Graphene oxide influences bacterial community and soil environments of Cd-polluted Haplic Cambisols in Northeast China
2021-07-15JiaxinRuGuoyouChenYongLiuYingSangJinfengSong
Jiaxin Ru·Guoyou Chen·Yong Liu·Ying Sang·Jinfeng Song
Abstract Graphene oxide (GO),a carbon nanomaterial that is widely used in the environment and other industries,may pose potential risks to ecosystems,especially the soil ecosystem.Some soils in Northeast China are frequently polluted with cadmium (Cd) metal.However,there is no study on the influence of GO on the Cd-contaminated soil microbial community and soil chemical properties.In this study,Cd (100 mg kg−1)-polluted soils were treated with different concentrations of GO(0,25,50,150,250,and 500 mg L−1,expressed as T1,T2,T3,T4,T5,and T6,respectively) for 40 days.The treatment without Cd pollution and GO served as the control (CK).Then,we investigated the influence of the GO concentrations on the bacterial community and chemical properties of Cd-polluted Haplic Cambisols,the zonal soil in Northeast China.After GO addition,the richness and diversity indexes of the bacterial community in Cd-contaminated Haplic Cambisols initially increased by 0.05–33.92% at 25 mg L−1,then decreased by 0.07–2.37% at 50 mg L−1,and then increased by 0.01–24.37%within 500 mg L−1 again.The species and abundance of bacteria varied with GO concentration,and GO significantly increased bacterial growth at 25 and 250 mg L−1 .GO treatments influenced the bacterial community structure,and the order of similarity of the bacterial community structure was as follows:T4=T5 > T1=T6 > T2 > T3 > CK.Proteobacteria and Acidobacteria were the dominant bacteria,accounting for 36.0% and 26.2%,respectively,of soil bacteria.Different GO treatments also significantly affected the metabolic function of bacteria and further influenced the diversity of the bacterial community structure by affecting several key soil chemical properties:soil pH,organic matter and available potassium,phosphorus,and cadmium.Our results provide a theoretical basis for scientific and comprehensive evaluation of the environmental impacts of GO on the zonal forest soils of Northeast China.
Keywords Cadmium pollution·Haplic cambisols·Graphene oxide·Bacterial community·Soil environmental factors
Introduction
Currently,with the rapid development of urbanization,industrialization and agricultural intensification,heavy metal pollution has become increasingly serious (Govil et al.2008).Heavy metal pollution of soils has become one of the most widespread and harmful environmental problems worldwide (Zhao et al.2007;Li et al.2008).At present,the cultivated land area polluted with heavy metals in China is about 20 million hm2,accounting for about one fifth of the cultivated land area,among which the area polluted by Cd is 13,000 hm2(Sun 2005).
Cadmium (Cd),one of the most common heavy metals in the environment,is a refractory organic pollutant that is also one of the most poisonous and widely distributed of the heavy metal pollutants.Because it rapid accumulates in organisms and is resistant to degradation,Cd has caused serious environmental problems and toxicity to human beings and other living organisms (Solgi et al.2012;Keng et al.2014).Studies have shown that a Cd content of 0.35–0.50 g in the human body can be lethal (He et al.2004;Zukowska and Biziuk 2010;Lion and Olowoyo 2013).Cd is also more easily absorbed and enriched by plants,such asIris ensatavar.hortensis,Vinca rosea,andJuncus effusus,resulting in dwarf stature,leaf chlorosis and reduced photosynthesis,with an eventual decline in biomass (Fu et al.2010;Najeeb et al.2011;Khan et al.2017).
Graphene oxide (GO),a derivative of graphene,is a single-layer or multilayer nanomaterial formed from the exfoliation of graphite oxide.It is only 0.6 nm thick and has a specific surface area of 2600 m2g−1(Mkhoyan et al.2010).It also has many oxygen-containing functional groups,such as hydroxyl,carboxyl and epoxy groups,which endow many unique chemical properties,such as the high specific surface area and performance,strong adsorption capacity,good dispersion stability in water and most polar organic solvents,good hydrophilicity and mechanical properties (Zhang et al.2018).Therefore,GO is widely used to inhibit bacteria in the environment (Ji et al.2016),as a sensor (Zhao et al.2016),in photovoltaic cells (Jariwala et al.2013;Acik and Darling 2016;Yang et al.2017),and disease diagnosis (Tonelli et al.2015).
During its production and application,GO inevitably is released into the environment,but it is more in contact with the soil ecosystem than with water or air (Nowack and Bucheli 2007;Dinesh et al.2012;Shrestha et al.2013).GO usually first enters the soil,including heavy metal-contaminated soil,then interacts with soil components,thus having an important impact on organisms and soil properties (Dinesh et al.2012).A study has shown that carbon nanotubes can inhibit the biological effects of pollutants in soil (Zhou et al.2013).Similarly,GO has a strong adsorptive capacity for soil pollutants and can quickly contact and stably adsorb various types of heavy metal ions,especially polyvalent metal ions (Machida et al.2006;Xu et al.2009;Gao et al.2011;Wang et al.2018).In an area polluted with Cd,the entry of GO into the soil also affects the chemical properties of the soil,the migration behavior and ecological toxicity of Cd in the soil,and the soil microbial communities (Hu et al.2017).The adsorption of Cd to GO mainly occurs on the oxygen functional groups,such as–OH and–COOH,which play a vital role in the adsorption of Cd(II) (Bian et al.2015).
The microbial community structure is an important index to measure the effects of exotic substances on soil activity and metabolic capacity (Yu et al.2019) and is also an effective index to measure the influence of GO on soil properties (Li et al.2016;Wang et al.2019),on its migratory nature and toxicity in soils (Bian et al.2015;Wang et al.2018) and on soil bacteria,which are known to be affected by GO (Hu et al.2010,2017).Li et al.(2016)found that GO at a certain concentration inhibited bacterial growth,but bacterial abundance and community diversity were impacted very little.
