were degraded,the surface vegetation changed from trees to herbs,and the soil became more permeable but with lower nutrients such as total nitrogen and total carbon.In this study,the relative abundance ofEurotialesandAcidobacteriasoil microbes increased dramatically butBasidiomycotalevels were reduced significantly,indicating an obvious enhancement of microbe community diversity (Li et al.2020).
Among various disturbances,fires have quite different effects on soil microbial community structure which are a continuous process (Villadas et al.2019;Luo et al.2020).Microbial communities are first directly affected by high temperatures and indirectly by changes in physical and chemical properties (Cai et al.2014;Wang et al.2020).Change in organic matter quantity and quality,fluctuations in carbon and nitrogen contents (Shen et al.2013;Kaiser et al.2016),increases in pH (Holden et al.2016;Wang et al.2016;Schmidta et al.2019),and variations in water contents are key factors changing the microbial communities (Shigeto et al.2008;Man et al.2010;Rasche et al.2011;Vries 2012).Forest fires have two forms of impacts on microbial communities (Brais et al.2000;Wallenius et al.2004):one,the structure and functions of microbial communities can be destroyed under high severity fires,while,low severity fires optimize the forming of microbial communities,contributing to the stability of ecosystem structure (Akburak et al.2018;Van et al.2019).
In Daxing’anling forest,boreal and the mixed wood species are widely distributed (Tian et al.2018).This is a region of high fire risk.In Hulunbuir City where the study area is located,fires occurred over 600 times from 1990 to 2018.Therefore,it is necessary to study the microbial restoration characteristics of the forest soils of the Daxing’anling area after fire.High-throughput sequencing for 16S rDNA and ITS rDNA were applied to analyze the changing characteristics and relative driving factors of the soil bacterial and fungal communities in burned areas under different fire severities.PICRUSt2 software was used to predict the functional characteristics of the soil element cycle in burned areas under different fire severity.The purpose of this study was to determine the relationship between structure and function of soil bacterial and fungal communities,the fire severity,and post-disturbance restoration time,to provide information for understand the restoration process of soil structure and function,and to propose targeted management measures.
Materials and methods
Study area
The research was located in Hulunbuir City,northwest of Daxing’anling in the northernmost part of China.It is a humid temperate zone with average annual temperatures of −5.4 °C,and average annual precipitation of 500 mm,mainly concentrated in June to September,and average altitudes of 976.5 m a.s.l.It has an average 75% forest cover consisting ofLarix gmeliniiassociated withBetula platyphylla,Populus davidianaandPinus sylvestris(Tian et al.2018).The study area has a high incidence of fires,over 677 fires from 1990 to 