The Safety of Polysorbate 80 for Injection was Improved by Removing Macromolecular Substances
2021-07-01XIAHengDUANJinlianLIYueQIULingHANYilunCHENHuanLIYongkunDUANWeigang
XIA Heng,DUAN Jinlian,LI Yue,QIU Ling,HAN Yilun,CHEN Huan,LI Yongkun,DUAN Weigang
(Yunnan Provincial Key Laboratory of Molecular Biology for Sinomedicine,Yunnan University of Chinese Medicine,Kunming 650500,China)
ABSTRACT:Objective The major aim of the present study were to prove that macromolecular impurities in polysorbate 80 for injection are a risk factor,and determine the main physical indexes of polysorbate 80 free of macromolecular substances.Methods The original polysorbate 80 for injection,with a theoretical molecular weight of 1 310 Da,was treated with ultra-filters to obtain component A(>100 kDa),B(10~100 kDa),and C(<10 kDa).The components were lyophilized to obtain their contents.Each component was prepared with normal saline at a concentration of 10%,anaphylactic and anaphylactoid reactions on guinea pigs that may be caused by the original polysorbate 80 and its components were recorded according to Chinese Pharmacopoeia of 2015th edition.Kunming mice were intravenously injected with them for 5 days'administration and 5 days’recovery to observe their organic toxicity.Results The proportion of substances with a molecular weight above 10 kDa in the original polysorbate 80 was above 85%.The original polysorbate 80 and its component A and B resulted in positive reactions in both anaphylactic and anaphylactoid guinea pigs,while component C didn't.In Kunming mice,component A and B caused an obvious retard of body weight increase in the 5 days’recovery.The original polysorbate 80 and its components caused mild but significant toxicity in the liver,kidney,spleen,and lung at organ indexes both in 5 days administration and recovery.Their mild toxicity on the liver and kidney was verified by histological assay.However,toxicity caused by component C was much milder and more recoverable.The physicochemical constants of component C,including solubilization capacity,viscosity,density,absorption spectrum,pH,and particle size were improved.Conclusion All the results suggested that the main substances in polysorbate 80 for injection are macromolecular impurities that are an important risk factor to cause anaphylactoid reactions and organic toxicity,and macromolecular impurity removal improved its safety and physicochemical constants.
KEY WORDS:polysorbate 80 for injection;macromolecular impurities;anaphylactoid reactions;organic toxicity;critical micelle concentration;solubilization
1 INTRODUCTION
The injection is a necessary dosage form for drugs with low bioavailability or used on emergent occasions.Medicines administrated through subcutaneous tissue,muscular tissue,or vein usually act much faster than those via oral administration,though the way of injection is more dangerous than oral administration.Injections include the solution for injection,suspension for injection,and powder for injection that can be dispersed quickly by other solutions.However,the first choice of the dosage form is the solution for injection,if possible.Solubilizers were frequently applied in injections to increase the solubility of insoluble substances in water[1].Polysorbate 80,also named Tween 80,was one of the solubilizers most frequently used and believed to be a safe excipient[2].
Polysorbate 80,as a nonionic surfactant,was a popular emulsifier used world-wide in the food industry and cosmetics industry,and so was in the pharmaceutical industry.The excipient has been approved for injection by authorities in many countries,including China,the United States,and the United Kingdom.
Polysorbate 80 for injection is a popular excipient in the modern injection industry[3].Medicines like taxol injection[4](an antineoplastic agent)and amiodarone injection[5](an antiarrhythmic agent)contain this excipient.In China,polysorbate 80 for injection was abused in traditional Chinese medicine injections(TCMIs)[3],like Yuxingcao injection[6],and resulted in a series of nonspecific adverse reactions.Since the adverse reactions of the injections often happen more frequently and more seriously than those of oral drugs with similar formulae,the excipient added in the injections could be an extra risk factor[7-9].According to our previous study[10],if the symptoms caused by injection are different from those caused by an oral drug with the same formula,the symptoms could be anaphylactoid reactions,more likely caused by macromolecular impurities which can be kept out by the gastrointestinal tract.The macromolecular impurities may be derived from the raw materials,the contaminated substances,and the excipients[11].
Polysorbate 80 for injection is a viscous liquid,almost colorless or light yellow.Theoretically,1 mole of polysorbate 80 is synthesized from 1 mole of sorbitol,20 moles of ethylene oxide,and 1 mole of oleic acid[12].There are three steps to synthesize the excipient.The first step is to synthesize sorbitan from sorbitol by dehydration,the second is to obtain a series of polymers from sorbitan and ethylene oxide,and the third is the polymers esterified with oleic acid.According to the synthetic route,polysorbate 80 must be a mixture of polyoxyethylene sorbitan oleic acid ester,with a theoretical molecular weight of 1 310 Da.The remaining substrates and the substances caused by unwanted polymerization should be the impurities.According to their molecular weight,the impurities can be largely grouped into“micromolecular”impurities and macromolecular ones.The micromolecular impurities in the polysorbate 80 for injection have been strictly controlled by authorities.However,the macromolecular impurities,which could be a big contributor to the nonspecific adverse reactions of injections,were almost ignored by the modern pharmaceutical industry.
Are the macromolecular impurities in the polysorbate 80 for injection a risk factor for injections?The present study tried to find the answer and give suggestions.
2 MATERIALS AND METHODS
2.1 Materials
Polysorbate 80 for injection(Lot:20170501)was manufactured by Nanjing Well Pharmaceutical Co.,Ltd.(Nanjing,China),and was approved by the National Medical Products Administration of China.Total bilirubin (TBil)assay kits(Lot:20190611),alanine aminotransferase(ALT)assay kits(Lot:20190612),blood urea nitrogen(BUN)assay kits(Lot:20190617),and serum creatinine(Cr)assay kits(Lot:20190615)were produced by Nanjing Jiancheng Bioengineering Institute(Nanjing,China).Huperzine A(Lot:150313)was produced by Shanghai Yuanmu Biotech Co.,Ltd.(Shanghai,China).Anisole(Lot:H1520007)was by Shanghai Jingchun Biochemical Co.,Ltd.(Shanghai,China).α-Tocopherol(Lot:08/2022)was produced by Zhongshan Yuanyang Biotech Co.,Ltd.(Wuhan,China).Potassium bromide of spectral purity(Lot:20190328)was produced by Tianjin municipal Institute of Guangfu Fine Chemicals(Tianjin,China).