In Northeast China,soil in a relatively wide area is contaminated with Cd from mines,such as the Xilin Cd-Lead(Pb)-Zinc (Zn) Mine in Yichun,Heilongjiang Province where the zonal forest soil is a Typic Bori-Udic Cambisol(corresponding to Haplic Cambisols [Grayic,Dystric])with high organic matter content and well-developed horizons.Changbai larch (Larix olgensis) is a vital afforestation tree species in this area.The total mass of microorganisms in the rhizosphere ofLarix olgensisplantation was reported as 638.4 × 104g−1by Shao et al.(2011),and bacteria were the dominant group microbes,followed by actinomycetes and fungi.In the rhizosphere soils of a 29-year-old larch plantation and one with 2-year seedlings,the mass ofBacillus cereuswas 0.011 × 105g−1and 15 × 105g−1,respectively,and ofStreptomycesspecies was 6.4 × 104g−1and 7.7 × 104g−1,respectively (Ji et al.2008).In these areas,especially in some agroforestry ecotones,GO may enter Cd-contaminated Haplic Cambisols through various ways and thus affect the biological properties of the soil,especially the microbial characteristics.However,how GO affects microbial community characteristics and chemical properties of Cd-contaminated Haplic Cambisols has not been reported.In this paper,we added different concentrations of GO into Cd-contaminated Haplic Cambisols and investigated the effects of GO on bacterial community diversity and structure,and soil chemical properties to provide a theoretical basis for the comprehensive evaluation of the ecological and environmental effects of GO on Haplic Cambisols polluted by Cd in Northeast China.
Materials and methods
Experimental materials and treatments
Characteristics of the 5 mg mL−1pure GO solution samples that were purchased from Suzhou Tanfeng Graphene Technology Co.,Ltd.(Suzhou,Jiangsu Province,China)were brown,with particle size of 0.5–5 μm,0.7–1.2 nm thick,purity > 99 wt%,and monolayer rate > 98%.In our preliminary study,this specification had optimal effects on chemical and biological properties of Haplic Cambisols (our unpublished data).
Soils collected for the study were Haplic Cambisols from the loamy A1horizon of in a Changbai larch (Larix olgensis) plantation (127°30′–127°34′ E,45°21′–45°25′ N).Larix olgensisseedlings (‘1a’),purchased in May 2018 from the Maoershan Experimental Forest Station,Harbin,Heilongjiang Province,China,were then planted in the soil,rather than in soil from barren or uncultivated areas,to best resemble the actual plant growth substrate and to ensure suitability for future practical application in reforestation or nurserys usingL.olgensisseedlings in Northeast China.
In late May (budding stage),L.olgensisseedlings were planted in pots (upper diameter 18.4 cm,lower diameter 16.2 cm,height 20.0 cm) filled with loamy A1horizon of Haplic Cambisols without impurities (3.3 kg per pot).Soil properties determined (mean,n=3) were soil texture:loam;pH (KCl):4.02 ± 0.05;cation exchange capacity (CEC):40.12 ± 1.45 cmol kg−1soil;organic matter (OM):79.9 ± 0.12 g kg−1;hydrolytic nitrogen(HN):282.05 ± 0.31 mg kg−1;available phosphorus(AP):61.28 ± 1.09 mg kg−1(extracted with 50 mmol L−1HCl–25 mmol L−1H2SO4);available potassium (AK):304.06 ± 1.17 mg kg−1;available Cd:0.26 ± 0.02 mg kg−1.Twenty seedlings were raised in each pot.
After the seedlings had grown for 20 days in the pots in a greenhouse with natural light,the daytime temperature of 21–35 °C and nighttime temperature of 8–18 °C,an aqueous solution of CdCl2·2.5H2O was evenly applied to the soil to achieve 100 mg Cd2+kg−1soil,which simulates the very high end of the soil Cd concentrations measured in the Cd–Pb–Zn mining area of Yichun,Heilongjiang Province,China.The Cd concentration was also based on our previous finding thatL.olgensisis tolerant to heavy metals,especially Cd.To investigate the impacts of Cd application at different concentrations onL.olgensisseedlings in preliminary tests,we found that 100 mg kg−1Cd significantly and optimally influenced the growth and physiology of these seedlings (data not shown).The Cd concentration in the soil was estimated based on the concentration of CdCl2·2.5H2O in solution,the amount of solution,and the soil mass in each pot.After Cd addition,the available Cd content of the soil was 42.76 mg kg−1.
After Cd exposure for 10 days,which we previously showed to be a sufficient time for Cd uptake,GO treatments were initiated.GO was applied once daily at 8:00 a.m.for 4 days.On the first day,300 ml of GO solution was added to each pot,then 100 ml was added the next 3 days to adjust the soil water content to 70% of the maximum moisture capacity.GO solutions were evenly applied to the soils using a sprinkling can;the GO concentrations were 0,25,50,100,250 or 500 mg L−1(Wu et al.2015;Li et al.2016;produced by reverse osmosis [RO] water,pH 5.8,and expressed as T1,T2,T3,T4,T5,and T6,respectively) at a rate equivalent to approximately 0,7.58,15.15,30.30,75.76,and 151.52 mg kg−1of soil per GO concentration,respectively.The control (CK) pots were treated with the same amount of RO water without Cd application and without GO to compare the effects with those were Cd (T1),and T2 to T6 were used to investigate the effects of different concentrations of GO on the bacterial community and soil chemical properties.Three pots were employed per treatment,resulting in 21 pots in total.Seedlings growing in Cd-polluted soils were incubated at 25 °C in the greenhouse,and the soil water content was regularly adjusted to 70% of the field waterholding capacity.The growing season in Northeast China is short;our study could not be started until early June,andL.olgensisleaves turn yellow and begin to fall at the end of August.Thus,relevant indicators must be measured before late August.At 40 days after the last GO application,allowing sufficient time for the soil microbes to adapt to Cd treatments (Chung et al.2015;Song et al.2018),soils were sampled and analyzed.
