Factors involved in the success of Castanea henryi stem cuttings in different cutting mediums and cutting selection periods
2021-07-15WangzunChenLibingHeShiyiTianJosephMasabniHuanXiongFengZouDeyiYuan
Wangzun Chen·Libing He·Shiyi Tian·Joseph Masabni·Huan Xiong·Feng Zou·Deyi Yuan
Abstract Chinquapin (Castanea henryi) is a dual-purpose tree species in China valued for as a source of timber and starch.We investigated the effect of four cutting mediums(pure vermiculite;peat:river sand at 3:1 v/v;peat:krasnozem at 1:1 v/v;and pure krasnozem) and three stem cutting periods (March,May,and July) on rooting performance of C.henryi cuttings.Different cutting periods and cutting mediums greatly influenced the rooting rate of C.henryi,ranging from 3.35 to 77.31%.Principal component analysis indicated that the best combination of cutting period and cutting medium was semi-hardwood cuttings (May cuttings)+krasnozem.Histological evidence indicated that adventitious root initials were present by week 5–6,and that the site of root primordia initiation was observed in the vascular cambium.Stem anatomical structures observed at different periods indicated that a xylem/radius ratio of 29.90–37.42% and a fractured phloem fiber ring are indicative of rooting success.The relational model between rooting index and medium properties indicated that nutrient content and porosity significantly influenced callus production.However,pH strongly affected C.henryi root formation,with the Pearson correlation coefficients for May and July cuttings of −0.856 and −0.947,respectively.Our protocol is helpful to achieve mass clone propagation of improved C.henryi genotypes,thus overcoming a common hurdle in chinquapin breeding programs.
Keywords Adventitious roots formation·Castanea henryi·Medium pH·Phloem fiber ring·Xylem
Introduction
Chinquapin (Castanea henryi(Skan) Rehder &E.H.Wilson) is an economically and ecologically important species of theCastaneagenus,and is widely distributed in southern China,especially in the Fujian,Zhejiang,and Hunan provinces of China (Fan et al.2015).C.henryiis a multipurpose tree species,the wood is known for its high hardness and density,and the nut is popular for its nutritious and unique flavor (Wu et al.2008;Xiong et al.2018).In addition,C.henryiforests play an important role in preventing soil erosion and greening of barren hills (Zou et al.2019).
Like other Chinese chestnut species,C.henryihas resistance to ink and canker diseases (Corredoira et al.2017;Jeffers et al.2012).However,manyC.henryitrees have been infested in recent years by pathogenic bacteria and pests,which seems to imply a lack of stability of the disease resistance mechanism inC.henryi(Zhang et al.2018;Gabriele et al.2019).The introduction of chestnut blight in the early twentieth century eventually caused the functional extinction of American chestnut trees (Westbrook et al.2019).In Europe,several recent disease epidemics of native tree species have caused a widespread decline of European chestnut(Rigling and Prospero 2018).Therefore,severalC.henryibreeding programs have been launched in China to incorporate resistance.In these breeding programs,clonal propagation methods are necessary and have been used to multiplyC.henryiindividuals (Lopez-Villamor et al.2017).
Clonal propagation involves the use of asexual propagation techniques,such as tissue culture,grafting,and cutting propagation to generate new plants.Tissue culture has not been successful withC.henryi.Xiong et al.(2018) was able to achieve a high induction percentage of adventitious shoots (85.67%) and greater rooting (76.70%) with tissue culture inC.henryi.However,the explants were too young to be used in traditional breeding programs (Perkins et al.2019).Additionally,tissue culture requires advanced training of workers to master tissue culture propagation techniques.Grafting,on the other hand,has been widely used in propagation ofC.henryias the standard clonal propagation technique.However,this technology still has its shortcomings.Scion/rootstock compatibility (Fallik et al.2019;Wen et al.2011),labor-intensive grafting techniques (Lowe and Beineke 1969),and weather (Wei et al.2019) can influence grafting success and survival.Propagation via cuttings,therefore,would be the ideal alternative to grafting and tissue culture to increase numbers of desired clones.In principle,propagation via cuttings is more practical and cheaper(Corredoira et al.2017).However,chestnut species are difficult to propagate via the cutting technique (Tetsumura and Yamashita 2004),and little information is available specifically forC.henryi.