Guinea pigs weighing 250~350 g and Kunming mice weighing 18~22 g were provided by Kunming Medical University.Animals were housed at 22°C temperature,at 45%~55% humidity-controlled conditions,and under natural light.The mouse experiments were approved by the Committee on Experimental Animal Management and Use,Yunnan Provincial Key Laboratory of Molecular Biology for Sinomedicine,Yunnan University of Traditional Chinese Medicine;and the guinea pig experiments were approved by the Animal Care and Use Committee of Jiangsu Provincial Institute of Materia Medica,Nanjing Tech University,Nanjing China.
The multiple microplate reader of Infinite 200 Pro was manufactured by Tecan Group(Männedorf,Switzerland).The lyophilizer of FDU-1100 was manufactured by EYELA(Tokyo,Japan).Ultra-filter of 100 kDa(Lot:1806047VS)and 10 kDa(1808013VS)were manufactured by Sartorius AG(Hamburg,Germany).The capillary viscometer was manufactured by Shanghai Baoshan Qihang Glass Instrument Factory(Shanghai,China).An ultraviolet-visible spectrophotometer(UV-2450)was manufactured by Beijing Jingke Ruida Science&Technology,Ltd.(Beijing,China).A Fourier transform infrared spectrometer(IRTracer-10)was manufactured by Shimazu Co.,Ltd.(Hongkong,China).An automatic surface tension meter(BZY-2)was manufactured by Shanghai Hengping Instrument&Meter factory(Shanghai,China).A laser particle analyzer(90 Plus PAL)was manufactured by Brookhaven Instruments Co.,Ltd.(Hamburg,Germany).Ultrapure water was obtained from a Milli-Q water purification system(EMD Millipore Group,Darmstadt,Germany).Other instruments or reagents used in the present study,if not mentioned,were made in China.
2.2 Macromolecular Substance Preparation
The light yellow original polysorbate 80 for injection was diluted with ultra-purity water to obtain a 5% solution.The solution was gradually added to the upper bowl of the ultra-filter of 100 kDa.The ultrafilter was spun at 3 500 rpm and 4℃until a half volume was filtered through.Then,the solution passed through was collected as a component containing substances with molecular weight less than100 kDa,and the solution in the upper bowl was double diluted with ultra-purity water and spun again.When the solution in the upper bowl was diluted six times,the upper solution was collected as component A that contained substances with molecular weight more than 100 kDa.
The solution passed through the ultra-filter of 100 kDa mentioned above was gradually added to another upper bowl of the ultra-filter of 10 kDa.The ultra-filter was also spun at 3 500 rpm and 4℃.The solution passed through was collected as another component,component C,that contained substances with molecular weight less than 10 kDa,and the solution in the upper bowl was double diluted with ultra-purity water and spun again.When the solution in the upper bowl was diluted six times,the upper solution was collected as component B containing substances with molecular weight from 10 to 100 kDa.
The three components mentioned above were dried with the lyophilizer.Briefly,the solution was put in a plastic bottle of 250 mL,and frozen at-40℃overnight.Then,the frozen solution was put into the lyophilizer and lyophilized for more than 48 h.After that,the bottle was weighed every 3 h,and when the totalweightofthe bottle did notdecrease,the lyophilization was ended.The net weight of the thick liquid was calculated by subtracting the bottle weight from the total weight.
2.3 Anaphylactic reactions(allergy)and anaphylactoid responses detected in guinea pigs
The original polysorbate 80 for injection and its components were dissolved with normal saline for injection to a 10% solution.Every 6 guinea pigs,including 3 male and 3 female animals(without pregnancy)were randomly arranged in a group to detect anaphylactic or anaphylactoid reactions that may be caused by the original polysorbate 80 and its components.Animals administrated with normal saline for injection of the same volume were served as a negative control.The detection of the anaphylactic reaction was performed according to(Chinese Pharmacopoeia(CP)of the 2015 edition(Book 4)[13].Briefly,injection of 0.5 mL was intraperitoneally administrated every two days,and the injection of the same volume was intravenously administrated two weeks later to evoke the anaphylactic reaction.The symptoms appeared 30 min after the evocation was observed.The scores were recorded according to Table 1.If an animal appeared two symptoms of piloerection, tremble, frequent scratching nose,sneezing three times in a row,three coughs in a row,retching,cyanosis,and dyspnea,or appeared any symptom included the last five items listed in Table 1,the animal was regarded as a positive animal[10].
As for the detection of anaphylactoid reactions[10],animals were intravenously administrated with the injection of 0.5 ml,and the symptoms appeared in 30 min after injecting were observed.Scores were also recorded according to Table 1.

Table 1 Scores of symptoms
2.4 Organic toxicity caused by repetitive doses in Kunming mice
The original polysorbate 80 and its components were dissolved with normal saline for injection to a 10% solution.Every 30 mice,including 15 male and 15 female mice(without pregnancy)were randomly arranged into a group to detect organic toxicity caused by repetitive doses of the original polysorbate 80 and its components.Animals administrated with normal saline for injection of the same volume were served as the negative control.Mice were weighed every morning and injected 0.5 mL through their caudal vein for 5 days.On the sixth day,10 male and 10 female mice were anesthetized with urethane(1.0 g/kg),and their blood was drawn from their abdominal aorta without anticoagulation.Serum was obtained by spinning at 3 000 rpm and 4℃,to assay hepatic function and renal function.Their hearts,livers,spleens,lungs,and kidneys were harvested and weighed.The organ index was calculated by dividing the organ weight by its body weight.After weighing,their organs were immersed in a 4% formaldehyde solution for more than 48 h.Then,the fixed organs were routinely dehydrated with ethanol and embedded in paraffin.The sections of the organs were cut at 20 μm and stained with hematoxylin-eosin(HE)staining kits.The photos of the sections were recorded with a fluorescence microscope in a light mode.