Identification of soil bacteria
At 40 days after the last GO applications,soil samples were randomly collected from the middle of each pot(~ 2.0 g),immediately treated with liquid nitrogen,stored in a refrigerator at −80 °C,and transported on dry ice to Shanghai Parsonol Biotechnology Co.,Ltd.(Shanghai,China),for sequencing 16S rDNA gene of soil bacteria.Illumina high-throughput sequencing technology on the MiSeq platform was used to identify the bacterial community composition.As described by Song et al.(2018),the 16S rDNA gene for the bacterial community of each soil was amplified by PCR,then sequenced,and operational taxonomic units (OTUs) were determined,and beta and alpha diversities were analyzed.The Chao1 and ACE indexes were used to assess richness and the Simpson and Shannon indexes for diversity in each soil sample.Nonmetric multidimensional scaling (NMDS) was used to reveal the extent of the differences and similarities in bacterial changes.Cluster analysis was used to evaluate soil bacterial similarity.The detailed information of bioinformatics and statistical analysis are the same as those in Song et al.(2018).Finally,ordination plots from a redundancy analysis (RDA) were used to reveal the relationships between the soil bacterial community and environmental variables.
Soil chemical properties
At 40 days after the last GO applications,soil samples were randomly collected from each pot (~ 200 g),airdried,evenly mixed,sifted through 2-mm nylon screens,for analysis.The pH was determined using a pH S-2 acidity meter (Shanghai Precision Science Instrument Co.,LTD.,Shanghai,China) with 1.0 mol L−1KCl extraction.Organic matter (OM) content was determined using a TOC(total organic carbon) analyzer (Multi N/C 3100,Analytik Jena,Germany).Hydrolytic nitrogen (HN) content was determined via the alkali reduction diffusion method;the available phosphorus (AP) content was measured through the Mo-Sb colorimetric method,with a 0.05 mol L−1HCl–0.025 mol L−1H2SO4extraction;and the available potassium (AK) content was measured via the photometric method.Methods for all assays were described by Chen(2005).
The available cadmium (Cd) content was also obtained using an inductively coupled plasma mass spectrometer(ICP-MS;SCIEX ELAN 6000,Perkin Elmer,Waltham,MA,USA) with diethyltriaminopentaacetic acid (DTPA)extraction.All samples were measured in triplicate.
Statistical analyses
OriginPro 2019 software (OriginLab,Northampton,USA)was used to construct the figures.SPSS 18.0 software (IBM,Armonk,NY,USA) was used to analyze the data,and Tukey’s new complex range method was used to test for treatment effects (P< 0.05).
Results
Sequencing results and soil sampling depth verification
Based on high-throughput sequencing,a total of 7814 OTUs were detected in Haplic Cambisols receiving the control,and four GO and Cd treatments (CK–T2–T4–T5–T6),whereas T3 and T1 totaled 5732 OTUs (Fig.1).The number of OTUs for unique and common bacteria in CK,T2,T4,T5,and T6 was 506,431,362,336,and 448,and 4058,4464,4298,4281,and 4433,respectively.The number of OTUs for common and unique bacteria in the T1 and T3 treatments was 4267,4312 and 1420,1465,respectively (Fig.1).
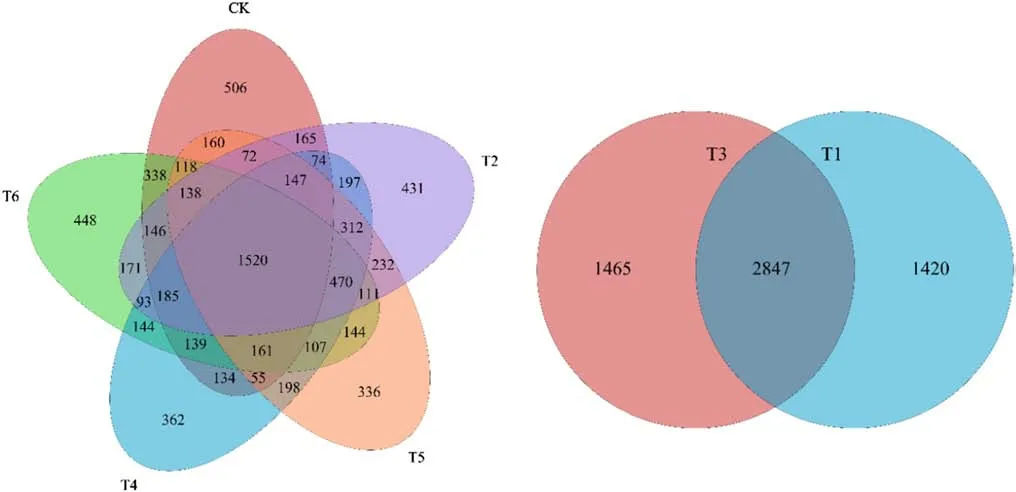
Fig.1 Venn diagrams of the total operational taxonomic units(OTUs) in Cd-polluted Haplic Cambisols treated with different concentrations of graphene oxide (GO).CK:0 mg L−1 GO without Cd;T1:0 mg L−1 GO+Cd;T2:25 mg L−1 GO+Cd;T3:50 mg L−1 GO+Cd;T4:100 mg L−1 GO+Cd;T5:250 mg L−1 GO+Cd;T6:500 mg L−1 GO+Cd
Alpha diversity analysis
The Chao1 and ACE indexes for richness and Simpson and Shannon indexes for diversity in the soil samples treated with Cd and different GO concentrations are presented in Table 1.Cd decreased the Chao1 index but the difference was not significant.Compared with T1,the GO treatments(except for T3) increased the Chao1 index,peaking in the T2 treatment,indicating that GO might promote the growth of some bacteria and increase the abundance of some bacteria to a certain extent,but none of the treatments were found to have a significant effect.During the incubation period,the Chao1 index ranking was T2 > T5 > T4 > CK > T6 > T 1 > T3,with the ACE index showing the same basic trend(Table 1).Compared with CK,the Simpson and Shannon indexes both increased after Cd treatment (T1),indicating that Cd increased the number of bacteria,but the difference was not significant (P> 0.05).The Shannon and Simpson indexes in all GO-treated soils also did not vary significantly(P> 0.05),but their specific changes differed by treatment(Table 1).For example,after GO treatment,the Shannon index increased initially,decreased and then increased with increasing GO concentration,reaching a maximum in T2;the ranking was T2 > T1=T6 > T4 > T5 > CK > T3 throughout the incubation period (Table 1).