2018.Fires generally occur in May and June (Zhang et al.2020).For this study,based on consistent site conditions,five burned blocks from the southern parts of Genhe City were selected in August 2018,where fires happened all by lightning (Table 1,Fig.S1) in May 1987,June 2003,May 2010,May 2015,and June 2018.These burned areas characterize the recovery status of the forest ecosystem after 30 years,15 years,8 years,3 years and 8 weeks,respectively,following the fire.
Setting of plots
Combining the forest resource features of Daxing’anling,(evolution history,forest age,tree species and forest type),the Composite Burn Index Investigation Contents and Evaluation Criteria (Table 2),which is applicable to the study area,was prepared according to the Composite Burn Index(CBI) proposed by Key and Benson (Key and Benson 2006).The field survey was conducted in August 2018.Sixty sampling sites (over 50 m intervals) were randomly established in every burned area based on the CBI of all sites and classified based on fire severity index (FSI) (Table 3),which was considered the FSI classification for burned areas of Daxing’anling.By calculating the CBI and grading the FSI of the burned areas created in different times,one low severity plot,one intermediate severity plot and one high severity plot were selected in each block.An unburned plot near the burned area was also selected which had the same site condition.There were 20 plots in total.
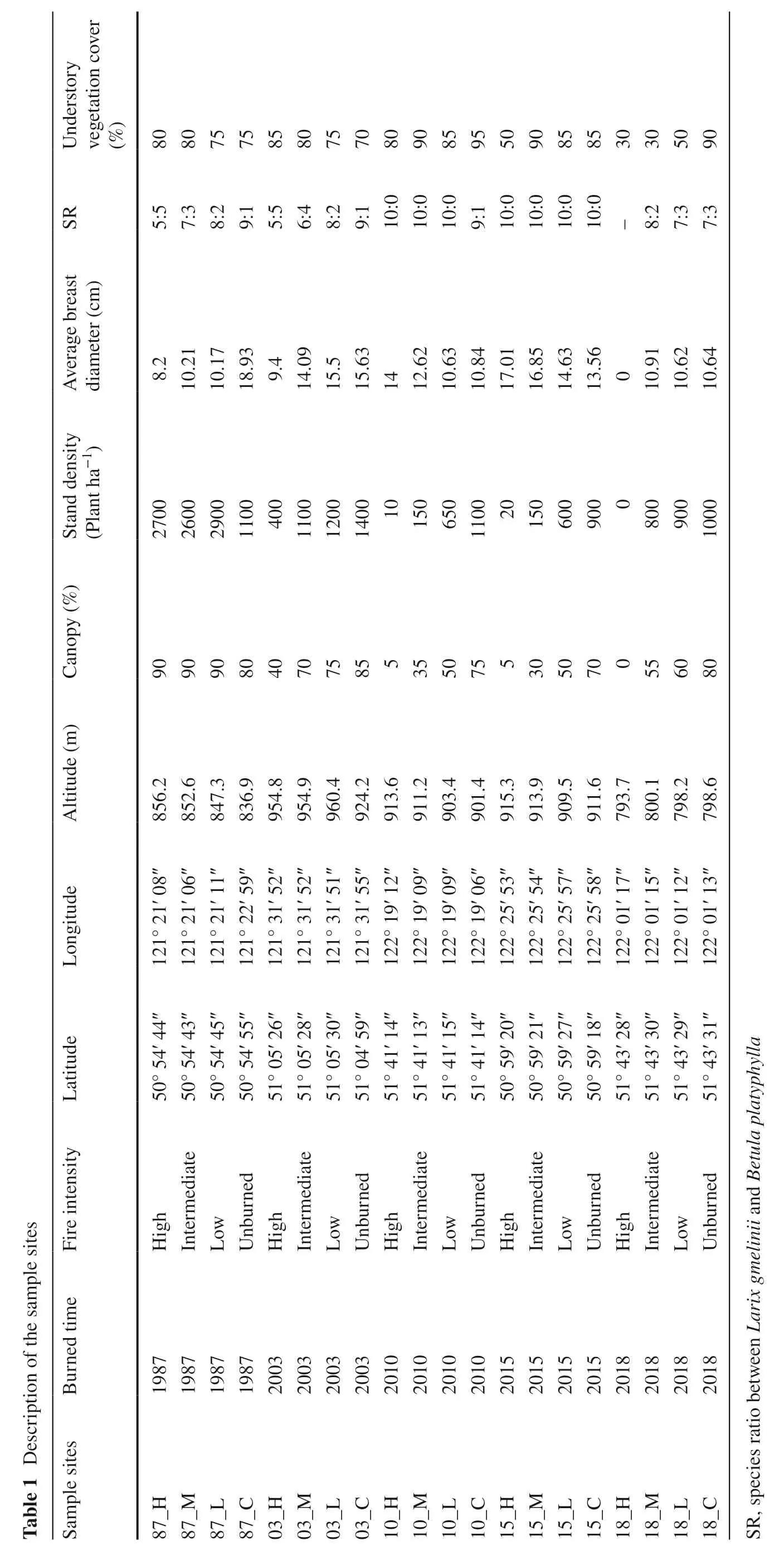
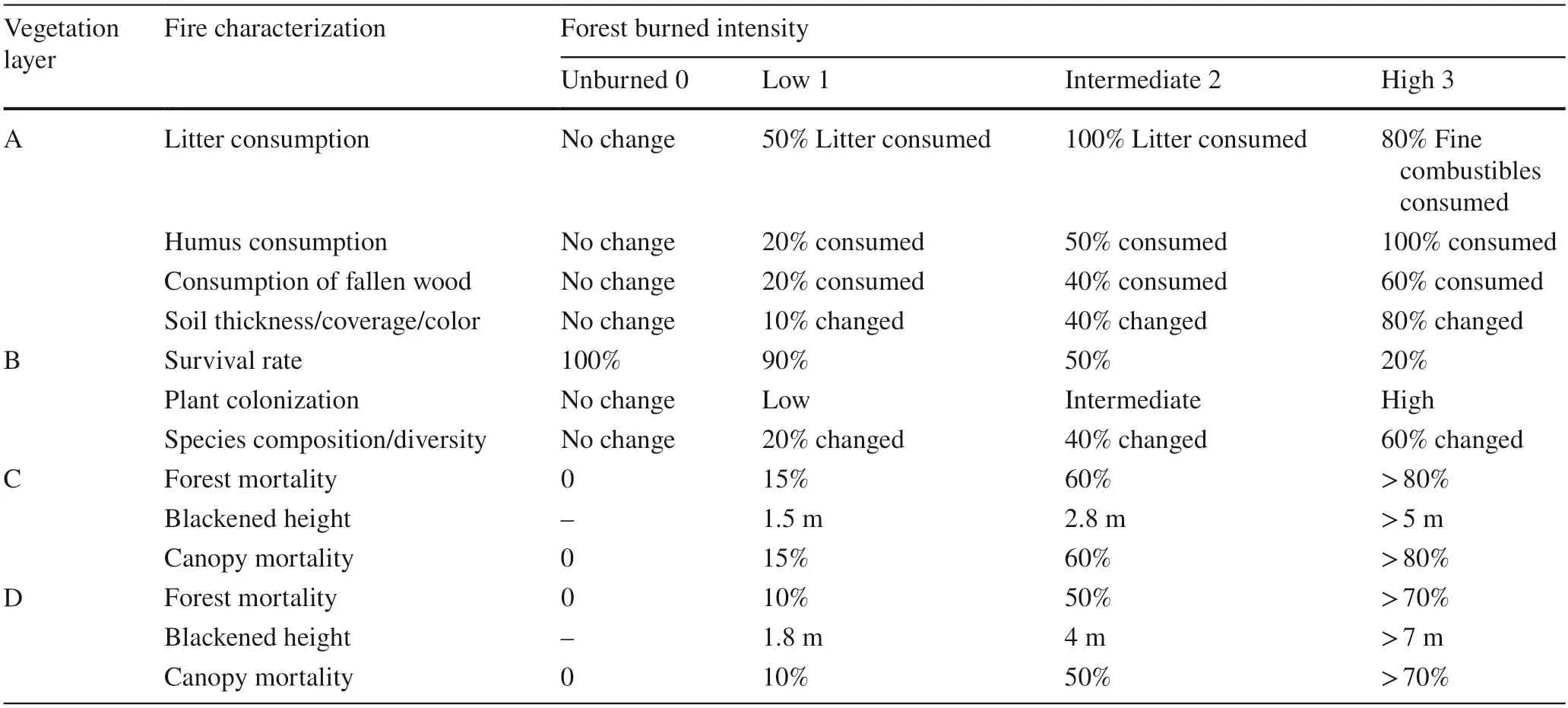
Table 2 Composite Burn Index survey content and evaluation criteria
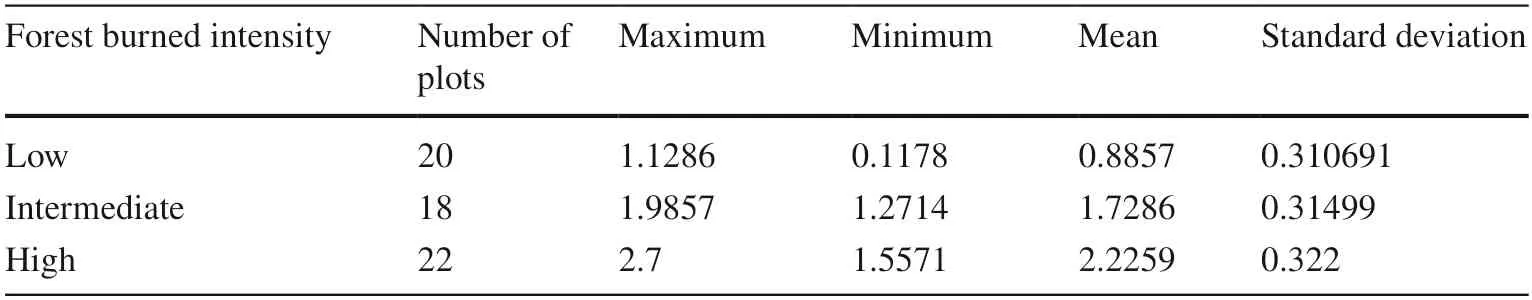
Table 3 Forest burned intensity classification based on CBI with 2015 burned area as an example
Sample collection
Three sampling points were randomly selected in each plot.With the sampling point as center,and within 3-m diameter,an auger with a 3-cm inner diameter was used to collect 10 samples at 1–10 cm depths.The samples were mixed and screened through a 2 mm-sieve,packed in self-sealing bags and stored at −20 °C.These samples were used for measuring the chemical properties,bacterial diversity,and fungal diversity of the soil.
Measurement of soil chemical properties
An aqueous extract of potassium chloride solution was used to determine pH (measurement standard:NY/T 1377-2007);an potassium dichromate volumetric method to measure total organic carbon (TOC) (measurement standard:NY/T 1121.6-2006);the Kjeldahl distillation was used to measure total nitrogen (TN) (measurement standard:NY/T 53-1987);the sodium hydroxide fusion-molybdenum stibium resist colorimetric method was used to measure total phosphorus (TP) (measurement standard:NY/T 88-1988) and total potassium (TK) (measurement standard:NY/T 87-1988);the alkaline hydrolysis diffusion method was used to measure available nitrogen (AN) (measurement standard:DB13T 843-2007);and,the sodium bicarbonate extraction-molybdenum stibium resist colorimetric method was used to measure available phosphorus (AP) (measurement standard:NY/T 1121.7-2014) (Table 4).
