Modeling the dynamics of aspruce forest and dwarf mistletoe population:a coupled system
2021-07-15DianguangXiongZhouyuanLiYingmeiLiangChengmingTian
Dianguang Xiong·Zhouyuan Li·Yingmei Liang·Chengming Tian
Abstract The parasitic plant dwarf mistletoe (Arceuthobium) is currently one of the most threatening infestations of coniferous forests worldwide,especially in Eurasia and North America,but its population dynamics in relation to one of its hosts (spruce) remain unclear.Here,toward understanding the population dynamics,differential equations were used to construct a life history model for the two populations,and two relatively independent subsystems,host and parasite,were generated from their symbiotic relationships.A suspected-infection model was used to couple them.The resulting models were used to analyze structural changes in the forest.When each infected spruce was assumed to support 1000 parasite shoots,the spruce population first increased rapidly,then slows.When 2000 parasite shoots were assumed,the forest declined dramatically,slipping to zero in the 10th year,and the spruce seedlings were unable to regenerate.Parasite shoot population curves transformed from exponential J-shapes to logistic S-shapes,reaching population limitations as germination rates changed.These results provide important clues to understanding developmental trends of the present parasite population and will assist in reconstructing invasion histories.
Keywords Dwarf mistletoe·Spruce·Population dynamic model
Introduction
The parasitic dwarf mistletoe (DM;Arceuthobium;Santalaceae),is a threat to coniferous forests worldwide,especially those in Eurasia and North America (Hawksworth and Wiens 1996;Watson 2001;Mathiasen et al.2008).It was first detected in the United States more than a century ago and had been well documented (Hawksworth and Wiens 1996;Norton and Carpenter 1998;Geils and Hawksworth 2002).Recently in northwestern China,it has evolved into one of the most serious threats to spruce forests (Ma et al.2007;Zhou et al.2007;Xia et al.2012).Importantly,it was first found onPinus tabulaeformis,an important afforestation tree species in China,in Qinghai Province (Ma et al.2019).This hemi-parasitic,seed-producing plant can photosynthesize,but it directly diverts water and nutrients from its host.Its life cycle consists of colonization,a latent period,growth,and then reproduction,and its seeds are dispersed mainly by explosive discharge (Geils and Hawksworth 2002;Hawksworth and Wiens 1996).DM infection of its host causes the formation of witches’ brooms,decreased vigor and lowered growth potential.The trees then become susceptible to bark beetle infestations,fungal infections,and other debilitating conditions.Infected host trees rapidly succumb (Brandt 2006;Robinson and Geils 2006;González-Elizondo et al.2018).
DM damage to coniferous forests has been studied using statistical models to plot DM development (Robinson and Punter 2001;Godfree et al.2002b;Robinson and Geils 2006).These models mainly focused on the parasite,rarely on host–parasite interaction synergies;thus,the interactions are not well understood (Godfree et al.2002a;Shaw et al.2005),and additional simulation models are needed.
Parasitic plants also play important roles in the evolution of local vegetation (Watson 2001;Press and Phoenix 2005;Shen et al.2006).Several models are presented to predict population growth of a parasitic plant,such as that used forRhinanthusspecies,explain the effects on host population development (Hautier et al.2010) and reveal a coupled host–parasite relationship.Although a number of studies described the DM life cycle,a systematic and holistic integration is needed to provide a targeted framework for host–parasite monitoring and assessment and a critical focus for model-guided fieldwork (Restif et al.2012).A general model to identify and quantify the severity of disease is needed to integrate current data and guide future surveys.The population dynamics of the host and parasite was modeled here using system dynamics (SD) methods to link a DM epidemic to the population dynamic of spruce (Picea crassifolia) forests.The model established a fundamental framework for the further investigation of the DM life cycle,which will help to understand DM,the hosts,and population development and characteristics along with the long-term disease cycles present in DM-infected forests.
Materials and methods
A high-order differential equation was used to establish a SD model which is commonly used for modeling population dynamics and environmental complex systems,primarily based on system theory principles and differential equations(Doyle and Ford 1998).Different scenarios were tested to evaluate possible control measures and the consequences(Xiu and Hesthaven 2005).The simulation software Vensim(Ventana Systems,Version 8.0,Harvard,MA,USA) was used to constructed and evaluate SD models for the different scenarios.Microsoft Excel (Redmond,WA,USA) and SPSS 18 (IBM,Armonk,NY,USA) further analyzed the data (Eberlein and Peterson 1992).Several different initial values and parameters were selected for the models which referenced common cases obtained from prior studies or from related field investigation results (Robinson and Punter 2001;Robinson and Geils 2006).Host parameters differed depending on the scale of the research.All were transfered into a spatial range of approximately 100 ha.A host tree number was obtained in prior fieldwork in Gansu Province,and the calculated spruce forest population density was used(Zhao et al.2010).