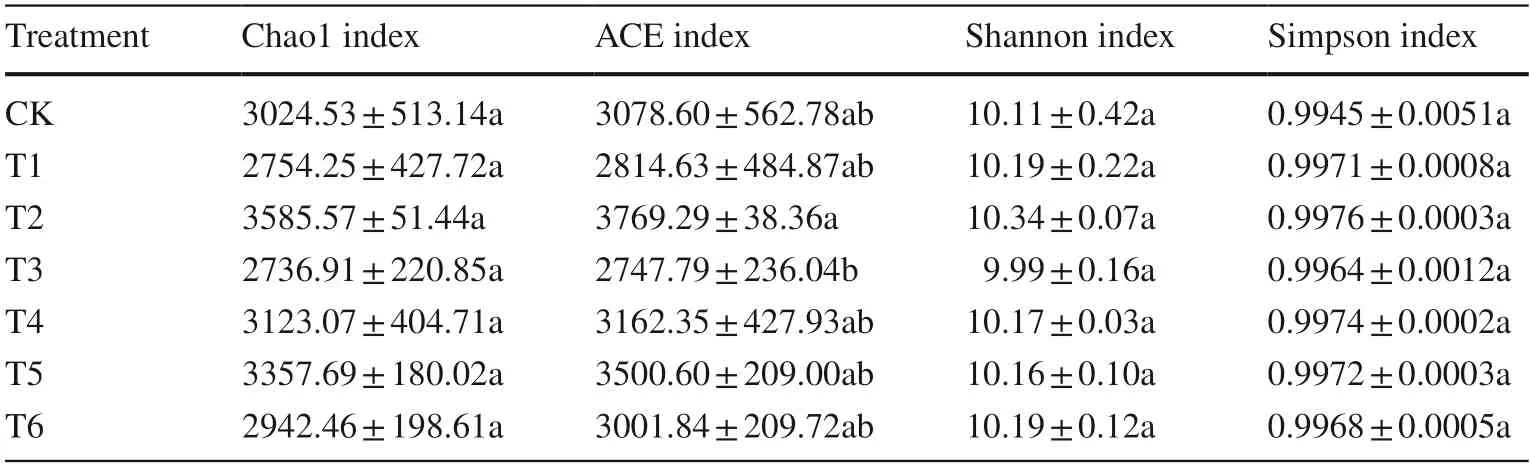
Table 1 Bacterial abundance and diversity indexes in soil treated with different concentrations of graphene oxide (GO)
Beta diversity analysis
Beta diversity analysis can examine the similarity of the bacterial community structure among different samples.Nonmetric multidimensional scaling (NMDS) of soil samples(Fig.2) was used to analyze the sequence of Cd-contaminated soil samples and reveal the extent of the differences and similarities in bacterial changes among different GO treatments.CK and Cd-treated soils (T1) were far apart,indicating that the bacterial community structure between the two groups was quite different.After GO treatment,the distance between T4 and T5 was small,which indicated that the similarity between them was high.The same was observed for T1 and T6 (but not for T1 and T6 with T2,T3,T4,and T5).The soil bacterial community structures under the six treatments were relatively similar irrespective of the GO treatment.Soil bacterial similarity was evaluated by cluster analysis (Online Appendix Fig.S1),which revealed a ranking of T4=T5 > T1=T6 > T2 > T3 > CK.Hence,GO had an effect on the beta diversity of Cd-polluted soils to some extent.
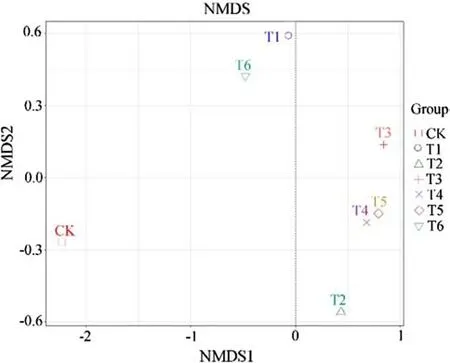
Fig.2 Nonmetric multidimensional scaling (NMDS) of Cd-polluted soil bacterial communities under different graphene oxide (GO) treatments (mean,n =3).CK:0 mg L−1 GO without Cd;T1:0 mg L−1 GO+Cd;T2:25 mg L−1 GO+Cd;T3:50 mg L−1 GO+Cd;T4:100 mg L−1 GO+Cd;T5:250 mg L−1 GO+Cd;T6:500 mg L−1 GO+Cd
Bacterial community composition
Based on the population statistics (Fig.3),the number of bacterial species in soil samples varied among Cd treatment and the different GO concentrations.Under Cd and GO treatments,bacteria were usually abundant and included Proteobacteria,Acidobacteria,Verrucomicrobia,Chloroflexi,Actinobacteria,Nitrospirae,Gemmatimonadetes,Bacteroidetes,Latescibacteria,Planctomycetes,and other bacteria.Except for a few unidentified bacteria,the relative abundance of Proteobacteria,Acidobacteria,Actinobacteria,Verrucomicrobia and Chloroflexi exceeded that of other co-occurring taxa (Fig.4).

Fig.3 Number of species estimated for different taxonomic levels of the soil bacteria in the various treatment samples(mean,n =3).CK:0 mg L−1 GO without Cd;T1:0 mg L−1 GO+Cd;T2:25 mg L−1 GO+Cd;T3:50 mg L−1 GO+Cd;T4:100 mg L−1 GO+Cd;T5:250 mg L−1 GO+Cd;T6:500 mg L−1 GO+Cd
The relative abundance of major bacterial phyla in Haplic Cambisols varied with Cd treatment and the applied GO concentration.Compared with the CK,the Cd treatment (T1) significantly increased the relative abundance of Proteobacteria,Acidobacteria and Planctomycetes,but the relative abundance of Verrucomicrobia,Chloroflexi,Actinobacteria,Nitrospirae,Gemmatimonadetes,Bacteroidetes and Latescibacteria decreased (Fig.4).After GO treatment,the relative abundance of Proteobacteria and Bacteroidetes decreased initially and then increased,that of Acidobacteria,Chloroflexi and Gemmatimonadetes decreased first,increased and then decreased,that of Verrucomicrobia and Planctomycetes increased first and then decreased,that of Actinobacteria increased with increasing GO concentration,while that of Nitrospirae and Latescibacteria increased first,then decreased and finally increased (Fig.4).Across the gradient of GO treatments,Proteobacteria and Acidobacteria were codominant,accounting for 36% and 26.2%,respectively,of all soil bacteria in our samples (Fig.4).