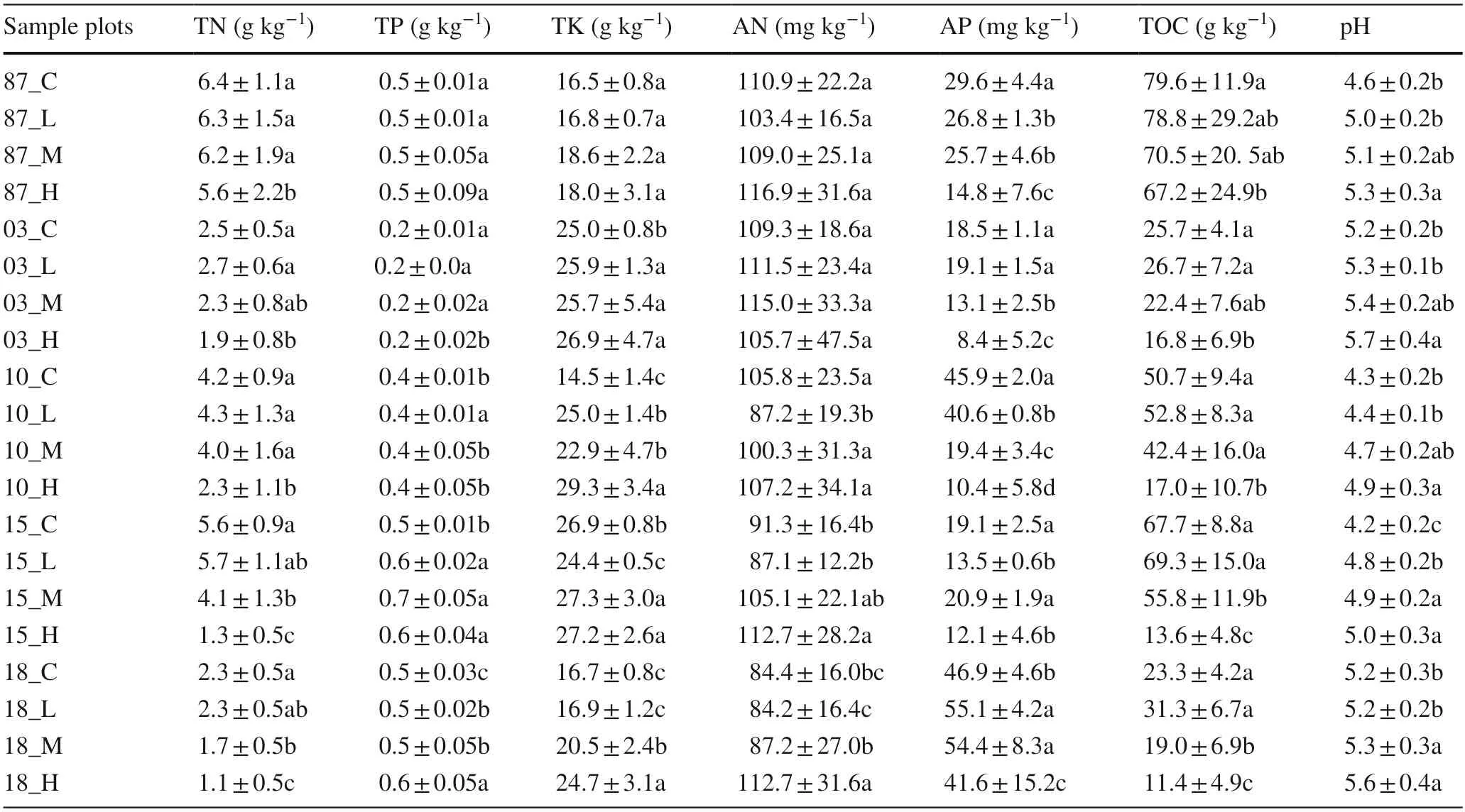
Table 4 Soil chemical properties
DNA extraction and PCR amplification
Total DNA of the microbial communities were extracted according to the instructions of E.Z.N.A.®soil DNA kit(Omega Bio-tek,Norcross,GA,USA.).A 1% agarose gel electrophoresis was applied to detect the DNA extraction quality while the NanoDrop2000 determined the DNAconcentration and purity.For bacterial samples,the 338F(5′-ACT CCT ACG GGA GGC AGC AG-3′) and 806R (5′-GGA CTA CHVGGG TWT CTAAT-3′) were used to carry out PCR amplification for the V3–V4 variable region of 16S rRNA gene;for fungal samples,ITS1 (5′-TCC GTA GGT GAA CCT GCG G-3′) and ITS4 (5′-TCC TCC GCT TAT TGA TAT GC-3′) were applied to perform PCR amplification for the ITS region of rRNA gene.Each operation was repeated three times on each sample.
High-throughput sequencing
The PCR products of the same sample were mixed and recycled using 2% agarose gel,and then purified the recycled products by AxyPrep DNA Gel Extraction Kit (Axygen Biosciences,Ltd,Union City,CA,USA).A 2% agarose gel electrophoresis was carried out and the recycled products quantified by Quantus™ Fluorometer (Promega,Ltd,Madison City,WI,USA).NEXTFLEX Rapid DNASeq Kit was next applied to set up a library and sequencing was performed by Miseq PE300 platform (Illumina,Ltd,San Diego City,CA,USA) of the company.The original data were uploaded to the NCBI SRA (Sequence Read Archive) database.
Biological information extraction
Trimmomatic software (version 0.39,http://www.usade llab.org/cms/?page=trimm omati c) was applied to control the quality of original sequencing,and the FLASH software used for splicing.UPARSE software (version 7.1,http://drive 5.com/upars e/) was used to carry out OTU clustering and to eliminate chimera on sequence according to 97%similarity.The RDP classifier (http://rdp.cme.msu.edu/) was then used to carry out species classification and annotation for each sequence,and the bacterial and fungal samples were compared with Silva database (Release132,http://www.arbsilva.de) and Unite database (Release 7.2,http://unite.ut.ee/index .php),respectively,for which the comparison threshold was set as 70%.
Data processing
SPSS (IBM,USA) software was used to calculate the mean and standard deviation of each chemical factor and to perform significance analysis.The mothur software package was used to calculate the diversity index and the R programming language (AT&T Bell Laboratories,Ltd,Hurray Hill City,NJ,USA) vegan package to carry out redundancy analysis (RDA) and canonical correspondence analysis (CCA)by taking the soil microbial OTU horizontal community composition as the response variable.Soil chemical characteristics were set as the explanatory variable.PICRUSt2 software (https://githu b.com/picru st/picru st2/wiki) predicted the functions of soil bacteria and fungi,screened the key functional enzymes in the soil carbon,nitrogen,and phosphorus cycles.Excel 2019 and R Studio software were used to process the data and Origin 2018 (Origin Laboratories,Ltd,Northampton City,MA,USA) software for drawing.
Results
Chemical properties of soils
The mixed forest soil of Daxing’anling has pH 4.5–5.2 with total organic carbon (TOC) content of 23.2–80.6 g kg−1,total nitrogen (TN) of 2.2–6.9 g kg−1,and total phosphorus(TP) of 0.2–0.5 g kg−1(Table 4).
Eight weeks after fire in soils subjected to intermediate/high severity burning,the TOC,TN,and AP contents dropped significantly,but soil pH rose dramatically.TP,TK,and AN levels increased slightly.In soil under a low severity fire,pH rose slightly and TOC,TN,TP,TK,AN,and AP levels increased slightly.Fifteen years after the fire,TP,TK,and AN contents were restored to pre-fire levels.Thirty years after fire,TOC and TN contents as well as pH were at pre-fire levels.Over time,the AP content gap between the burned area and the control plot increased significantly(Table 4).