As for the remaining mice,they were normally bred for another 5 recovery days.Then,they were anesthetized and followed the process mentioned above.
Total bilirubin (TBil)and alanine aminotransferase(ALT)in the serum were assayed with assay kits to evaluate the mouse’s hepatic function.Blood urea nitrogen(BUN)and serum creatinine(Cr)were assayed with assay kits to evaluate the mouse’s renal function.
2.5 Critical micelle concentration(CMC)determination of the original polysorbate 80 for injection and its components
The original polysorbate 80 for injection and its components were diluted to a solution of 100 mg/ml with pure water.Then,it was double diluted to obtain a series of concentrations.First,the CMC was determined with the iodine method[14].The solution of 2 mL was put in a tube with enough iodine powder and sealed with its lid.The tube was shaken on a shaker at 100 rpm for 4 h to make a saturated solution of iodine.Then,the tube was spun at 10 000 ×g and(20±1)℃for 5 min,and the supernatant was collected for absorbance assay.The solution of 200 μL was added in a well of 96-well UV-plate,and the absorbance of the supernatant at 352 nm was detected with the multiple microplate reader.If the absorbance was overflowed or higher than 3,the solution was double diluted with pure water and transferred to a new well,and its absorbance was assayed again.Iodine is almost insoluble in water,and a high absorbance at 352 nm means its high solubility.The absorbance of iodine was correlated with the logarithmic concentration of polysorbate 80.The curve was simulated by two linear equations with the help of GraphPad 5.0 software,and the logarithmic concentration at the cross-point of the two equations was obtained,the logarithmic value at which point was calculated to an arithmetic value that was regarded as the CMC.
CMC was also determined with surface tension[15].The surface tension of the solution was assayed with the automatic surface tension meter.CMC was also calculated with the same method mentioned above according to the surface tension affected by their series of solutions.
2.6 Solubilization capacity assay of the original polysorbate 80 and its components
The solutions of the original polysorbate 80 and its components were adjusted to a concentration of 9.76 μg/mL,lower than their CMC.The solution of 2 mL was put in a tube with enough huperzine A,anisole,or α-tocopherol powder and sealed with its lid.The tube was shaken on the shaker at 100 rpm for 4 h to make the saturation solution.Then,the tube was spun at 10 000×g and(20±1)℃ for 5 min.The supernatant for huperzine A and α-tocopherol and the subnatant for anisole were collected for absorbance assay.The solution of 200 μL was added in a well of 96-well UV-plate,and the absorbance at 312 nm for huperzine A,271 nm for anisole,and 286 nm for αtocopherol were detected with the multiple microplate reader.If the absorbance was overflowed or higher than 3,the solution was double diluted with pure water and transferred to a new well,and its absorbance was assayed again.The content of huperzine A,anisole,or α-tocopherol in the solution was calculated by their standard quantitative curves.
2.7 Viscosity,density,absorption spectrum,pH,and particle size assay of the original polysorbate 80 for injection and its components
According to the quality criterion documented in the CP of 2015 edition (Book 4)[16],viscosity,density,absorption spectrum,pH,and particle size of the micelle of polysorbate 80,and those in its components were assayed.
Viscosity assay.The viscometer and the solutions of original polysorbate 80 and its components were kept at(20±0.5)℃ or(25±0.5)℃ for more than 30 min.Then,the solution was infused in the bulb of the viscometer,the viscosity was calculated based on the time it flowed through the capillary column.
Density assay.Pure water was filled in a bottle with a tiny mouth at(20±0.5)℃.The net weight of the water in the bottle was weighed with a balance.Then,the bottle was dried and filled with solutions of the original polysorbate 80 or its components,and their net weight in the bottle was also obtained.Their density was calculated by dividing their net weight with the net weight of the water.
Absorption spectrum assay.The original polysorbate 80 and its components of 0.1 g were added into a volumetric flask of 25 mL,then dissolved with a mixed liquor(acetonitrile∶water=70∶30(v∶v))to 25 mL.The solution was filled in a quartz cell with an optical diameter of 1cm and scanned by the ultraviolet-visible spectrophotometer.
pH assay.The original polysorbate 80 and its components of 0.5 g was dissolved with pure water to 25 mL.The pH value of the solution was detected with a pH meter at(25±0.5)℃,which was calibrated by a standard solution(pH=7.00).
Particle size assay.The original polysorbate 80 and its components of 5 mg/mL were prepared.Their particle size was determined with the laser particle analyzer at 20℃.
2.8 Infrared spectroscopy assay of the original polysorbate 80 and its components
The original polysorbate 80 and its components were dissolved in anhydrous ethanol to obtain their 10% solution.The solution of 15 μL was piped and mixed with dried powder of potassium bromide.The mixed powder was kept at110℃ overnight and pressed into a fine piece.The piece was scanned by the infrared spectrometer from 650~4 000 cm-1,and the infrared spectrogram was recorded.
2.9 Statistics
Values were expressed as mean±standard error(SE)or mean±standard deviation(SD).As for data accorded with the normal distribution,one-way analysis of variance(ANOVA)was performed to compare means between groups.If there was a significance,post-hoc statistical tests(equal variance)or Tamhane’s tests(unequal variances)were used to compare the mean values between every two groups.As for data not accorded with the normal distribution,a non-parametric test of Mann Whitney test (two-tailed)was used to compare their median values between every two groups.Statistical significance was accepted at P<0.05.
The Prince thought some witchcraft8 must be at work, and he hastened away before the return of the shepherdess, who became that evening the butt9 of everybody s jests
3 RESULTS
3.1 Proportions of macromolecular substances in polysorbate 80 for injection
By treating with ultra-filters and lyophilizing,component A(>100 kDa,25.5 g,51.0%),component B(10~100 kDa,12.3 g,27.4%),and component C(<10 kDa,5.3 g,10.6%)were obtained from the original polysorbate 80 for injection of 50 g(Fig.1).To our surprise,more than 85% of substances in the polysorbate 80 were substances with molecular weight higher than 10 kDa,according to its chemical formula,which suggested that the major substances in the original polysorbate were macromolecular impurities!