Fig.4 Bacterial compositions of the 10 most abundant phyla in Haplic Cambisols with and without Cd after treatment with various concentrations of graphene oxide (GO) (mean,n =3).CK:0 mg L−1 GO without Cd;T1:0 mg L−1 GO+Cd;T2:25 mg L−1 GO+Cd;T3:50 mg L−1 GO+Cd;T4:100 mg L−1 GO+Cd;T5:250 mg L−1 GO+Cd;T6:500 mg L−1 GO+Cd
Online Appendix Fig.S2 shows a heatmap of the soil bacterial community under Cd and the GO treatments at the genus level.From the corresponding cluster analysis of the top 50 most-abundant bacterial genera,Anaeromyxobacter,Rhizomicrobium,Rhodanobacter,Candidatus Koribacter,Candidatus Solibacter,Bryobacter,Bradyrhizobium,Nitrobacter,Pseudolabrys,Reyranella,andAcidibacterwere relatively more abundant in most soil samples;Aquicella,Candidatus Xiphinematobacter,Reyranella,Anaeromyxobacter,Lysobacter,Rhizomicrobium,Rhodanobacter,Candidatus Koribacter,Candidatus Solibacter,Bryobacter,Bradyrhizobium,NitrobacterandPseudolabryswere less abundant in CK,but were higher in Cd-treated soil (T1) and other samples treated with GO (T2–T6;Online Appendix Fig.S2).Seven soil samples were clustered according to the relative abundance of the top 50 bacterial genera,and T1 and T6 were on the same branch,similar to T3 and T4,suggesting that the composition of bacterial communities was similar,consistent with the NMDS results (Fig.2).Pairwise comparisons of the number of sequences (i.e.,absolute abundance) of bacterial taxa at the phylum and genus levels were carried out (Table 2).The abundances among the samples (groups) differed significantly among Cd and the GO treatments.Specifically,comparisons of 0 vs.25 mg L−1(CK vs.T2),0 vs.50 mg L−1(CK vs.T3),0 vs.100 mg L−1(CK vs.T4) and 0 vs.250 mg L−1(CK vs.T5)were most different at the phylum level,while 0 vs.0 mg L−1(CK vs.T1 (with Cd)),0 vs.25 mg L−1(CK vs.T2) and 0 vs.100 mg L−1(CK vs.T4) had the most pronounced differences at the genus level (Table 2).

Table 2 Statistical results of Metastats for the pairwise comparison test among the different sample groups
Prediction of bacterial metabolic function
The Venn diagram of common functional groups (Online Appendix Fig.S3) was used to analyze the OTU numbers of common and unique functional groups in soil samples.A total of 6108 OTUs were found in five soil samples (CK,T2,T4,T5 and T6),and the number of OTUs of common bacteria were 5577 (91.31% of the total).The number of OTUs of common and unique bacteria in CK,T2,T4,T5 and T6 was 5754,5847,5894,5779,5764 and 12,50,90,23 and 16,respectively.A total of 6027 OTUs was found in T1 and T3 and that of common bacteria was 5613 (93.13% of the total).The number of OTUs of common and unique bacteria in T1 was 5635 and 6005 and 22 and 392 in T3,respectively.Hence,different concentrations of GO treatments significantly increased the number of functional groups in Cd-polluted Haplic Cambisols (Online Appendix Fig.S3).
Based on the cluster heatmap analysis of bacterial functional groups (Fig.5),it can be seen that,compared with 0 mg kg−1Cd (CK),the metabolic function for 100 mg kg−1Cd (T1) was basically stagnant.For Cd-treated soils under different concentrations of GO treatments (T1-T6),the metabolic function in the T2 treatment significantly increased,that in T3 almost stagnated,and that in T4 recovered to a certain extent;the maximum was reached in T5,whereas there was slight decrease in T6 (Fig.5).

Fig.5 Heatmap of bacterial community abundance for KEGG homologous gene clusters (KO) derived from the cluster analysis.The color gradient from green to red indicates increasing similarity.Each cell depicts the similarity for two soil samples in the matrix(Online Appendix S1).CK:0 mg L−1 GO without Cd;T1:0 mg L−1 GO+Cd;T2:25 mg L−1 GO+Cd;T3:50 mg L−1 GO+Cd;T4:100 mg L−1 GO+Cd;T5:250 mg L−1 GO+Cd;T6:500 mg L−1 GO+Cd
Changes in soil chemical properties
Compared with CK,Cd significantly reduced soil pH,which increased initially but decreased and then increased under GO treatment,reaching the maximum in T6 (500 mg L−1;Online Appendix Fig.S4).Cd significantly increased the OM content (P< 0.05),which increased initially and then decreased under GO treatment,being the highest in T5(250 mg L−1;P< 0.05;Online Appendix Fig.S4).
Cd also significantly increased the HN content (P< 0.05),and the AK and AP contents also increased,but the effects were not significant (P> 0.05;Fig.6).The HN,AK and AP contents in GO-treated soils initially decreased,increased and then decreased.There was a significant difference in the HN contents between T1 and other concentrations of GO treatments (except for T5;P< 0.05).The AK content in T1 was significantly different from that in T2 and T5 (P< 0.05).The AP content in T1 was significantly different from that in T2 and T6 (P< 0.05;Fig.6).