Assumptions
For simplicity,the two populations were defined as independent subsystems:subsystem H,the host coniferous forest;subsystem P,the parasite DM.Natural processes were simplified and discretized by locating the two populations at different stages.The two subsystems were connected,or linked,by constructing a relationship between their respective stock and flow variables.The number of DM seeds was assumed to determine the infection rate of host trees.The number of successfully germinated DM shoots on branches depended on the number of infected trees.Two parameters,one from the DM and the other from the host were established:a2,the infection ratio (number of infected trees/1000 DM seeds);a5,host seedling capacity for DM seedlings (number of DM seedlings/tree).
Model structure
The model constructed two relatively independent subsystems which were then coupled.The process is shown in the flow diagrams of the H-P system (Fig.1) for a 200-month simulation period.
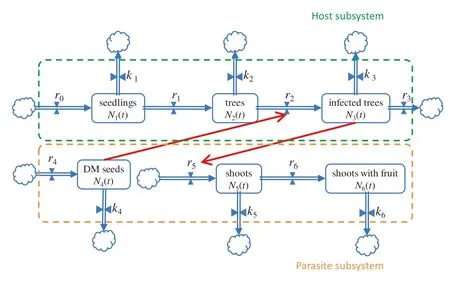
Fig.1 Diagram of host–parasite coupling system based on system dynamics methods.Details on parameters are given in the model structure section and Table 1
In the H subsystem,the flow diagram begins with seedlings,N1,which grow into healthy trees,N2,at a seedling growth rate,r1.These healthy trees then are available for DM seed infection at an infection rate,r2,and become infected trees,N3.Both healthy and infected trees produce a seed rain,which restores the forest at the seed rain rate,r0.Seedlings and healthy and infected trees have differing fractional mortalities that determine their respective death rates:k1,k 2,andk 3.Infected trees,once detected,have a removal rate,r3.
The P subsystem flow diagram starts with DM shoots,N5,which germinate on host tree branches at a DM shoot germination rate,r5.DM shoots grow and reproduce at a DM shoot growth rate,r6.They become shoots with fruits,N6,and contain DM seeds,N4.Their dispersal rate isr4.DM seeds and shoots have fractional death rates,k 4andk 5,respectively.
When H-P coupling is done,an infection rate,r2,in the H system is determined by the number of DM seeds,N4,in the P system.The number of infected trees per DM seed isa2.The germination rate,r5,of the P system depends on the number of infected trees,N3.The DM ratio of shoots to infected hosts isa5.
Variables and their symbols and parameters are presented in Table 1.The differential equation set used for modeling is also presented.

Table 1 Symbol list of the host-parasite coupling system

Results
H system structural changes and the setting of a series of values for the parameters shown in the graphs (Fig.2) were studied.For simulated years,a5=1000 anda2=1,no parasite impacts on host forests were obvious.Healthy trees(N2) increased to a peak before year 10 (t=120 months).After that peak,healthy trees (N 2) began decaying.About 10 months later,the number of infected trees (N3) peaked.During the forest’s life,the number of seedlings (N1)always increased.

Fig.2 Structural changes in the host subsystem under a series of values for the parameters.Parameter a 2 is the number of infected trees/1000 DM seeds,a 5 is the number of DM seedlings/tree
As expected,during the test period,healthy trees became infected.Parametersa5anda2were established to confirm and demonstrate the infection.There was a gradient of 250 fora5,and stock variable numbers changed gradually to another typical pattern.When host load-carrying capacity reached 1500 seedlings/tree or the infection ratio reached 4 trees/1000 DM seeds,indicated bya5≥ 1500 and a2≥ 4,no healthy trees remained.This event took place by the first quarter of year 13.At that moment,there were an inadequate number of healthy trees to regenerate trees at the rates needed to maintain forest.
Seedling numbers (N1) over time returned to an S-shaped logistic curve indicating that the forest had reached a limit.Infected trees (N3) reached a maximum point just beyond theN2switching point.The interval between those two points was 10–40 months in the middle of the test period.It also declined by the end of the period.A curve family for the number of DM shoots (N5) under parameter valuesa5ora2was prepared to illustrate P system behaviors (Fig.3).As thea5/a2ratio increased from 1000/1 to 2000/7,DM shoot growth curves transformed from exponential J-shape to logistical S-shape.The parasite population had reached its limit.No more host trees were available for colonization.

Fig.3 The curve family for the number of DM shoots in the parasite subsystem.Parameter a 2 is the number of infected trees/1000 DM seeds,a 5 is the number of DM seedlings/tree
Discussion
In the climax forest community,healthy spruce death rates were very low.Long-term DM infestation,however,weakens host communities and significantly depresses forest growth and regeneration because the parasite absorbs nutrients from the host (Filip 1992).DM also affects host abscisic acid and auxins levels,promoting rapid parasite growth and reproduction,which results in witches brooms (Hawksworth and Wiens 1996).These changes seriously affect host nutrient transport and greatly reduce host growth in height,diameter,and volume (Godfree et al.2002a).Many studies show that parasitic plants do not kill their host rapidly to maintain a balance with the host to assure parasite population survival(Dawson et al.1994;Maloney and Rizzo 2002;Parker and Mathiasen 2004).When faced with drought,environmental changes,and human disturbances,DM increases water,nitrogen and metabolite uptake from the host,leading tohost death (Hawksworth and Wiens 1996;Livingston et al.1999;Reblin et al.2006;Xia et al.2012).Although DM do not usually kill their host rapidly,the host is gradually weakened;bark beetle infest the debilitated conifer trees and increase the number of dead trees within a decade or two.