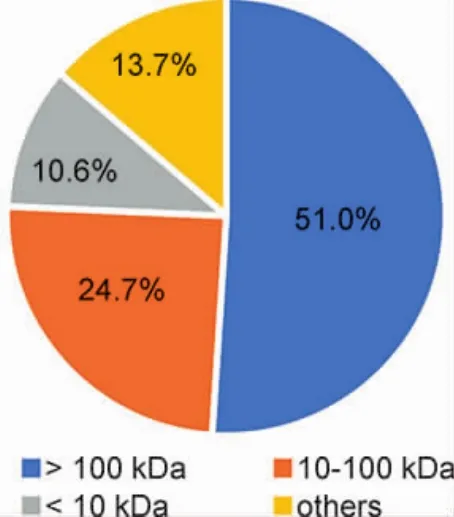
Figure 1 Components in the original polysorbate 80 for injection.Others were the substances lost during component preparation.Component A,>100 kDa,25.5g,51.0%;component B,10-100 kDa,12.3g,24.7%;component C,<10 kDa,5.3g,10.6%;others,substances lost during preparation,13.7%.
3.2 Anaphylactic reactions(allergy)and anaphylactoid responses detected in guinea pigs
According to the criterion listed in Table 1,the scores of positive symptoms were recorded and showed in Fig.2.The results showed that the original polysorbate 80 for injection and its component A and B,which contained a large number of macromolecular substances(>10 kDa),were able to result in positive reactions in both anaphylactic and anaphylactoid animal models and got higher symptom scores.However,its component C,only containing substances with molecular weight less than 10 kDa,was unable to do so,suggesting that the substances with higher molecular weight(>10 kDa)were a risk factor to cause anaphylactic or anaphylactoid reactions,and component C was much safer.
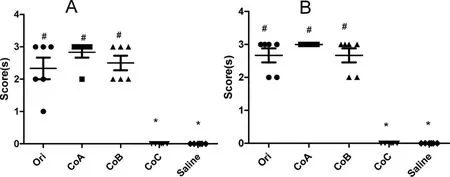
Figure 2 Anaphylactic reactions(A)and anaphylactoid reactions(B)detected in guinea pigs(n=6).Ori,the original polysorbate 80 for injection;CoA,Component A,> 100 kDa;CoB,component B,10-100 kDa;CoC,component C,<10 kDa;and Saline,normal saline for injection.*,P<0.05,vs original polysorbate 80;#,P<0.05,vs normal saline for injection;two-independent-samples test with Mann-Whitney U method(a nonparametric test).
3.3 Organic toxicity caused by repetitive doses in Kunming mice
All the animals survived at the end of the animal experiment.The animal’s body weight at the day before administration was set as“1”.During 5 days’administration,there was no significant difference in body weight between the groups(Fig.3).However,in the recovery period,body weight in components A and B groups increased more slowly than that in the other groups(Fig.3),suggesting that the macromolecular substances in polysorbate 80 are toxic since bodyweight is reliable evidence of toxic reaction.
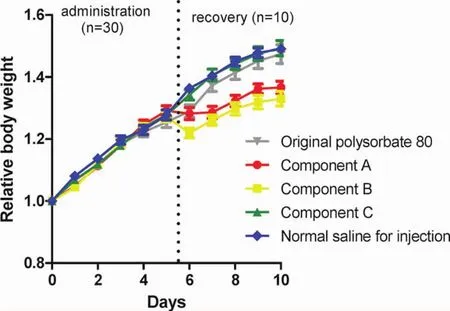
Figure 3 Relative body weight(mean±SE)in the original polysorbate 80 for injection and its component groups during 5 days’administration(n=30)and 5 days’recovery(n=10).
The mice’s organ indexes were calculated and showed in Fig.4.Mice’s liver index in original polysorbate 80 and its component groups significantly increased after 5 days’administration,suggesting that polysorbate 80 and its components revealed hepatic toxicity to some extent(Fig.4A).After 5 days’recovery,the toxicity could be recovered to some extent(Fig.4B).By contrast,the toxicity of the original polysorbate 80 and its component C was relatively milder.
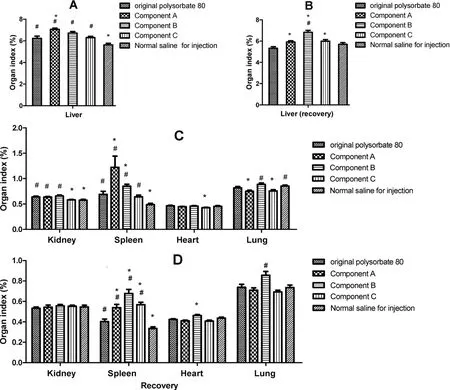
Figure 4 Organ index in groups treated by the original polysorbate 80 for injection and its components(mean±SE).Animals intravenously administrated with the original polysorbate 80 for injection and its components of 0.5 g for 5 days(A and C,n=20),and then were allowed 5 days for recovery(B and D,n=10).Normal saline for injection was served as negative control.Component A,> 100 kDa;component B,10-100 kDa;and component C,< 10 kDa.*,P<0.05,vs original polysorbate 80;#,P<0.05,vs normal saline for injection;one-way ANOVA.
After 5 days’administration,mice’s kidney index,spleen index,and lung index in the original polysorbate 80 and its component groups were also increased(Fig.4C).After 5 days’recovery,apart from the spleen index,their kidney and lung indexes recovered,similar to that in the normal saline for injection group(negative control)(Fig.4D).It seemed that the heart was the insensitive organ to the original polysorbate 80 and its components,and the toxicity on the spleen was more obvious(Fig.4C)and would maintain a longer time(Fig.4D).