Soil bacterial community characteristics
Structural characteristics
The 16SrRNA high-throughput sequencing was carried out on all 60 soil samples of 20 groups.There were 3,302,646 high quality sequences with 432 bp mean reading length obtained after quality control and chimera removed,and were clustered into 4771 OTUs (97% similarity),belonging to 33 phylum,234 orders,and 604 genera.
The predominant bacterial phylum,(relative abundance ratio in each sample above 0.1%) in unburned soils include:Acidobacteria(28.2% ± 3.8%),Proteobacteria(25.1% ± 4.0%),Actinobacteria(16.5% ± 1.6%),Chloroflexi(10.0% ± 4.5%),Verrucomicrobia(3.3% ± 2.2%),Planctomycetes(3.0% ± 1.7%),Gemmatimonadetes(2.6% ± 1.8%),Bacteroidetes(2.1% ± 1.6%),Cyanobacteria(0.2% ± 0.1%),Firmicutes(0.4% ± 0.2%),andNitrospirae(0.2% ± 0.3%).The total relative abundance ratio is over 95% (Fig.1,Fig.S2).

Fig.1 Soil bacterial community composition at the phylum level
Fire results in significant changes in bacterial community structure.In soils under high severity fire,the relative abundance ratios of a portion of the dominant soil bacteria decreased dramatically,including:Acidobacteria(14.7% ± 5.2%),Actinobacteria(14.8% ± 0.5%),Planctomycetes(2.8% ± 0.9%) andFirmicutes(0.1% ± 0.1%),while other parts increased significantly;Proteobacteria(35.5% ± 8.3%),Chloroflexi(16.9% ± 4.9%),Verrucomicrobia(5.9% ± 2.6%),Cyanobacteria(0.6% ± 0.2%),andNitrospirae(0.3% ± 0.1%).With an increase of recovery time,the changes to the bacterial community structure gradually decreased.Thirty years after the fire,it was essentially restored to pre-fire levels.In soils with low/intermediate severity fire,the relative abundance ratios ofActinobacteria(20.0% ± 2.9%) andPlanctomycetes(3.4% ± 1.5%) increased significantly.With an increase in recovery time,the bacterial community structure gradually recovered and was restored to pre-fire levels.The changes by other bacterial phylum were similar to those of bacteria under high severity fire.
Bacterial diversity characteristics
Chao and Shannon indexes,respectively,characterize the abundance ratio and diversity of bacterial species (Fig.2).Eight weeks after the fire,both the richness index and diversity indexes were reduced dramatically;3 years after the fire,the two indexes increased beyond pre-fire levels;15 years later,the two indexes dropped to pre-fire levels.The β diversity characteristics of soil bacteria in all plots is shown by RDA analysis.All plots are located in five agglomeration areas which take the sampling site as the center (Fig.3).Soil bacteria are capable of resisting disturbance and able to maintain a relatively stable community structure and restore rapidly after fire.
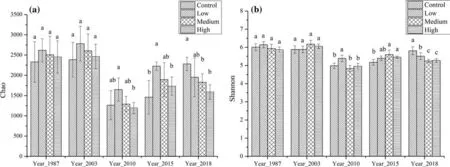
Fig.2 Soil bacterial α diversity characteristics:a Chao indexes;b Shannon indexes
Influence of environmental factors on bacterial communitiesFrom the redundancy analysis (RDA) (Fig.3),it is clear that the explained variable in the first and second axes are 17.7%and 7.7%,respectively,and the cumulative contribution rate is 25.45% (p< 0.01).Soil pH,TOC,and AP are explained by 19.31%,18.21%,and 17.69% of the variance,respectively.
The cover vegetation has less effect on soil bacterial community (Fig.3);the explained variable in the first and second axes are 17.75% and 7.7%,respectively,and the cumulative contribution rate is 25.45% (p< 0.01).

Fig.3 Results of RDA analysis of bacteria:a redundancy analysis (RDA) of bacterial community structure and soil chemical properties;b RDA of bacterial community structure and vegetation factor redundancy analysis
Characteristics of soil fungal community
Structural characteristics
The ITS rRNA high-throughput sequencing was carried out on all 60 soil samples of 20 groups.There were 3,302,646 high quality sequences with 432 bp mean reading length obtained after quality control and chimera removal,which were clustered into 4771 OTUs (97% similarity),belonging to 33 phylum,234 orders,and 604 genera.
The predominant fungal orders,(relative abundance ratio in each sample is above 0.3%),in unburned soils wereHelotiales(36.7% ± 18.0%),Thelebolales(3.4% ± 3.3%),Eurotiales(0.7% ± 0.9%),Hypocreales(0.3% ± 0.2%),Archaeorhizomycetales(0.2% ± 0.2%) ofAscomycotaphylum;Agaricales(15.6% ± 13.0%),Russulales(10.6% ± 6.5%),Thelephorales(8.8% ± 8.0%),andAtheliales(1.2% ± 1.4%) ofBasidiomycotaphylum;andMortierellales(3.4% ± 2.6%) ofMortierellomycotaphylum.The total relative abundance ratio was over 70% (Fig.4).
Fire causes significant changes in the soil fungal community structure.In soil under high severity fire,the relative abundance ratios ofArchaeorhizomycetales(38.1% ± 19.4%),Eurotiales(5.8% ± 6.4%),andHypocreales(0.8% ± 0.9%) were significantly increased,while that ofHelotiales(19.0% ± 14.4%),Thelebolales(1.6% ± 1.1%),Agaricales(1.4% ± 1.4%),Atheliales(0.1% ± 0.1%),Russulales(2.3% ± 3.7%),andThelephorales(1.5% ± 1.5%) were significantly reduced.With an increase of recovery time,changes to community structure gradually decreased.Thirty years post-fire,the community structure had not been restored to pre-fire levels.In soils under low/intermediate severity fire,the relative abundance ratios ofArchaeorhizomycetales(6.1% ± 12.1%) andEurotiales(2.2% ± 5.5%) increased significantly while that ofAgaricales(5.9% ± 5.8%) andRussulales(6.8% ± 6.5%)were significantly reduced;there was no change forHelotiales(38.8% ± 20.3%).With an longer recovery time,the bacterial community structure gradually recovered to prefire levels after 30 years (Fig.4).