Fig.6 Available phosphorus(AP),available potassium (AK),and hydrolytic nitrogen (HN)contents of Cd-polluted soils treated with different concentrations of graphene oxide (GO)(mean ± 1 SD,n =3).Values followed by the same letter are not significantly different at P < 0.05 according to Tukey’s multiple range test.The Ck treatment served as a non-Cd contaminated control without GO;T1,T2,T3,T4,T5 and T6 represent0,25,50,100,250 and 500 mgL−1 GOtreatment,respectively,with 100 mg kg−1 Cd
Compared with CK,the available Cd content in Cd-polluted soil increased significantly (P< 0.05;Online Appendix Fig.S5).An initial decrease was followed by an increase after different concentrations of GO treatments were applied,and different concentrations of GO resulted in significant differences (P< 0.05;Online Appendix Fig.S5),whichindicated that GO had a certain effect on Cd adsorption in Haplic Cambisols.
Correlations with soil environmental factors
Pearson correlations (Table 3) and redundancy analysis (RDA,Fig.7) were used to examine the relationships between the soil bacterial community and soil chemical properties and environmental factors as well as the relationships among various environmental factors.There was a certain correlation between the top 10 most-abundant bacteria phyla and the soil chemical properties.Positive correlations were observed between soil pH and Proteobacteria(P< 0.05),while negative correlations occurred between soil pH and Acidobacteria (P< 0.05).Notable positive correlations (P< 0.01) were found between the AK content and Acidobacteria,whereas negative correlations occurred between the OM content and Chloroflexi (P< 0.05) and also between the available Cd content and Nitrospirae and Latescibacteria (Table 3).
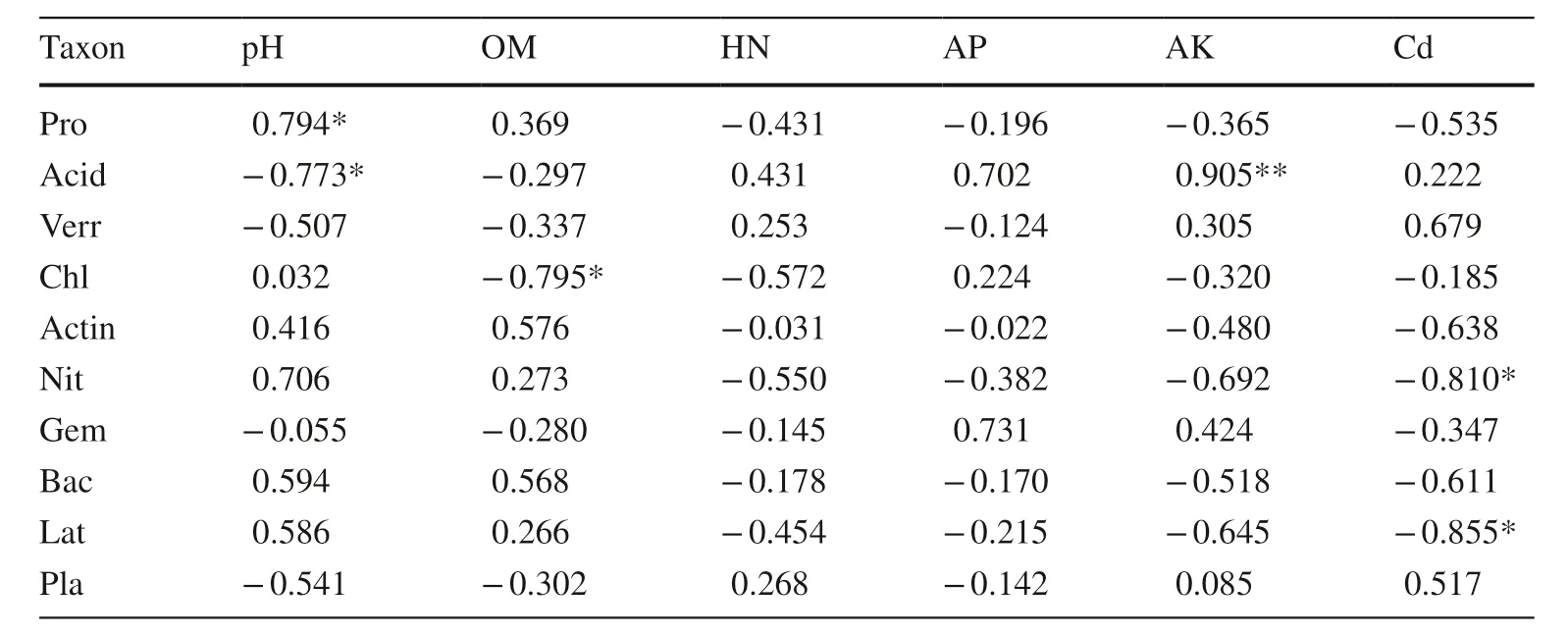
Table 3 Pearson correlations between the soil bacterial community for the top 10 most-abundant bacteria at the phylum level and environmental variables (n =3)
Based on the RDA results (Fig.7),as expected,many of the environmental variables were strongly correlated with each other.Positive correlations and negative correlations occurred between some parameters.For example,soil pH and the OM content were positively correlated,as were the HN,AK,AP and available Cd contents.Positive correlations also occurred between AK content and AP and available Cd contents and between available Cd and AP contents.However,negative correlations occurred between soil pH and HN,AK,AP and available Cd contents,and between OM and HN,AK,AP and available Cd contents.There were some similarities among the top 10 most-abundant bacterial phyla.Nitrospirae,Latescibacteria,Bacteroidetes,Actinobacteria and Proteobacteria were close to each other,so the bacterial community structure among them was similar,and Planctomycetes and Verrucomicrobia also had similar community structures (Fig.7).