The toxicity of the original polysorbate 80 and its components on the liver and kidney(Fig.5)after 5 days’administration was further verified by histological examination.Mild injuries in the liver and kidney caused by the original polysorbate 80 and its components were found,including swelling hepatocytes,interstitial edema,enlarged or collapsed renal capsule,and swelling renal tubules.After 5 days’recovery,the mild injury could be recovered to some extent(Fig.6).Injuries in other organic sections,including the heart and lung,were not obvious(data not shown).To our surprise,no obvious injuries in the spleen were found(Fig.7),though the organ index in polysorbate 80 and its components increased after 5 days’administration and 5 days’recovery.However,it can be found that the number of splenocytes and its volume increased in groups treated with polysorbate 80 and its components(Fig.7),suggesting that the function of the spleen was upregulated.
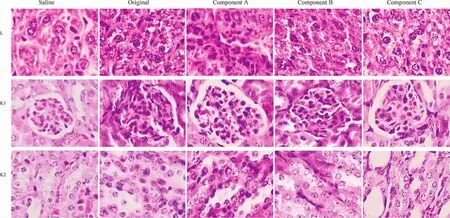
Figure 5 Histological sections of liver and kidney stained by HE after their hosts intravenously administrated with the original polysorbate 80 for injection or its components for 5 days.The row of L showed hepatic sections,the row of K1 showed the glomeruli,and the row of K2 showed the renal tubules.Saline was served as negative control.Saline,normal saline for injection;original,the original polysorbate 80 for injection;Component A,>100 kDa;component B,10-100 kDa;and component C,< 10 kDa.Picture=65 μm × 87 μm
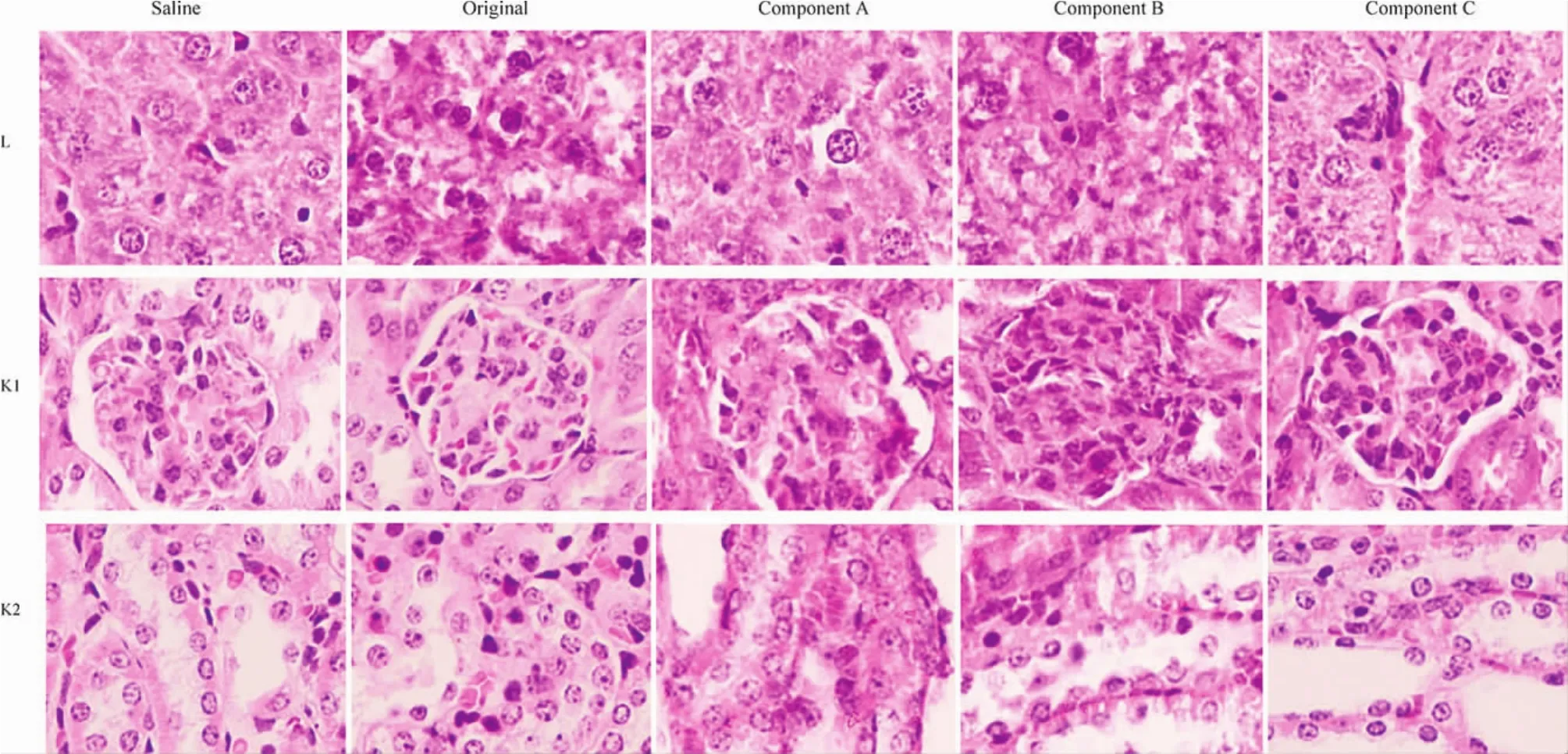
Figure 6 Histological sections of liver and kidney stained by HE after 5 days’administration of the original polysorbate 80 for injection or its components with 5 days’recovery.The row of L showed hepatic sections,the row of K1 showed the glomeruli,and the row of K2 showed the renal tubules.Saline was served as negative control.Saline,normal saline for injection;original,the original polysorbate 80 for injection;Component A,>100 kDa;component B,10-100 kDa;and component C,< 10 kDa.Picture=65 μm × 87 μm