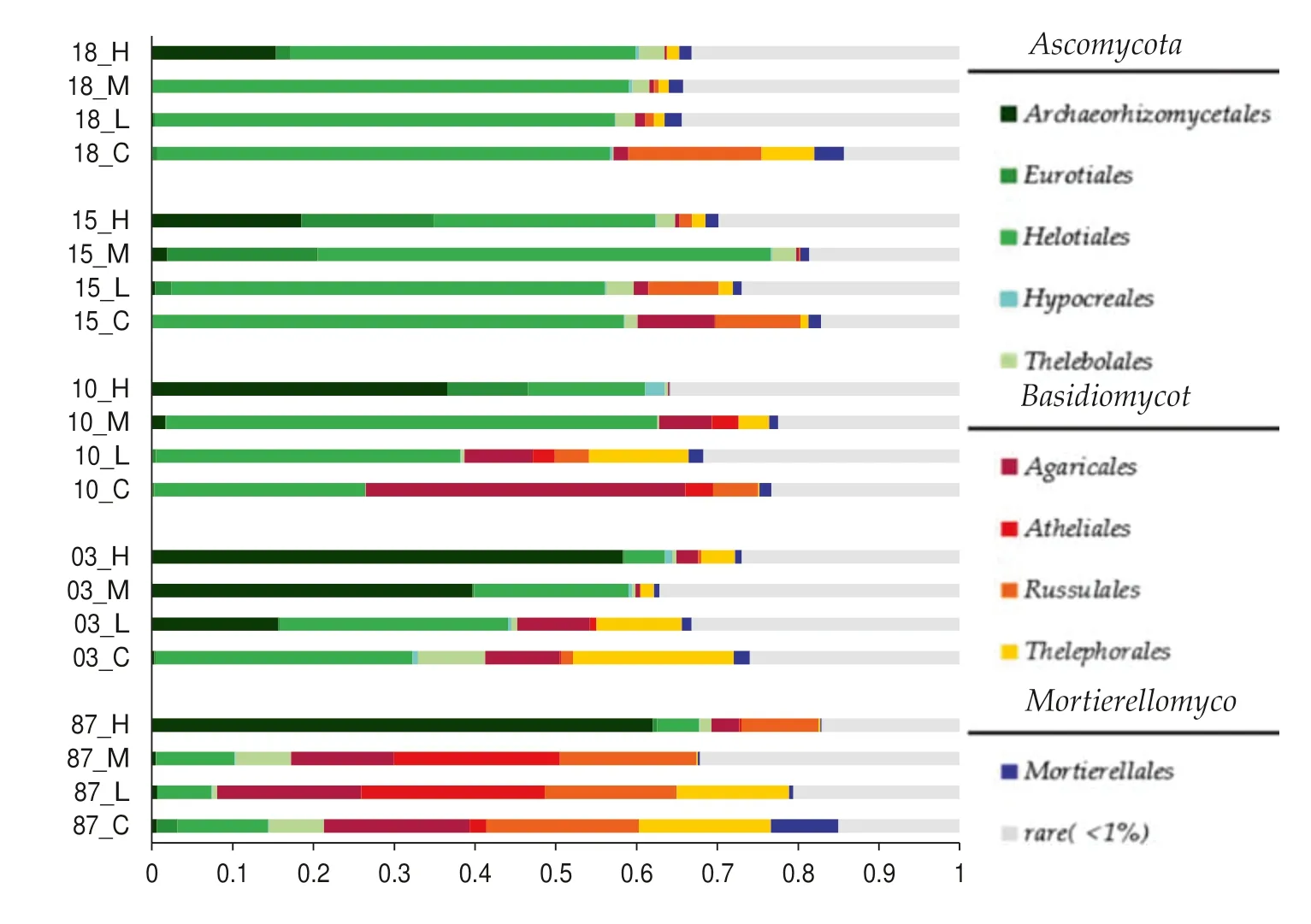
Fig.4 Soil fungal community composition at the order level
Fungal diversity characteristics
The soil fungal diversity index (value range:1.5‒3.5) was significantly lower than the soil bacterial diversity index(value range:5.0‒6.5) (Fig.5).After fire,fungal richness and diversity indexes were dramatically reduced.With increased recovery time,abundance ratios and diversity index gradually recovered;the abundance ratio was restored to pre-fire levels after 30 years while the diversity index did not (Fig.5).From the β diversity characteristics of soil fungi,it is apparent that:the high severity burned and the low/intermediate severity burned areas have fungi in two clusters(Fig.6).Fungi are more sensitive to environmental changes than bacteria after fire.This is because soil fungi have a homogeneous structure and are mainly distributed over a few predominant phyla.They are unstable and have poor adaptability to disturbance,and easily show significant changes in community structure after fire,requiring a length period for restoration.
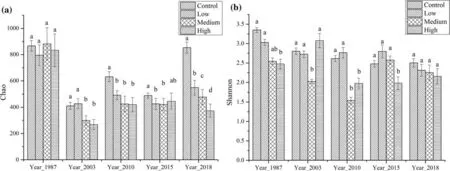
Fig.5 Soil fungal α diversity characteristics:a Chao indexes;b Shannon indexes
Influences of environmental factors on fungal communitiesThe results of the canonical correspondence analysis (CCA)(Fig.6) show that soil factors have little influence on the fungal community.The relative explained variable in the first and second axes are 7.1% and 6.4%,respectively.The cumulative contribution rate is 13.6% (p< 0.01).Soil pH,TOC and AP contents explain 9.0%,8.9% and 6.7% variance,respectively,which indicates that these are the main factors affecting soil fungal communities.

Fig.6 Results of RDA analysis of fungi:a canonical correspondence analysis (CCA) of fungal community structure and chemical properties;b CCA of fungal community structure and vegetation factor redundancy analysis
The vegetation factors such as tree biomass,herbaceous biomass,and herbaceous diversity can greatly affect the fungal community.The explained variable in the first and second axes are 14.4% and 11.9%,respectively.The cumulative contribution rate is 26.4% (p< 0.01).Tree biomass,herbaceous biomass,and herbaceous diversity respectively explain 18.7%,16.0%,and 15.6% variance,respectively,showing that they are the main influencing factors on soil fungal communities (Fig.6).
Prediction on soil functions
PICRUSt2 was applied to identify the functional characteristics of soil microbes related to element cycling.Among the functions that the soil bacteria are involved in,the dominating genes were those relating to the anaerobic acetyl-CoA pathway and the 3-hydroxypropionic acid pathway in carbon fixation,and those involved in cellulose degradation,and organic phosphorus hydrolysis.Relative abundances of other functions were comparatively low.The analysis on carbon cycling functions showed that:after fire,with increasing severity,Calvin fixation was enhanced to an ever-increasing extent.Among them,the relative abundance ratios (relative abundance ratio refers to the ratio of the abundance of the soil enzyme to the sum of the abundance of all enzymes in the soil) of 1,5-ribulose bisphosphate carboxylase [EC:4.1.1.39] ([EC:] represents the ID of each enzyme in the KEGG database.) involved in the Calvin cycle,carbon-monoxide dehydrogenase[EC:1.2.7.4] involved in anaerobic acetyl-CoA pathway,acetyl CoA carboxylase [EC:6.4.1.2] and propionyl-CoA carboxylase [EC:6.4.1.3] involved in 3-hydroxypropionate pathway were increasingly enhanced.The relative abundance ratios of 4-hydroxybutyryl-CoA dehydratase[EC:4.2.1.120] involved in succinyl-CoA pathway,and the pyruvate carboxylase [EC:6.4.1.1] involved in oxaloacetate pathway were increasingly weakened.After increasing fire severity,cellulose and hemicellulose degradation were further enhanced.The relative abundance ratios of endoglucanase [EC:3.2.1.4],endo 1,3(4) beta glucanase[EC:3.2.1.6] and beta glucuronidase [EC:3.2.1.31]involved in cellulose degradation were increasingly weakened.The relative abundance ratios of xylan 1,4-betaxylosidase [EC:3.2.1.37] and endo-1,4-beta-xylanase involved in hemicellulose degradation were also weakened.After increasing fire severity,the relative abundance of catechol 1,2-dioxygenase [EC:1.13.11.1] involved in lignin degradation was enhanced.The analysis on nitrogen cycling showed that after fire,with increasing severity,the relative abundance ratio of a rate-limiting,enzyme-ammonia monooxygenase [EC:1.14.99.39] involved in nitrification was enhanced in ever increasing amounts,while the key enzymes involved in nitrogen fixation–nitrogenase iron protein NifH [EC:1.18.6.1] and nitrite reductase (NO-forming) [EC:1.7.2.1] involved in denitrification were increasingly weakened.Phosphorus cycling analysis showed that with an increase in fire severity,the relative abundance ratios of alkaline phosphatase [EC:3.1.3.1]and trehalose 6 phosphate hydrolase [EC:3.2.1.93] were enhanced,while acid phosphatase [EC:3.1.3.2] weakened(Fig.7).