Cutting medium and selection period (henceforth called cutting period) are examples of internal and external factors that influence success of cuttings (Mukhtar 2019).Monder et al.(2019) recorded the rooting index at four phenological stages ofRosa beggerianacuttings and reported that cutting selection at 7–14 days after petal drop was ideal for rooting success.In addition,Aghdaei et al.(2019) reported that the best medium for pepino cuttings was peat and sand.However,little research has been done on the influence of cutting period and cutting medium onC.henryi.This study aimed to improve success ofC.henryicutting propagation by testing various cutting mediums and cutting period to:(1)determine the best combination of cutting medium and cutting period suitable forC.henryipropagation;(2) document the histological origin of adventitious root formation in three cutting periods and develop a spatially explicit timeline of adventitious root formation;and (3) better understand factors influencing adventitious root initiation and development through anatomical observations and statistical analyses.
Materials and methods
Plant material
Seeds of the chinquapin cultivar ‘Youzhen’ were harvested in mid-September 2016 from an orchard at Central South University of Forestry and Technology (lat.28°09′ N,long.112°59′ E).Mature seeds were dehusked,cleaned,and stratified for 120 d in moist sand at 5 °C in the dark.Nuts were then germinated and grown in seedling trays(33 cm × 34 cm) in peat:perlite:vermiculite potting mix(2:1:1,v/v/v).Seedling plants were watered as needed and fertilized with organic fertilizer during the growing season.In winter 2018,plants were heavily pruned in order to get more branching and thus more shoots for use in our research.
Cutting medium
Four mediums were used in this study,including pure vermiculite,peat:river sand at 3:1 v/v,peat:krasnozem at 1:1 v/v,and pure krasnozem.Vermiculite was purchased from the Wan Yun Co.Ltd.,China,while peat was purchased from the Klasmann-Deilmann Co.,Germany.Krasnozem was collected in Changsha City,Hunan Province,China,from the topsoil of a local forest.All mediums were sterilized at 121 °C for 30 min to eliminate the potential of harmful pathogenic microorganisms onC.henryicuttings.
Experimental design
Experiments were conducted using the following parameters to determine their effects on the rooting rate ofC.henryiseedlings:(1) Selection of 2-year old seedlings as test plants,all of similar height.All test plants were marked after shoot selection to avoid repeated sampling from the same plant;(2) All experiments were performed in a phytotron with air temperature and humidity maintained at 25 °C and 70%,respectively;(3) Comparing the rooting ability of cuttings collected on 15 March,15 May,and 15 July in 2019;and(4) Comparing the rooting ability in four different mediums.Additionally,our research intended to determine:(1)The rooting process ofC.henryishoots through anatomical analysis;(2) The consistency of rooting ability as affected by different cutting mediums,using medium characteristic analysis;and (3) The main factors influencing rooting and the best treatment ofC.henryicutting,using Pearson correlative analysis and principal component analysis (PCA)(He et al.2019).
Rooting of cutting
Rooting of cuttings was performed as follows,except when otherwise noted.The experiments were conducted from mid-March to mid-July.Shoots were collected in the morning and immediately cut into 12-cm-long pieces.Test plants were marked after sampling to avoid repeated sampling from the same plant.Leaves close to the base of a cutting were removed,and the remaining upper leaves were cut in half,to reduce risk of wilting.Cuttings were treated by dipping for 10 min in a 0.5% carbendazim solution to prevent infection of pathogenic fungi.Then the base of the cuttings was dipped in a solution containing 2 g L−1indole-3-butyric acid (IBA) (Sigma) for 3–5 min(Lopez-Villamor et al.2017).Cuttings were then inserted vertically into the prepared treatment substrate to a depth of 8 cm into the medium.Then a plastic bag was placed over the pot and tied with a rubber band (Fig.1).Finally,pots were moved into a phytotron room,where air temperature was maintained at 25 °C and photoperiod at 16 h(06:00–22:00).White LED T5 lights (Shanghai,China)with a photosynthetic photon flux density (PPFD) of 200 μmol m−2s−1were used in this work.Each treatment consisted of 30 cuttings and three replications.When quantifying the rooting of plants,cuttings with at least one adventitious root were considered as successfully rooted.

Fig.1 Propagation of stem cuttings of Castanea henryi in a phytotron room
Parameters used in determining rooting ability
Using the procedure of Gomes et al.(2018),we recorded the rooting parameters at the 49th and 56th day after initial setup,including rooting,mortality,callus formation,root number,and root length as the average of three largest roots.