DM populations are determined by the host population(Hawksworth and Wiens 1996),and can reach extreme values after a long-term infection.Explosive discharge of DM seeds is the primary mode of short-range dispersal in a spruce forest.Some seeds successfully attach to host twigs,germinate,and penetrate the twigs (Hawksworth and Wiens 1996;Geils and Hawksworth 2002;Brandt 2006).After 7 years of colonization,latency,and growth within the host,DM new shoots will generate and produce fruit and seed.DM seed rain is great even though the percentage of successful germinations is quite small and is influenced by several factors,such as light,host,and seed health.Host infection rates are relatively low;thus DM infection and transmission are relative slow (Hawksworth and Wiens 1996;Brandt et al.2004,2005,2006;Robinson and Geils 2006).As described above,host load-carrying capacity determines DM populations.Lower canopies gradually wither,and parasitic plants drop offthe host as damage to the tree increases.The large number of seeds assures that DM can infect an entire forest eventually.If the number of hosts are relatively stable,the DM population growth curves are S-shaped.
Generally,parasite and host populations maintain a dynamic balance and mutually interact in the absence of addititional pressures (Price 1980;Mutikainen and Koskela 2002).The number of healthy hosts quickly declines when the infection rate is high and host community growth peaks and thus regeneration of the host population declines.Because DM growth and reproduction relies on the host,long-term DM infection leads to host canopy death,and the paraiste dies along with its host(Hawksworth and Wiens 1996;Sala et al.2001).Thus,the DM population determines the infection rate in the absence of other harmful factors such as significant human disturbances or other environmental changes.The higher the infection rate,the sooner DM population saturates the forest.When the parasite population peaks,the number of healthy host declines.At this point,almost all susceptible hosts will have been infected and begun to die,and no new trees can be infected.Host death leads to DM death and their mutual extinction.
The fitted models and their results can help recreate the history of the DM invasion and assist in managing the woodland and preventing DM infection,which will be especialy useful for seriously infected woodlands with have limited invasion events.It is necessary to clarify the current situation of host and parasite populations and how they develop so that suitable silvicultural measures can be taken to prevent and manage DM infestations.For example,simple culling or pruning in woodlands that are moderately damaged by DM may be useless.Inversely,after felling,or pruning,canopy transparency or forestry canopy density declines,and thus may lead to DM outbreaks (Shaw et al.2005;Mathiasen et al.2008).These models may also be used to identify and evaluate development stages of the parasite and predict DM disease progress over the long term.
Setting the parameters and testing the models using measurements of natural samples is necessary and should improve the practical utility of the methods used here (Hautier et al.2010).This model has yet to be validated with real-world regional values due to a lack of field data,especially longterm observations,and to delayed recognition of infections.The simple models using spruce forests and DM is in its initial stage of development and will have greater potential for recreating invasion history,diagnosing disease stage,and predicting population trends as more observational data are collected and statistically analyzed.Future work using the results in this study might be used to determine the relationships between DM ratings (DMR) (Hawksworth 1977)and the number of shoots/tree or infection rate.The models could also be applied more widely when combined with spatial analysis technology and LANDIS models to simulate dynamic forest succession under conditions of interference(Mladenoff2004).Because UAV-based infrared thermography has shown that the temperature of the host plant canopy,mistletoe,and mistletoe-infected host plants differ (Maes et al.2018),this technique might also be used to determine the DM infection in the forests.
Conclusion
This model separated symbiotic relationship states and used a suspected-infection model to couple the host and parasite subsystems by using population dynamics and differential equations.Using central key assumptions,the model found that for a host subsystem,the higher the infection rate,the earlier forest growth peaked.After their population peaked,healthy trees stopped reproducing at rates required for regeneration,and tree numbers began to decline.For the parasite subsystem,the higher the infection rate,the earlier parasite population reached its limits from the pressure from fewer available host.The results of this study provide the primary step in reconstructing invasion histories.However,our model needs to be verified with the actual field data,instead of using estimated values to depict the overall progress of infection and generational growth in this spruce-dwarf mistletoe system.
杂志排行
Journal of Forestry Research的其它文章
- Flexible transparent wood enabled by epoxy resin and ethylene glycol diglycidyl ether
- Diversity and surge in abundance of native parasitoid communities prior to the onset of Torymus sinensis on the Asian chestnut gall wasp (Dryocosmus kuriphilus) in Slovenia,Croatia and Hungary
- Ozone disrupts the communication between plants and insects in urban and suburban areas:an updated insight on plant volatiles
- Testing visible ozone injury within aLight Exposed Sampling Site as aproxy for ozone risk assessment for European forests
- Logging and topographic effects on tree community structure and habitat associations in a tropical upland evergreen forest,Ghana
- Spatial pattern dynamics among co-dominant populations in early secondary forests in Southwest China