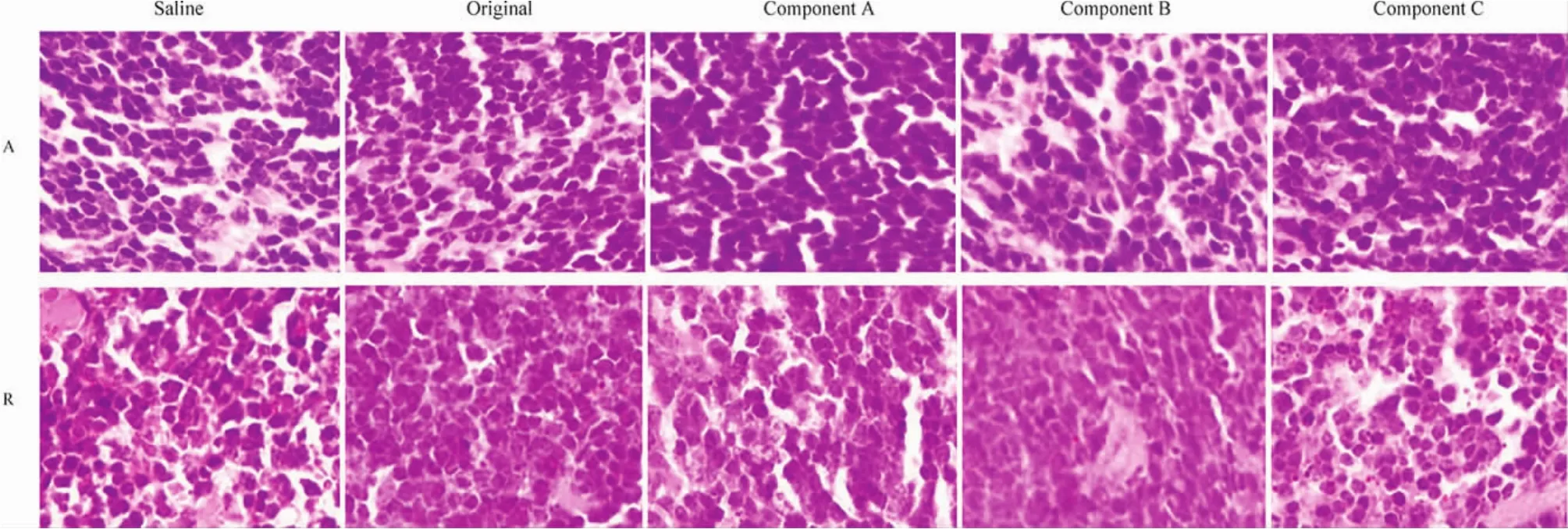
Figure 7 Histological sections of spleen stained by HE after 5 days’administration of the original polysorbate 80 for injection or its components(the row of A),and after 5 days’recovery(the row of R).Saline was served as negative control.Saline,normal saline for injection;original,the original polysorbate 80 for injection;Component A,>100 kDa;component B,10~100 kDa;and component C,< 10 kDa.Picture=65 μm × 87 μm
All the liver function indexes and renal function indexes assayed were in the normal ranges,though some significanceswere also found between the groups(Fig.8).
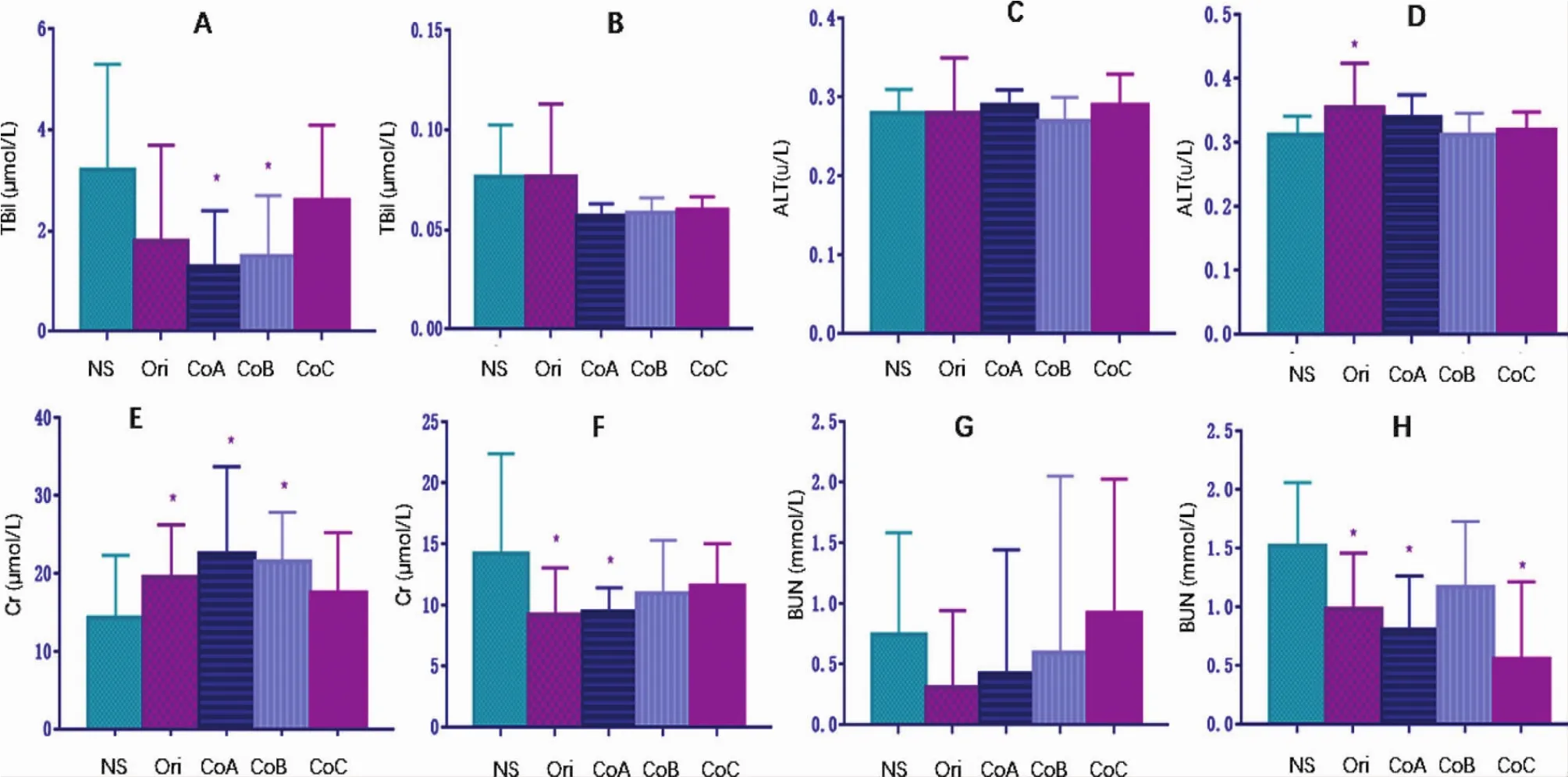
Figure 8 Mice’s liver function and renal function affected by the original polysorbate 80 for injection and its components(Mean±SD,n=20).Mice were intravenously administrated with the original polysorbate 80 for injection or its components of 0.5 g for 5 days and with another 5 days for recovery.Mice’s liver function was not affected by the administration of injections,whether evaluated by total bilirubin(TBil,A)or alanine aminotransferase(ALT,C)after 5 days’administration.So was after 5 days’recovery(B,D).Mice’s renal function was not affected by the administration of injections either,whether evaluated by serum creatinine(Cr,E)or blood urea nitrogen(BUN,G)after 5 days’injection,though there some significances between groups.So was after 5 days’recovery(F,H).Normal saline for injection(NS)was served as negative control.Ori,the original polysorbate 80 for injection;CoA,Component A,>100 kDa;CoB,component B,10-100 kDa;and CoC,component C,<10 kDa
3.4 CMC of the original polysorbate 80 for injection and its components