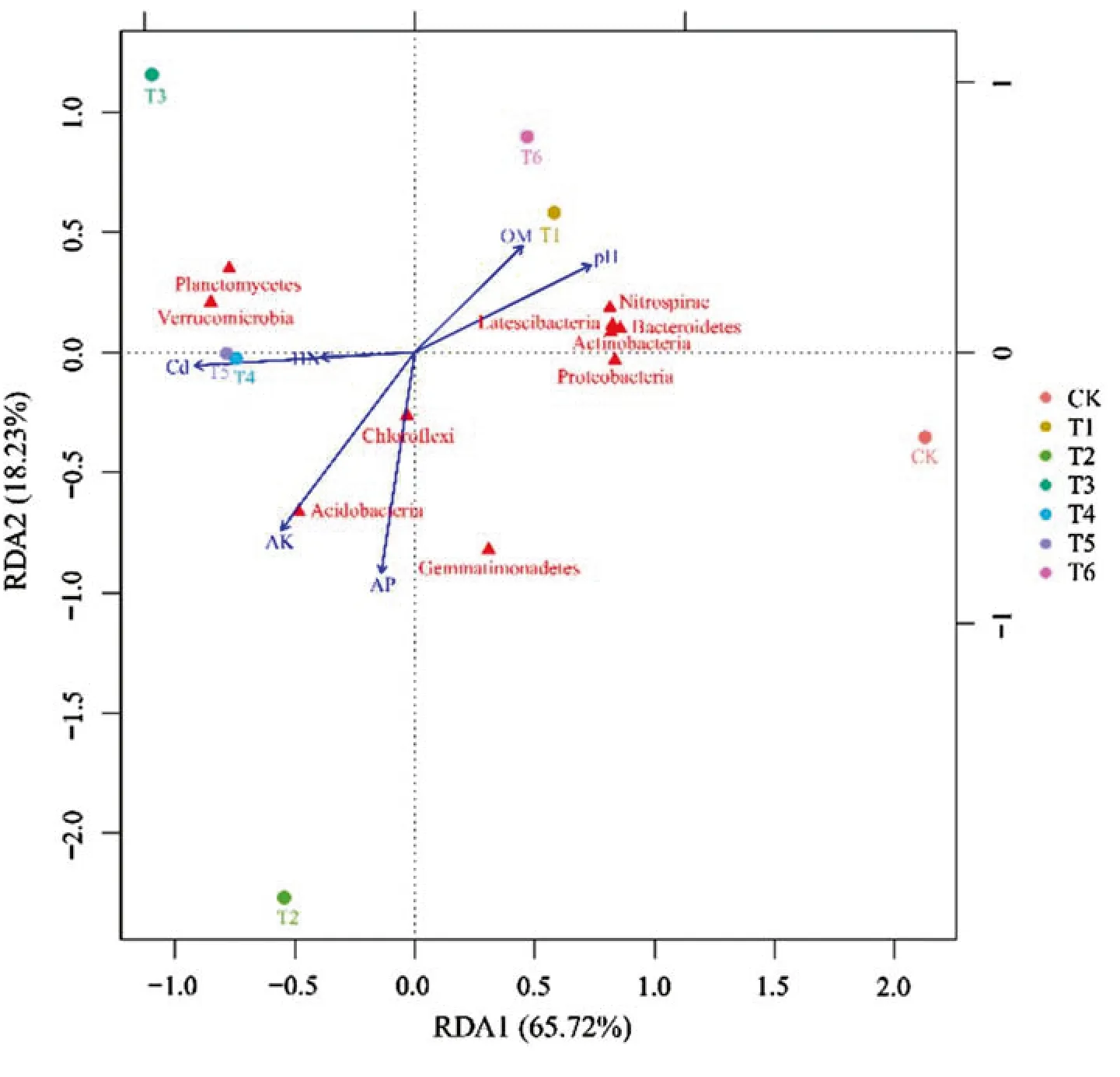
Fig.7 Redundancy analysis(RDA) was used to explore the relationships between the soil bacterial community and environmental variables.The arrows indicate the lengths and angles between explanatory and response variables and reflect their correlations.Soil samples from different concentrations of GO treatments are marked with different colors.OM,organic matter;HN,hydrolytic nitrogen;AP,available phosphorus;AK,available potassium;Cd:available cadmium.CK:0 mg L−1 GO without Cd;T1:0 mg L−1 GO+Cd;T2:25 mg L−1 GO+Cd;T3:50 mg L−1 GO+Cd;T4:100 mg L−1 GO+Cd;T5:250 mg L−1 GO+Cd;T6:500 mg L−1 GO+Cd
Discussion
At 100 mg kg−1Cd,the Simpson and Shannon indexes for diversity of bacteria increased in Haplic Cambisols(Table 1),indicating that Cd addition could promote the growth of some bacteria,which is consistent with Wang et al.(2003) and Jiang et al.(2018),who found that under 100 mg kg−1of Cd treatment Shannon index of yellow cinnamon soils in whichXanthoceras sorbifoliumgrew much taller than in the 10 mg kg−1Cd treatment,but partly agrees with Guo et al.(2010),who found that heavy metals severely decreased the diversity of microbial community,but the number of dominant populations rose.As for why 100 mg kg−1Cd increased the diversity of bacteria in our study,high concentrations of heavy metals might reduce the dominance of dominant species in soil,thus eliminating their competitive inhibition of other species,and thus increase bacterial diversity (Ipsilantis and Coyne 2007).
After incubation of plants in Cd-polluted soils with different concentrations of GO,the bacterial community structure,diversity,richness,and relative abundance of their main bacterial phyla all changed.GO generally increased bacterial richness and diversity indexes(Table 1),but the available Cd contents decreased (Online Appendix Fig.S5),suggesting that GO has a strong capacity to absorb Cd and promote the growth and proliferation of some bacteria,similar to the findings of Zhou et al.(2019).The effects of GO on microbes are closely related to the GO concentrations.The diversity and richness increased,decreased,increased,and then decreased with an increase in the GO concentration,reaching the maximum in T2,whereas the lowest diversity and richness were observed in the T6 treatment (Table 1).It may well be that GO treatments with different concentrations changed soil chemical properties by influencing microorganisms,thus altering microbial selection (Duan et al.2020).Our results also suggested that although GO could adsorb Cd,when GO reached a certain concentration,it inhibited bacterial growth,and the effect on bacterial abundance and diversity was limited,consistent with the results of Li et al.(2016).
Our NMDS and cluster analyses results revealed potential changes in the bacterial community structure in response to the Cd-and GO-treated Haplic Cambisols.The similarity between CK and T1 was low,whereas that between T1 and T6 was higher,as was that between T2,T3,T4 and T5.In particular,the T4 and T5 treatments had the most similar bacterial community structure (Figs.2,3,4),indicating that Cd significantly influenced soil bacteria,and could change the structure of bacterial communities,but soil bacteria seemed to be resistant to GO addition after GO concentration reached a certain level,and the soil bacterial community changed negligibly as more GO was applied (Figs.2,3,4).