Fig.7 PICRUSt2 function prediction results of soil bacterium
Among the processes that soil fungi are involved in,the dominating genes are those relating to the oxaloacetate pathway and organic phosphorus hydrolysis in carbon fixation,while other functions were comparatively low abundances.The analysis of carbon cycling showed that with increasing fire severity,the relative abundance ratios of pyruvate carboxylase [EC:6.4.1.1],beta glucuronidase [EC:3.2.1.31]involved in cellulose degradation,and xylan 1,4-betaxylosidase [EC:3.2.1.37] involved in hemicellulose degradation were gradually weakened.Catechol 1,2-dioxygenase enzymes [EC:1.13.11.1] involved in lignin degradation were increasingly enhanced.The analysis of phosphorus cycling showed that after increasing fire severity,the relative abundance ratios of alkaline phosphatase [EC:3.1.3.1]were enhanced while acid phosphatase [EC:3.1.3.2] was depleted (Fig.8).

Fig.8 PICRUSt2 function prediction results of soil fungi
Discussion
Characteristics and driving factors of soil bacterial community structure after fire
The soils of the Daxing’anling mixed forest contained similar bacterial communities,dominated byAcidobacteria,Proteobacteria,andActinobacteria.After high severity fire disturbance,the two bacterial indexes of abundance ratio and diversity both declined dramatically.The structure also changed considerably to a community dominated byProteobacteriaandChloroflexi.Over time,the indexes of abundance and diversity increased significantly to pre-fire levels 30 years after the fire.After low/intermediate-severity fire,Acidobacteriadecreased significantly,while other bacteria increased slightly.Over time,the bacterial community which was originally dominated as noted above,was dominated byProteobacteria,ActinobacteriaandChlorofl exi.Mild fire disturbance only resulted in small changes to the soil bacteria.
When the environment changes,different bacteria show different coping strategies (k-strategy,r-strategy) due to their different survival characteristics.Acidobacteria,Actinobacteria,andFirmicutesare gram-positive bacteria (Fierer et al.2007),which follow a“k-strategy (Kapazitätsgrenzestrategistis)”for survival,and were characterized by good growth in a stable environment but with a weak capability in adapting to a changed environment (Mickan et al.2017);Proteobacteria,Gemmatimonadetes,and mostChlorofl exigram-negative bacteria (Chaudhry et al.2012) with“r-strategy (rate-strategistis)”for survival,which features rapid growth and capable of adapting to new environment(Mickan et al.2017).Fontaine et al.(2003) considered that fast-growing“r-strategy”species dominated in the early stages of environmental change,and later,these species were replaced by“k-strategy”species when easy-to-degrade components of nutrition were depleted.In the study area,the soil environment was relatively stable.The“k-strategy”species likeAcidobacteriaandActinobacteriadominated the soil bacteria communities.After fire disturbance,nutrition from organic matter were greatly reduced (Table 4),and the bacteria species changed.In this event,“r-strategy”species such asProteobacteriaandChloroflexi,multiplied and became dominant.Then,when the soil environment tended to be stable again,“k-strategy”species gradually resumed a dominating status.
Among the environmental factors,vegetation has little effect on soil bacteria;pH,TOC (total organic carbon)and AP (available phosphorous) have obvious effects.Soil pH was negatively correlated with the relative abundance ofAcidobacterialesof theAcidobacteriaphylum,Actinobacteria andCorynebacterialesof theActinobacteriaphylum,andGemmatalesandIsosphaeralesof thePlanctomycetesphylum It was positively correlated withGaiellalesandIMCC26256of theActinobacteriaphylum,Betaproteobacteriales,Elsterales,MyxococcalesandRhizobialesof theProteobacteriaphylum,AD3andKD4-96of theChloroflexiphylum,Chloroplastof theCyanophytaphylum,Nitrospiraof theNitrospiraephylum,Chitinophagalesof theBacteroidetesphylum,andChthoniobacteralesof theVerrucobacteriaphylum (Fig.9).Austin and Smith (1990) found that pH had a direct physiological gradient on organisms,i.e.,the growth of organisms is subject to the restraining level of pH.The ability of different organisms to adapt to low pH is anacid tolerance response (ATR).The optimal pH forAcidobacteriagrowth is 4–5,having high ATR,but most bacteria grow better at a pH 7‒8 environment,having comparatively low acid tolerance.Soil pH in the study area was 4.2‒5.2 so that,compared to other bacteria,Acidobacterialesand other bacteria of the same kind,which possessed higher ATR,were more competitive.But due to the alkaline hydrolysis effect of ash contents after fire,pH rose slightly to 4.6‒5.6.At this pH,the competitiveness ofAcidobacteriawas weakened while the relative abundance of other bacteria increased accordingly.

Fig.9 Correlation coefficients of soil bacterial community and environmental factors (**represents a significant difference between the factors at the 0.01 level;*a significant difference at the 0.05 level)
Soil TOC (total organic carbon) was positively correlated with the relative abundance ofSubgroup_11of theAcidobacteriaphylum,GaiellalesandCorynebacterialesof theActinobacteriaphylum,andBacillalesof theFirmicutesphylum (Fig.9).Soil TOC is the main carbon source for heterotrophic microorganisms such asAcidobacteria,Actinobacteria,andFirmicutes.After fire,the loss of soil TOC showed significant depressing effects on these bacteria.
Available soil phosphorous was positively correlated with the relative abundance ratios ofAcidobacterialesof theAcidobacteriaphylum,SolirubrobacteralesandActinobacteriaof theActinobacteriaphylum,Bacillalesof theFirmicutesphylum,andGemmatalesof thePlanctomycetesphylum,but negatively correlated toKD4-96of theChloroflexiphylum,Chloroplastof theCyanobacteriaphylum,andElsteralesandMyxococcalesof theProteobacteriaphylum(Fig.9).Phosphorus is an important element of biological cells.Available soil phosphorous is directly absorbed and utilized by soil microorganisms,so that its level in the soil has an important influence on the growth of microorganisms.Total phosphorus (0.21‒0.49 g kg−1) is lower than the total phosphorous (0.41‒1.5 g kg−1) of the upper 10-cm layer in generally all Chinese forest soils (Gholz et al.2000;Mcgroddy et al.2004;Han et al.2005;Parton et al.2007;Li et al.2018) which confirms that soils of the study area lack phosphorus.In the meantime,after fire disturbance and due to appropriate environmental conditions,bacteria such asChloroflexi,Cyanobacteria,andProteobacteriamultiplied and consumed any available phosphorous and allowed for the production of weak bacteria likeAcidobacteriaandActinobacteria(Parton et al.2007).
According to previous studies,total soil nitrogen and available nitrogen are strongly correlated toPlanctomycetes,Firmicuates,andProteobacteriabacteria (Mao and Qi 2015).However,the results of this study show that the levels of total and available nitrogen had little influence on bacteria,which is different from previous research findings.Total soil nitrogen (2.24‒6.93 g kg−1) in the study area is relatively higher than the total nitrogen levels (1.2‒6.35 g kg−1) of the upper 10 cm layer in Chinese forests (Gholz et al.2000;Mcgroddy et al.2004;Han et al.2005;Parton et al.2007;Li et al.2018),which indicates that the soils of the study area are not lacking in nitrogen;after the fire,however,total soil nitrogen decreased,the available nitrogen which is directly absorbed by organisms increased.Therefore,the level of nitrogen resources was not restricted by soil bacteria.