CMC of the original polysorbate 80 and its components were determined with the method of iodine absorbance at 352 nm(Fig.9A)and with the method of surface tension(Fig.9B).Their CMC were calculated in Table 2.The CMC of the original polysorbate 80 provided by the manufacturer is 12 mg/L,and the present results were near to the value.According to the results showed in Fig.9 and Table 2,component C had the biggest CMC,and component A and B had a smaller CMC than the original.The results suggested that the macromolecular substances were easier to form micelles than the micromolecular ones.
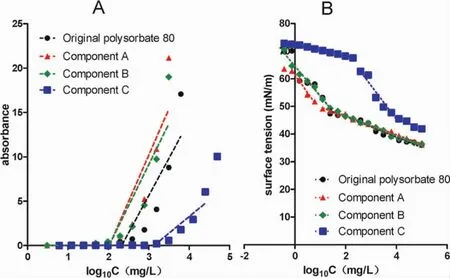
Figure 9 Critical micelle concentration(CMC)curve determination of the original polysorbate 80 and its components determined with iodine absorbance at 352 nm(A)or with their ability to decrease surface tension(B).Their CMC values were calculated in Table 2.Component A,>100 kDa;component B,10-100 kDa;and component C,<10 kDa
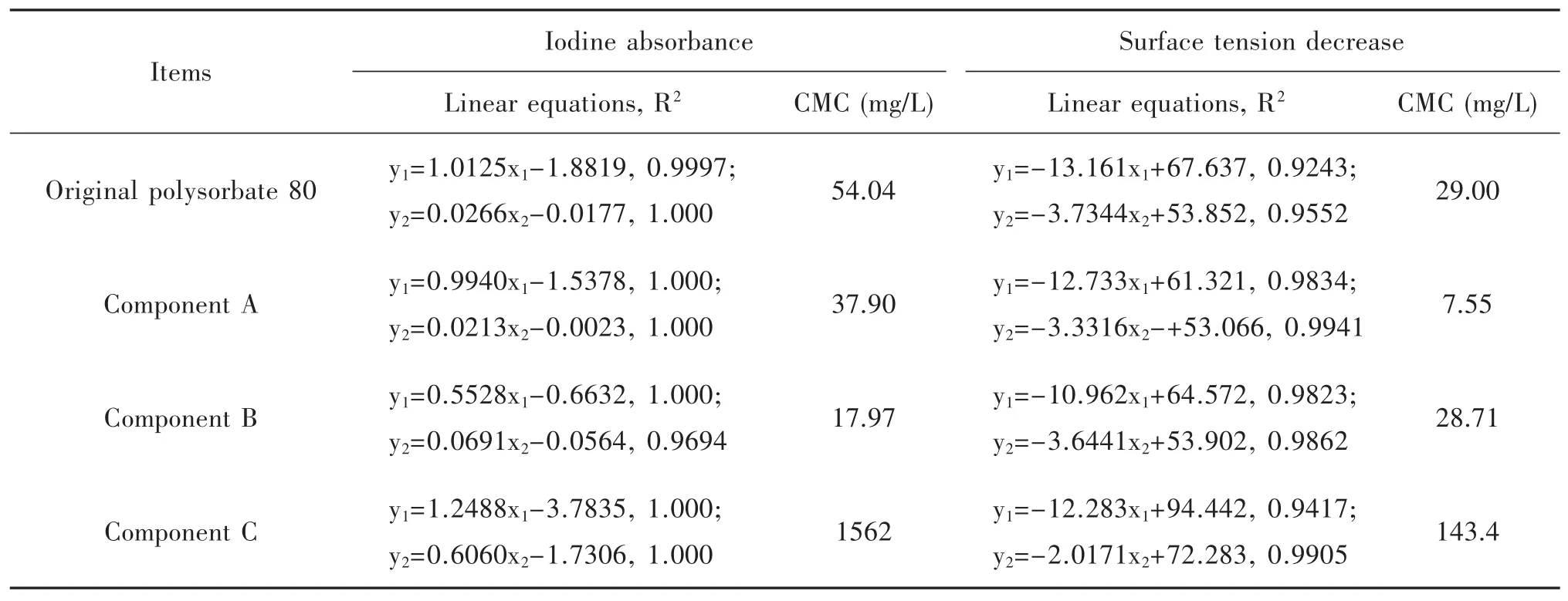
Table 2 Linear equations and critical micelle concentration(CMC)
3.5 Solubilization capacity of the original polysorbate 80 and its components
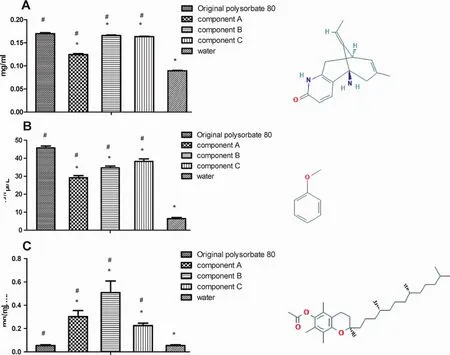
Figure 10 Solubilization capacity of the original polysorbate 80 and its components at 9.76 mg/L for huperzine A(A),anisole(B),and α-tocopherol(C)(mean ± SE,n=3).The structures of huperzine A,anisole,and α-tocopherol at the right were cited from https://pubchem.ncbi.nlm.nih.gov/.*,P<0.05,vs original polysorbate 80;#,P<0.05,vs water
3.6 Physicochemical constants including viscosity,density,absorption spectrum,pH,and particle size assay of the original polysorbate 80 for injection and its components
Component C had the lowest viscosity,and component B had the highest both at 20℃ and 25℃.The viscosity of component A and B were higher than that of the original polysorbate 80(Fig.11).