Anatomical analysis
Firstly,the samples were fixed in FAA (70% alcohol:formalin:acetic acid at 18:1:1,v/v/v) for 24 h,and then macerated in the mixed solution of hydrogen peroxide and glacial acetic acid (1:1,v/v) for 48 h to soften the materials,after which samples were dehydrated in a graded ethanol series,and embedded in paraffin wax with a 58–60 °C melting point.The samples were sectioned into 10 μm sections using a Leica RM 2265 microtome (Wetzlar,Germany).Lastly,samples were stained with 1% Safranin O and Fast Green (Xiong et al.2019).Histological sections were observed using a light Leica DMi8 microscope(Wetzlar,Germany),and Leica Application Suite (LAS)software (Leica,Germany).
Medium characteristic analysis
Physical propertiesBulk density (BD),total porosity (TPS),aeration porosity (APS) and water-holding porosity (WHP) of the four mediums were determined by the ring knife method (Chen et al.2020).
Chemical properties
An extract solution was prepared by adding 100 mL of distilled water to a 10 g sample (1:10 ratio of sample to water on a weight by volume basis),and mixing thoroughly for 30 min on a shaker.The solution was then filtered through filter paper.The pH and electrical conductivity (EC) were measured using a PHS-3E pH meter (Shanghai,China)and DDS-307A EC meter (Shanghai,China),respectively(Chen et al.2020).
Nutrient properties
Mineral nitrogen (N) was extracted with 2 M KCl and followed by colorimetric analysis of NH4+-N and NO3−-N,which were expressed per g of dry weight of sample.According to Maluf et al.(2018) and Zou et al.(2019),the contents of water-soluble potassium (K),water-soluble calcium (Ca),water-soluble sodium (Na),water-soluble magnesium (Mg),and water-soluble iron (Fe) were determined using an atomic absorption spectrophotometer model TAS-990 (Beijing,China),while other metals were determined using inductively coupled plasma mass spectrometry (ICP-MS) model ICAP Q (USA).In addition,the content of water-soluble P was determined using the Automated Discrete Analyzers Model Smart Chem 200(Italy) (Zou et al.2019).
Statistical analysis
Pearson correlative analysis (He et al.2019) was used to test for significant effects of rooting indexes and medium characteristics.Principal component analysis was used to evaluate the scores and rankings of each treatment (Wang et al.2019).Mean comparisons were performed using the least significant difference (LSD) test.For anatomical data analysis,a one-way analysis of variance (ANOVA) followed by Duncan’s test,at α=0.05,were performed using Leica Application Suite (LAS) software (Leica,Germany).
Results and discussion
Rooting indexes in different treatments
Cuttings that were collected in March rotted within the first 2 weeks of initiating the trial.This was caused by a high decay rate of cuttings (data not shown).Both the cutting period and cutting medium greatly influenced the rooting ofC.henryi(Fig.2 and Table 1).May cuttings showed a high rooting rate,and cuttings grown in krasnozem showed the highest rooting rate of 77.31% and an adventitious root number of 8.22.In July,only the cuttings inserted in vermiculite had not yet rooted.

Table 1 Effect of medium and cutting period on rooting potential of Castanea henryi cuttings

Table 2 Variance and variance cumulative contribution rate of each composition

Table 3 Component matrix
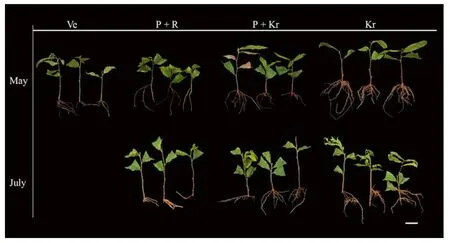
Fig.2 Representative images of rooted Castanea henryi cuttings in different rooting media and cutting period.Ve vermiculite,P+R peat+river sand,P+Kr peat+krasnozem,Kr krasnozem.Scale bar is 4 cm
Principal components analysis synthesis estimate model
After all data were standardized with SPSS for their Z-score,principal component analysis was carried out for all the five indicators processed.The variance and cumulative contribution rate of variance of each component are presented in Table 2,and the component matrix in Table 3.SPSS extracted two principal components whose eigenvalues were greater than 1.00,and the cumulative contribution rate of their variance was 87.1% (Tables 2,3).