Proteobacteria are the most tolerant of heavy metals(Gillan et al.2005),and under 100 mg kg−1of Cd treatment,relative abundance of this phyla was highest (Fig.4).Nonetheless,along with Proteobacteria,Acidobacteria,Verrucomicrobia,Chloroflexi,and Actinobacteria were also dominant bacteria in Cd-polluted Haplic Cambisols (Fig.4),which is consistent with the results of Guo et al.(2010).As typical acidophilic bacteria,members of Acidobacteria are negatively correlated with soil pH,and the richness of this phylum rises with soil pH (Duan et al.2020).In our study,100 mg kg−1Cd decreased soil pH (Fig.A3),so the relative abundance of Acidobacteria increased.In Cd-polluted Haplic Cambisols with different GO additions,Proteobacteria,Verrucomicrobia,Acidobacteria,Actinobacteria and Chloroflexi were also variously dominant (Fig.A3),consistent with those in Haplic Cambisols with different graphene treatments (Song et al.2018).Buckley and Schmidt(2003) and Tang et al.(2015) found that Proteobacteria,Acidobacteria,Actinobacteria,Bacteroidetes,and Planctomycetes were the dominant phyla in agricultural soils.In this study,three of these were among our five dominant phyla,which indicated similarities and differences in soil microbes between Cd-polluted Haplic Cambisols and agricultural soils.Furthermore,at the phylum and genus levels,the abundances of the groups in the samples differed significantly among the GO treatments (Table 2).The effects of the GO treatments on the soil properties and on the relative abundance of the various groups of bacteria in Cd-polluted Haplic Cambisols in turn likely further alter the bacterial community composition.
Changes in the soil microbial biomass and metabolic function from the effects of exotic substances,such as GO,also affects the stability of soil ecosystems (Doran and Zeiss 2000;Fan et al.2017;Yan et al.2017).Chung et al.(2015)found that soil microbial biomass showed little change in response to GO treatment up to 1 mg GO g−1soil.But in our study,GO treatments significantly affected the metabolic function of bacteria in Haplic Cambisols polluted by Cd,and the change in the major bacterial flora was closely related to the GO concentration (Fig.5).In our study,Cd significantly inhibited the soil bacterial metabolic function,but metabolic function in the T2 treatment was significantly improved,indicating that this concentration of GO alleviated the inhibition of Cd on the metabolic function through GO adsorption of Cd.In the T3 treatment,the metabolic function significantly decreased,whereas the metabolic function improved in T4 and T5 and basically stagnated in T6(Fig.5).Note that hormesis occurred in some of the assessed characteristics/traits,especially under low concentrations of GO (e.g.,Table 1,Figs.3,4 and 5),which suggested that when the concentration of GO is low,microbial growth is stimulated,but as the concentration increases,effect becomes inhibitory,likely due to changes in soil properties as mentioned earlier.
Soil bacterial diversity is closely related to soil chemical properties,and the response of bacteria to soil environmental factors varies greatly (Lauber et al.2008;Chu et al.2010;Griffiths and Philippot 2013).The relative abundance of Acidobacteria in soil was negatively related to soil pH(Table 3),similar to other studies.Acidobacteria are acidophilic bacteria that play an important role in ecosystems;these bacteria can grow in soils at very low pH,optimally when the pH is less than 3.Therefore,acidic soil,such as a Haplic Cambisol,is beneficial for the physiological activities of these bacteria.The relative abundance of Acidobacteria was positively correlated with the AK content (Table 3),similar to the results of Huang et al.(2018).The abundance of Proteobacteria was positively correlated with soil pH(Table 3),indicating that Proteobacteria abundance was affected by pH,also consistent with the results of Huang et al.(2018).Shen et al.(2018) found that Cd was negatively correlated with the abundance of Nitrospirae,as in this study,showing that Cd-polluted soil is not conducive to Nitrospirae survival.For the first time,our empirical study provides experimental evidence that reveals the relationships between GO application and bacterial communities and chemical properties of Haplic Cambisols polluted by Cd in Northeast China.For soils polluted by other heavy metals in China and elsewhere,our results can provide guidance for the environmental assessment of GO.
Conclusions
GO influenced the richness and diversity indexes of the bacterial community in Cd-polluted Haplic Cambisols,and the effects varied with GO concentration.Only 25 and 250 mg L−1GO significantly increased the species and quantity of bacteria.Lower concentrations (i.e.,50 mg L−1) of GO significantly increased the beta diversity of bacterial community.The relative abundances of Proteobacteria and Acidobacteria in Cd-polluted Haplic Cambisols treated with GO were the highest.
Soil chemical properties were also closely related to the bacterial community diversity in the Cd-polluted Haplic Cambisols.GO may affect bacterial diversity by changing soil pH,organic matter,hydrolytic nitrogen,available potassium,phosphorus and Cd contents.GO at different concentrations may adsorb Cd to different degrees.These environmental factors are likely to affect the bacterial community assembly in Cd-contaminated Haplic Cambisols.Our results can provide theoretical support for ecological assessment of GO on the soil environment of Northeast China.
杂志排行
Journal of Forestry Research的其它文章
- Flexible transparent wood enabled by epoxy resin and ethylene glycol diglycidyl ether
- Diversity and surge in abundance of native parasitoid communities prior to the onset of Torymus sinensis on the Asian chestnut gall wasp (Dryocosmus kuriphilus) in Slovenia,Croatia and Hungary
- Ozone disrupts the communication between plants and insects in urban and suburban areas:an updated insight on plant volatiles
- Testing visible ozone injury within aLight Exposed Sampling Site as aproxy for ozone risk assessment for European forests
- Logging and topographic effects on tree community structure and habitat associations in a tropical upland evergreen forest,Ghana
- Spatial pattern dynamics among co-dominant populations in early secondary forests in Southwest China