Characteristics and driving factors of fungal community structure after fire
The dominant soil fungi orders areHelotiales,Agaricales,andRussulales.Eight weeks after a high intensity fire,the abundance ratios and diversity decreased significantly and the community structure showed significant change from the original fungi to fungi dominated byArchaeorhizomycetales,Helotiales,andEurotiales.Over time,the community structure enlarged.Thirty years after fire,the fungal community changed to a community dominated byArchaeorhizomycetales,Russulales,andHelotiales.After low/intermediate fire,the abundance ratios and diversity decreased but the community structure was only slightly changed from being dominated byHelotiales,Agaricales,andRussulalesto being dominated byHelotiales.With greater recovery time,the bacterial community gradually recovered and was restored to pre-fire levels.
Different species of soil fungi have various survival characteristics and specific relationships with vegetation.Therefore,changes in vegetative cover could be a significant influence on soil fungi.Archaeorhizomycetaleswas negatively correlated with tree biomass,and positively with herbaceous diversity.EurotialesandHelotialesare negatively correlated with tree biomass while positive with herbaceous diversity.AgaricalesandRussulalesshow positive correlation with biomass and negative correlation with herbaceous diversity (Fig.10).Agaricomycetesof theAgaricalesphylum andRussulaof theRussulalesphylum are the main ectomycorrhizal fungi of pines and also able to use saprophytic fungi using litter (Zhang et al.2016).Archaeorhizomycetalesis commonly distributed in the rhizosphere ofPinus,Rhododendron,and other species which can either coexist with mycorrhizal fungi or provide organic matter for saprophytic fungi (Rosling et al.2011).The mixed forest of Daxing’anling is largely homogeneous plant species dominated by Chinese larch associated with birch and aspen.Their roots are densely distributed in the soil which is rich in organic matter.AgaricalesandRussulalesfungi multiply rapidly through symbiosis and saprophytic relationship with roots.After fire,most vegetation was killed and roots decreased accordingly.Soil organic matter was largely consumed and further reduced the quantityAgaricalesandRussulalesdue to the loss of nutrients.For low/intermediateseverity fire,the surface vegetation structure had not been greatly changed and recovered quickly,so that the number ofAgaricalesandRussulalescould gradually restore to prefire levels.For high-severity fire,the restoration process of surface vegetation should be:open herb community → shrub and grass community → mixed forest.AgaricalesandRussulalesare specific to ecosystems and host habitats and unable to adapt to frequent changes to surface vegetation and failed to restore to pre-fire level.However,Archaeorhizomycetales,due to its powerful adaption capability dominated among soil fungi (Fig.10).
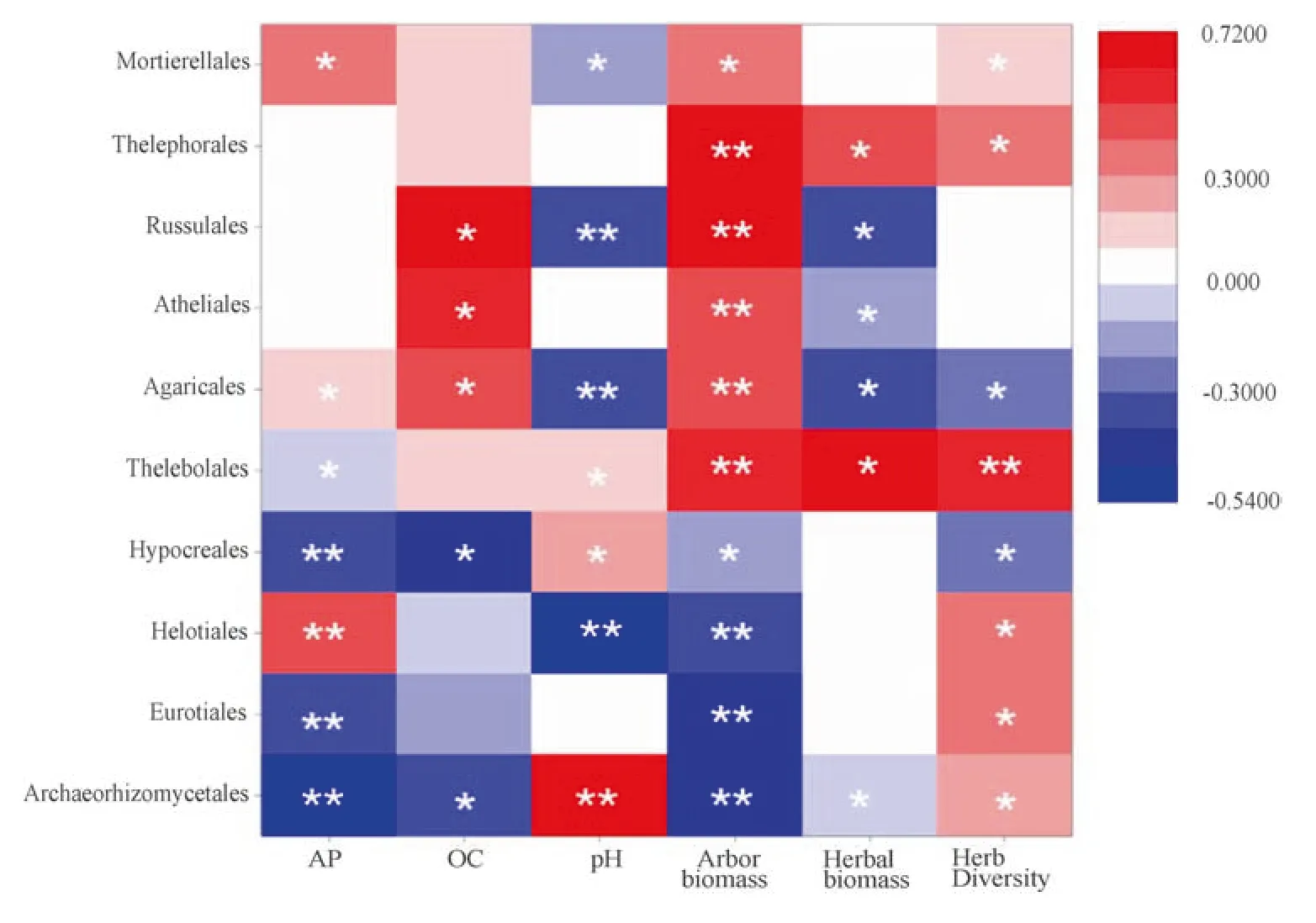
Fig.10 Correlation coefficient of soil fungal community and environmental factors (**a significant difference between the factors at the 0.01 level;*a significant difference between the factors at the 0.05 level)
The influence of the soil environment on soil fungi cannot be ignored.Archaeorhizomycetalesis positively correlated with pH,whileHelotiales,Agaricales,andRussulalesare negatively correlated (Fig.10).Soil fungi also show a prominent physiological gradient to pH.The optimal pH forAgaricalesandRussulalesis 3‒5,while the optimal forArchaeorhizomycetalesis around 7 (Xiong et al.2019).Archaeorhizomycetaleswas negatively correlated with available phosphorous and total organic carbon;Eurotialeswas negatively correlated with available phosphorous;RussulalesandAgaricaleswere positively correlated with total organic carbon;Helotialeswas positively correlated with available phosphorous (Fig.10).After fire disturbance,the pH rose which promoted the growth ofArchaeorhizomycetaleswhich grows best in a neutral soil.Organic matter in the soil was consumed by the fire so that the growth ofAgaricalesandRussulaleswere significantly reduced due to the lack of saprophytic nutrition.Soil available phosphorous was consumed and reduced byArchaeorhizomycetalesandEurotiales,which dramatically contained the growth ofHelotiales.