According to Tables 2 and 3,the formulas of scoring calculation could be developed as follows (Wang et al.2019):

whereC1represents the score of principal component 1,C2represents the score of principal component 2,Crepresents the comprehensive score;X1throughX5represent the average rooting rate,average rooting quantity,average root length,callus rate,and decay rate,respectively.λ1andλ2respectively represent the variance of principal component 1 and principal component 2,as shown in Table 2,withλ1=64.75 andλ2=22.37.
SubstitutingX1throughX5of formulas (1) and (2) with the five index values of each treatment will calculate the score and ranking of each treatment (Table 4).The rooting index of cuttings collected in May was higher than that of July,suggesting that the cutting selection period was more important than the medium used.This result indicated that the combination of May cuttings and pure krasnozem had the highest comprehensive score.
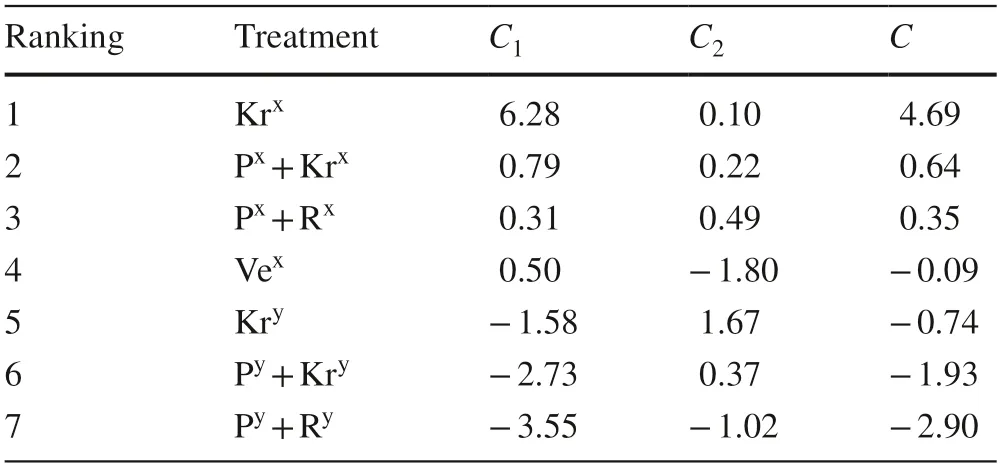
Table 4 Principal component scores and comprehensive scores of each treatment
Cuttage is a simple way to obtain seedlings of elite genotypes in a short time.AlthoughCastaneaspecies are known for their difficulty to propagate from cuttings (Tetsumura and Yamashita 2004),a higher rooting rate (77.31%) inC.henryiwas achieved in this study.Results indicated that even after the traditional two-year breeding period (Perkins et al.2019),propagation ofC.henryiis feasible.However,various cutting periods and cutting mediums greatly influence the rooting ofC.henryicuttings.
Anatomical analysis of cuttings in different periods before rooting
The anatomical structure from the three cutting periods before rooting are shown in Fig.3.The thickness of epidermis,cortex,phloem fiber,phloem,xylem,pith radius,and pith ray of the three cutting periods are shown in Fig.4.The stem anatomy of March,May,and July cuttings was similar in terms of cell type and tissue organization,but significantly different in their thickness (Table A1).Stem segments ofC.henryiexperienced an obvious thickening of pith radius and xylem cells (Fig.4) between March and July,while the width of pith ray cells decreased significantly.The width of phloem,cortex,and epidermis varied among the three cutting periods (Fig.4),but were not statistically different (Table A1).The ratio of xylem radius to stem radius increased continuously between March and July,while the ratios of pith,phloem,cortex,epidermis,and pith ray width to stem radius had the opposite trend (Fig.5).Finally,the ratio of phloem fiber radius to stem radius decreased initially and then increased (Fig.5).

Fig.3 Anatomy of shoot base of Castanea henryi in three cutting periods before rooting.a–c Whole transverse sections of shoot bases of C.henryi in three cutting periods (a March,b May,c July);d,f,h partial transverse sections in three cutting period (d March,fMay,h July);e,g,i close-up of phloem fibers in three cutting periods (e March,g May,i July);white arrowheads show gaps in phloem fibers ring.Ep epidermis,Co cortex,PF phloem fiber,Vc vascular cambium,Ph phloem,Xy xylem,Pi pith,PiR pith ray.Scale bar of a–c 200 μm and d‒ i 100 μm

Fig.4 The different tissue thickness of shoot base of C.henryi in three cutting periods before rooting
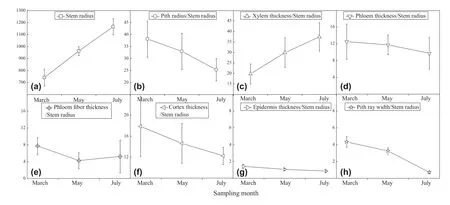
Fig.5 Stem radius thickness (a) and ratio of anatomy structure thickness to stem radius thickness of shoot base of C.henryi in three cutting periods before rooting (b–h)
Although prior research had documented the importance of stem anatomy structure to rooting ability in woody species,few studies addressed the relationship betweenC.henryistem anatomy and rooting ability.The anatomical observations collected in this study indicated that the growth ofC.henryistems may lead to a decline of their rooting ability.When the stem of aC.henryiis growing,a decrease in width of pith and pith ray would be unfavorable in regulating water and nutrient absorption during rooting,and an increase in lignification would definitely lead to a reduction of rooting progenitor cells plasticity (Zhang et al.2010).
Anatomical observations on rooting of Castanea henryi cuttings
The stem anatomy of May and July cuttings was similar in terms of tissue organization and cell type (Figs.3,4).Observations in the first three weeks after May and July cuttings showed no detectable anatomical changes (data not shown).On the 21st day,both cutting periods displayed a callus formation in the lenticels and at the base of the cut,which was differentiated from the cortex cells (Figs.6 a,b;7 a,b).On the 35th day,a significant cell proliferation occurred in the phloem region of May cuttings,and root primordia were visible in the vascular cambium (Fig.6 c,d).The root primordia in July cuttings were evident a week later than May cuttings,with a similar site of root primordia initiation in the vascular cambium (Fig.7 c,d).After that,the root primordium that grew outward forced its way between fiber strands,crushing the phloem fibers ring and reaching the outer cortex,followed by a progressive differentiation of the vascular system.

Fig.6 Anatomy of shoot base of Castanea henryi collected in May.a,b Callus differentiation from the cell of cortex on the 21st day of growth and its close-up;c,d root primordia on the 35th day of growth and its close-up;e,femergence of an adventitious root on the 49th day of growth and its close-up.Ep Epidermis,Co Cortex,PF phloem fiber,Vc vascular cambium,Ph phloem,Xy Xylem,Ca Callus,RP root primordia,AR adventitious root.Scale bar is 200 μm

Fig.7 Anatomy of shoot base of Castanea henryi collected in July.a,b Callus differentiation from the cell of cortex on the 21st day and its close-up;c,d root primordia on the 42nd day of growth and its close-up;e,femergence of adventitious root on the 56th day of growth and its close-up.Ep epidermis,Co cortex,PF phloem fiber,Ph phloem,Vc vascular cambium,Xy Xylem,Ca callus,RP root primordia,AR adventitious root.Scale bar is 200 μm
The origin of adventitious roots in stem cuttings has been reported to be in various tissues and varies among species (Naija et al.2008;Agullo-Anton et al.2014).In woody plants,cells in the vascular cambium have an enormous potential for the formation of root primordia (Legué et al.2014).Anatomical analyses elucidated the origin of adventitious root development of cuttings in our study.Our histological evidence also showed that the origin-point ofC.henryiadventitious roots can be traced back to the vascular cambium in May and July cuttings (Figs.6 c,d;7 c,d).
This is the first reporting of the origin of adventitious roots inC.henryi,although similar results have been reported in other difficult-to-root species such as feijoa (Acca sellowiana) (Zhang et al.2010) and olive (Olea europea)(Denaxa et al.2019).In addition,there was no callus rooting observed in this study.