Prediction of soil functions after fire disturbance
According to the prediction of PICRUSt2,soil microbe functions related to carbon fixation,cellulose degradation and organic phosphorus hydrolysis were in relatively high abundance.In contrast,hemicellulose and lignin degradation,nitrogen fixation,nitrification and denitrification were low.This indicates that,in the soil of the study area,microbes are capable in decomposing cellulose but weak in decomposing hemicellulose and lignin,and weak in functions related to nitrogen cycling.
The carbon cycling process in the soil occurs with both bacteria and fungi simultaneously.Carbon fixation by the soil converts inorganic carbon to organic carbon,thus enhancing total organic carbon.In the soil of the study area,the Calvin cycle pathway,involving participation of photoautotrophic microorganisms likeCyanobacteria,the 3-hydroxypropionate pathway involving input byChlorofl exi,the anaerobic acetyl-CoA pathway with anaerobic bacteria,and the oxaloacetate pathway involving of symbiotrophism fungi like ectomycorrhizal fungi and arbuscular mycorrhizal fungi,were the main ways to increase total organic carbon (Yuan et al.2011).The degrading cellulose,hemicellulose and lignin represented microbe capability in decomposing and re-utilizing organic matter in animal and plant residues (Yuan et al.2011),among which,Acidobacteriahelped degrade plant carbon like cellulose and hemicellulose efficiently (Fierer et al.2007).Actinobacteriaplayed an important role in lignin degradation (Pan et al.2011).After fire disturbance,the rise of soil pH led to an increase in the relative abundance ofCyanobacteria,Chloroflexi,andAcidobacteria,which further enhanced microbe carbon fixation and lignin degradation;the relative abundance ofAcidobacteriadecreased,which weakened microbe capability in degrading cellulose and hemicellulose.In fact,the enhanced capability in carbon fixation and lignin degradation were favorable for total organic carbon accumulation.The forest fire played a positive role in soil carbon cycling.
Soil nitrogen cycling was accomplished by soil bacteria.Soil nitrogen cycle mainly includes nitrogen fixation,nitrification,and denitrification,etc.Nitrogen fixation converts nitrogen in the air into ammonia,which is beneficial for the accumulation of nitrogen in the soil (Sierra 1992;Chen et al.2006).Nitrogen fixation in the study area soil was achieved through symbiotic nitrogen fixation bacteria likeFrankia.Nitrification is an important step for organic nitrogen mineralization,for soil microbes in decomposing and reutilizing nitrogen in animal and plant residues (Weier et al.1993;Galloway et al.2004).In the study area,nitrification is mainly achieved by nitrifying bacteria ofNitrospiraandNitrosomonadaceae.Denitrification converts nitrates and nitrites into nitrogen to return it to the atmosphere (Weier et al.1993;Galloway et al.2004).In the study area,denitrification is mainly achieved by denitrifying bacteria.Research indicates that most denitrifying bacteria are heterotrophic which take organic matter as carbon and energy sources(Mao and Qi 2015).After fire,Frankiadecreased due reduction in available phosphorous and weakened nitrogen fixation.The increase in pH resulted in an increase in nitrifying bacteria such asNitrospiraandNitrosomonadaceaewhich enhanced nitrification.The decrease total organic carbon levels resulted in an inadequate supply of denitrifying bacteria,weakening the denitrification effect.The strengthening of soil nitrification and nitrogen fixation were important for the accumulation of nitrogen;the weakening of denitrification reduced nitrogen consumption and fire played a positive role in soil nitrogen cycling.
Organic phosphorus hydrolysis hydrolyzes macromolecular organic phosphorus into available phosphorus that can be directly absorbed by organisms (Podgorskii 1988;Liu et al.2015).This occurs in both soil bacteria and fungi simultaneously.After fire,soil pH (4.6–5.6) rose slightly but was still weakly acidic.This had little effect on acid phosphatase but and weakened the inhibitory effect of alkaline phosphatase.Overall levels of phosphatase increased,the hydrolysis of organic phosphorus was enhanced,and the availability of phosphorus in the soil was also increased.Fire had positive influence on phosphorus cycling.
Conclusions
Fire of different intensities has various effects on soil bacterial communities.Severe fires negatively impact soil bacterial communities.Fire changes the nutrients levels,damages original bacterial communities,and replaces them by bacteria with more powerful adaption capabilities.Such kinds of changes require at least 30 years to restore to the original communities.Low/intermediate fires enhances diversity and abundances of bacterial communities and optimizes composition by improving the soil environment.
Changes brought by high intensity fires on soil fungal communities are irreversible in the short-term because surface vegetation is destroyed and there is a loss of soil nutrients.This results in the decrease or loss of dominant fungi likeAgaricalesandRussulaleswhileArchaeorhizomycetalesbecomes dominant fungi by its strong adaptability.Over time,these changes become worse and are not restored in 30 years.However,low/intermediate-severity fires only affects the environment to a small extent.The relative amounts ofAgaricalesandRussulalesare slightly reduced but are restored essentially after 30 years.
After fire disturbance,soil carbon fixation,lignin degradation,mineralization of organic nitrogen and hydrolysis of organic phosphorus are enhanced,while denitrification is weakened.This positively promotes soil carbon,nitrogen,and phosphorus cycles to some extent.
AcknowledgementsWe thank the Inner Mongolia Daxing’anling Forest Ecosystem Research Station for support.We thank Yongliang Zhang (Inner Mongolia Daxing’anling Forest Ecosystem Research Station) and Zhenggong Yu (Inner Mongolian University) who helped measure the data.
Open AccessThis article is licensed under a Creative Commons Attribution 4.0 International License,which permits use,sharing,adaptation,distribution and reproduction in any medium or format,as long as you give appropriate credit to the original author(s) and the source,provide a link to the Creative Commons licence,and indicate if changes were made.The images or other third party material in this article are included in the article’s Creative Commons licence,unless indicated otherwise in a credit line to the material.If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use,you will need to obtain permission directly from the copyright holder.To view a copy of this licence,visit http://creativecommons.org/licenses/by/4.0/.
杂志排行
Journal of Forestry Research的其它文章
- Flexible transparent wood enabled by epoxy resin and ethylene glycol diglycidyl ether
- Diversity and surge in abundance of native parasitoid communities prior to the onset of Torymus sinensis on the Asian chestnut gall wasp (Dryocosmus kuriphilus) in Slovenia,Croatia and Hungary
- Ozone disrupts the communication between plants and insects in urban and suburban areas:an updated insight on plant volatiles
- Testing visible ozone injury within aLight Exposed Sampling Site as aproxy for ozone risk assessment for European forests
- Logging and topographic effects on tree community structure and habitat associations in a tropical upland evergreen forest,Ghana
- Spatial pattern dynamics among co-dominant populations in early secondary forests in Southwest China