Xylem and phloem fibers ring are important for rooting of Castanea henryi cuttings
Xylem is important in rooting of cuttings.In our work,almost all March cuttings died before the base of the cuttings produced a callus.The narrow xylem (Fig.5 c) might be the cause of the highest death rate of these cuttings.The highly lignified cells in the xylem might be the main barrier to protect the wound from decay before a callus was produced.Prior research has indicated that cell lignification could improve the cuttings tolerance against fungal pathogens (Eskandari and Sharifnabi 2020).Ride (1978)suggested that lignin may make plant cells more resistant to mechanical penetration and can increase its resistance to dissolution by fungal enzymes.InC.henryi,roots originated in the vascular cambium,and the increase in lignification would definitely lead to a reduction in plasticity of cambium progenitor cells (Zhang et al.2010).This also may be the major factor for the lower rooting rate ofC.henryicuttings collected in July.From these observations,although a wider xylem would decrease the rooting rate forC.henryicutting,we still suggest usingC.henryistem cuttings with a 29.90–37.42% xylem ratio (Fig.5 c).
Several anatomical studies suggested a correlation between rooting difficulty and the presence of a ring of phloem fibers (Avidan and Lavee 1978;Amissah et al.2008;Porfirio et al.2016).Figure 3 a–i show the changes of phloem fibers ring inC.henryistems from March to July.In March,the phloem fibers are manifested as a continuous and thick ring structure (Fig.3 a,d,e).In May cuttings,the division and differentiation of vascular cambium cells and a concurrent increase in stem diameter resulted in compressed and thinner phloem fibers (Fig.3 b,f,g).Some gaps have been observed in phloem fiber rings in May cuttings which could be due to the growth of vascular bundle and especially in the xylem and phloem (Fig.3 b,f,g).In July cuttings,when the cell volumes increased and cell walls between the two phloem fibers thickened,the ring structure tended to be integral again (Fig.3 c,h,i).
A phloem fiber ring could inhibit adventitious root formation in different ways.In a study on the anatomical structure of feijoa cuttings,Zhang et al.(2010) reported that the phloem fibers ring acts as a mechanical barrier during rooting and thereby inhibits adventitious root formation.Stevens and Pijut (2017) indicated that the cross section of mature black walnut stems revealed a continuous ring of phloem fiber cells,thereby eliminating potential sites for the origin of adventitious roots present in juvenile cuttings.In a recent study,Denaxa et al.(2019) observed that the presence of a phloem fibers ring acted as a barrier to exogenously applied auxin from reaching the sites of root primordia induction and differentiation.In this work,the higher rooting rates in May cuttings could be partially explained as a breakage in the phloem fibers ring,which was only observed in thisperiod.Anatomical observations suggested that the phloem fibers ring does not act as a mechanical barrier for rooting because it was easily penetrated by the growing primordium(Figs.6 c,d;7 c,d).Unlike black walnut,C.henryiadventitious roots do not originate from gaps between phloem fiber bundles but from the region of the vascular cambium(Figs.6 c,d;7 c,d).Therefore,we speculate that the impediment of auxin lateral movement may be the major factor for the inhibition of adventitious root formation inC.henryicuttings.
Physio-chemical characteristics and water-soluble nutrient content of the different cutting mediums
Table 5 lists differences in water-soluble nutrient content of the different substrates.Peat had the highest content of NO3−-N (137.24 mg kg−1),NH4+-N (12.60 mg kg−1),P (12.95 mg kg−1),K (165.83 mg kg−1),and Ca(115.26 mg kg−1),while krasnozem had the lowest content in NH4+-N (2.03 mg kg−1) and Ca (24.75 mg kg−1).Vermiculite had the lowest content in NO3−-N (2.24 mg kg−1) and K (5.50 mg kg−1).Water-soluble P was not detected in krasnozem and vermiculite.In terms of micronutrients,watersoluble Cu and Mn were not detected in all four substrates.Peat had the highest content of Na (18.83 mg kg−1),Mg(12.13 mg kg−1),Zn (1.98 mg kg−1) and Fe (1.67 mg kg−1),while krasnozem and vermiculite had a lower content of micronutrients.
Differences in physical and chemical properties between different matrices are presented in Table 5.Krasnozem had the lowest pH (4.62),total porosity (45.81),water holding porosity (31.25) and aeration porosity (14.56).Peat had the highest EC (304.16 mS cm−1) and water holding porosity(48.12) while vermiculite had the highest pH (6.18).
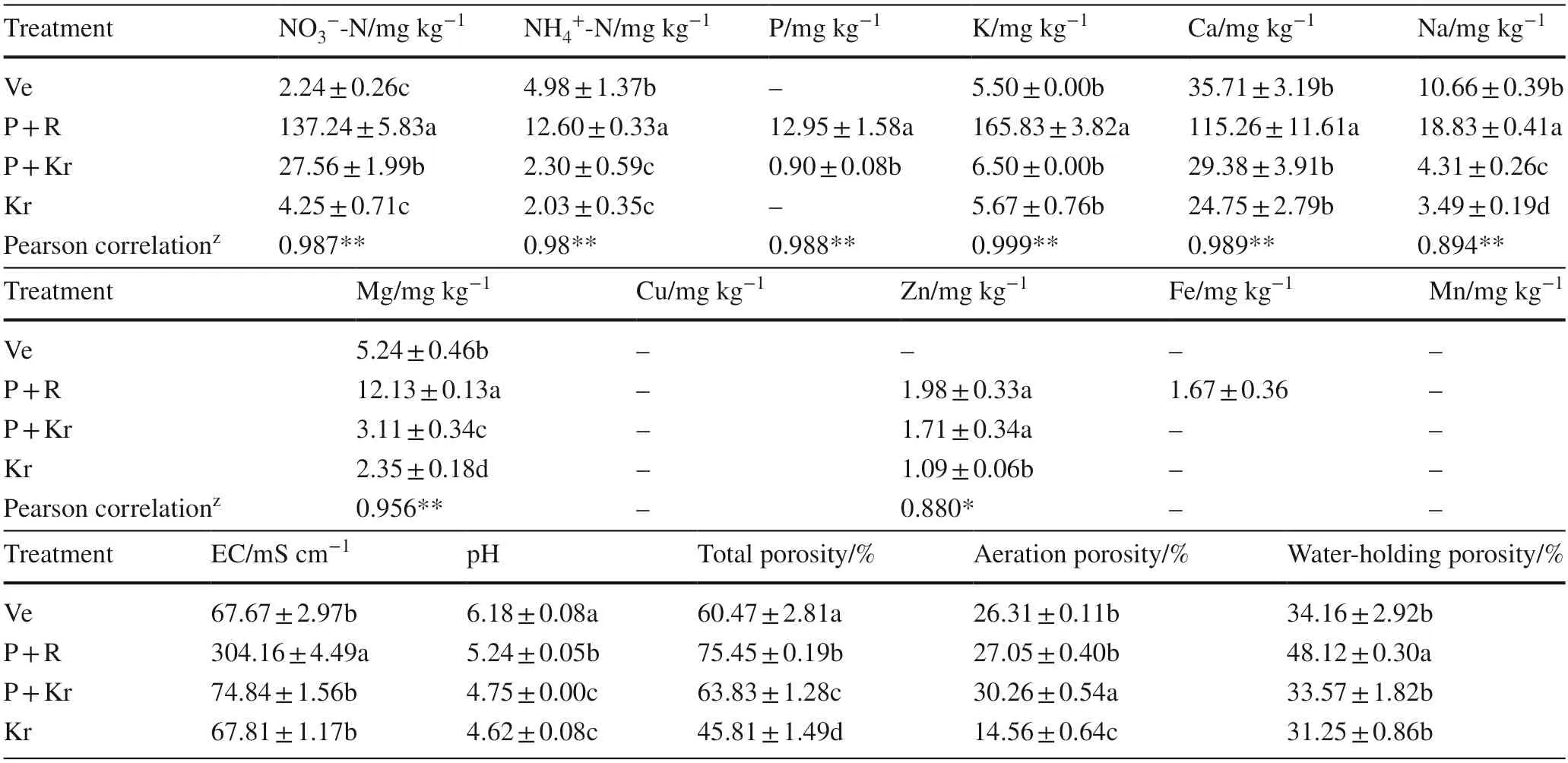
Table 5 Water-soluble nutrient content and physio-chemical characters of rooting media
In general,peat had a high content of organic matter,which is conducive to the formation of a porousenvironment.Previous research has indicated that a proper ventilation around the roots in a culture medium containing peat moss is effective in the induction and growth of roots in cuttings (Aghdaei et al.2019).Therefore,peat is widely used as the cutting medium in woody plants.In many developing countries,vermiculite has become one of the most popular cutting medium due to its low cost and high porosity.Although all mediums were acidic,krasnozem had the lowest pH value.In China,the geographical distribution is similar for krasnozem andC.henryiplanting area (Xiong et al.2018),so krasnozem is readily available during the idealC.henryicutting period.Therefore,krasnozem has a certain advantage for use as a medium for cutting propagation ofC.henryi.
Effects of medium properties on root indexes base on Pearson’s correlation coefficient analysis
The Pearson correlations among different rooting indexes and physio-chemical characters of cutting medium are presented in Fig.8.Due to the high correlation between water-soluble nutrients and EC values (Table 5),we used EC value in correlation analysis instead of the water-soluble nutrients.When comparing the Pearson matrix of two rooting months (May,July),eight strong correlations between rooting index and medium characteristics were found in both rooting months (Fig.9).
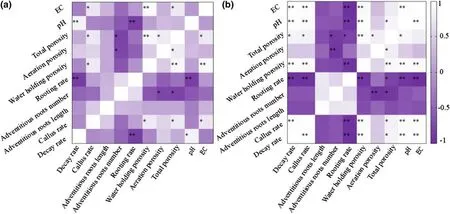
Fig.8 Pearson’s correlations between different rooting indexes and physical characters of cutting medium.a Cutting rooting in May;b cutting rooting in July.* p < 0.05;and ** p < 0.01
Callus formation at the base of the cuttings is a natural reaction of stems to wounding caused by cutting,both in woody (Strzelecka 2007;Trofimuk et al.2020) and herbaceous plants (Wróblewska 2013).In the present study,a higher water-soluble nutrient content and WHP significantly increased the callus formation rate inC.henryicuttings (p< 0.05) (Fig.9).However,callus rooting was not observed in this research,therefore the production of callus did not seem to have a positive effect on the rooting ofC.henryicuttings.Some studies indicated that a high level of cytokinin and a low level of auxin in callus would lead to a continuous formation of callus and to a large consumption of nutrients and energy,thus inhibiting the formation of adventitious roots (Du et al.2019;Druege et al.2016).Therefore,callus formation may inhibit the production of adventitious roots inC.henryicuttings.

Fig.9 The relational model between rooting index and medium characteristics,based upon the Pearson matrix of two rooting months.Black numbers on each line between boxes are the Pearson correlation coefficients in May;blue numbers are the Pearson correlation coefficients in July.* p < 0.05;and ** p < 0.01
A significantly negative correlation (p< 0.01) between medium pH and the rooting rate ofC.henryicuttings taken in May and July indicated that a low pH seems to be the key factor for rooting ofC.henryi.Similar results were found in prior research.Kumar et al.(2011) considered that a low pH may improve the internal cellular environment of target cells leading to initiation and growth of adventitious roots inGmelina arboreaRoxb.cuttings.Lee et al.(1976) also reported that northern California black walnut cuttings could be 100% rooted with an acid pre-treatment prior to application of IBA.Decreasing the pH can reduce the restriction of lateral movement of auxin across cross sections of cuttings,because of the lower ionization percentage of auxin in an acid environment (Kumar et al.2011).Additionally,the acidification of cell wall may promote the production of adventitious roots to some extent (Alexander et al.2017).In this work,a lower pH of cutting medium significantly increased the rooting rate ofC.henryicuttings.
Conclusion
In this report,studying how the cutting period and cutting medium affect the rooting indexes ofC.henryi,we demonstrated that:(1) the best combination of cutting period and cutting medium was May and krasnozem;(2)the adventitious root initials ofC.henryicuttings appeared by weeks 5–6,and the sites of initiation of root primordia were identified in the vascular cambium;and (3) the rooting rate ofC.henryicuttings were closely related to the cuttings lignification degree,medium pH value,and the structural change of phloem fiber ring.Future studies are encouraged to decipher the molecular mechanism ofC.henryiadventitious roots formation,which is therefore essential for the improvement of existing propagation protocols.
杂志排行
Journal of Forestry Research的其它文章
- Flexible transparent wood enabled by epoxy resin and ethylene glycol diglycidyl ether
- Diversity and surge in abundance of native parasitoid communities prior to the onset of Torymus sinensis on the Asian chestnut gall wasp (Dryocosmus kuriphilus) in Slovenia,Croatia and Hungary
- Ozone disrupts the communication between plants and insects in urban and suburban areas:an updated insight on plant volatiles
- Testing visible ozone injury within aLight Exposed Sampling Site as aproxy for ozone risk assessment for European forests
- Logging and topographic effects on tree community structure and habitat associations in a tropical upland evergreen forest,Ghana
- Spatial pattern dynamics among co-dominant populations in early secondary forests in Southwest China