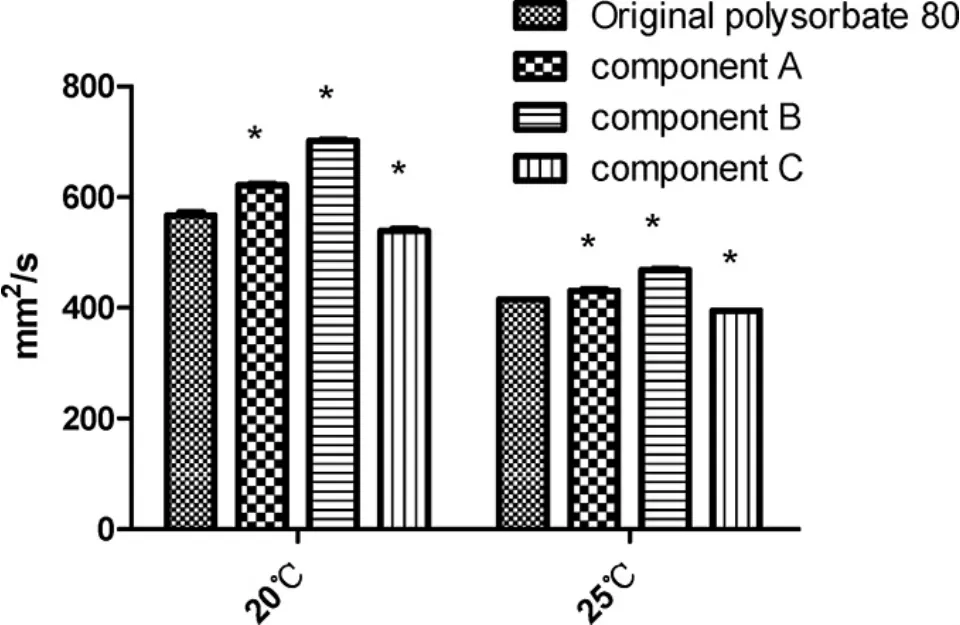
Figure 11 The viscosity of the original polysorbate 80 for injection and its components detected according to the methods documented by China Pharmacopeia(CP)of 2015 edition Book 4(mean±SE,n=3).The reference range permitted by China Pharmacopeia(CP)is 350~450 mm2/s at 25℃.Component A,> 100 kDa;component B,10-100 kDa;and component C,< 10 kDa.*,P<0.05 vs original polysorbate 80
The density of the original polysorbate 80 and its components A-C are 1.0 724,1.0 644,1.0 663,and 1.1 489 g/ml,respectively.Component C had the highest density.The reference range permitted by CP is 1.06~1.09 at 20℃,of course,the density of component C seemed not to be acceptable by CP.
As shown in Fig.12A,the ultra-violet spectrum of component C was weaker than others.According to Fig.12B,there were no chromophores in its structure,and polysorbate 80 should be colorless.They only could cause a very weak absorption at ultra-violet wavelengths.Since component C resulted in a weaker absorption than others,the ultra-violet absorption might come from the substances with higher molecular weight.
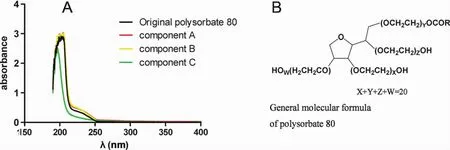
Figure 12 The ultra-violet spectrum of the original polysorbate 80 for injection and its components scanned according to the methods documented by China Pharmacopeia(CP)of 2015 edition Book 4(A),and their general molecular formula(B).Component A,> 100 kDa;component B,10~100 kDa;and component C,< 10 kDa.
The pH of the original polysorbate 80 for injection and its component A-C was 5.29,6.94,5.45,and 5.13,respectively.Since the pH range allowed by CP was 5.0~7.5 at 25℃,all of the pH were acceptable.
Considering the maximal amount of polysorbate 80 for injection used was 5 g/L,their particle size at the concentration was scanned by the laser particle analyzer.As showed in Fig.13,sodium dodecyl sulfate(SDS),an anionic surfactant,tended to form large micelles,benzalkonium bromide,a cationic surfactant,tended to form small micelles,and the particle size of the micelles formed by polysorbate 80,a nonionic surfactant,was between them.There was no obvious difference between the particle sizes formed by polysorbate 80 and its components.However,the particle sizes of micelles formed by component C distributed in a much narrower range,suggesting that the micelles had a better homogeneity.
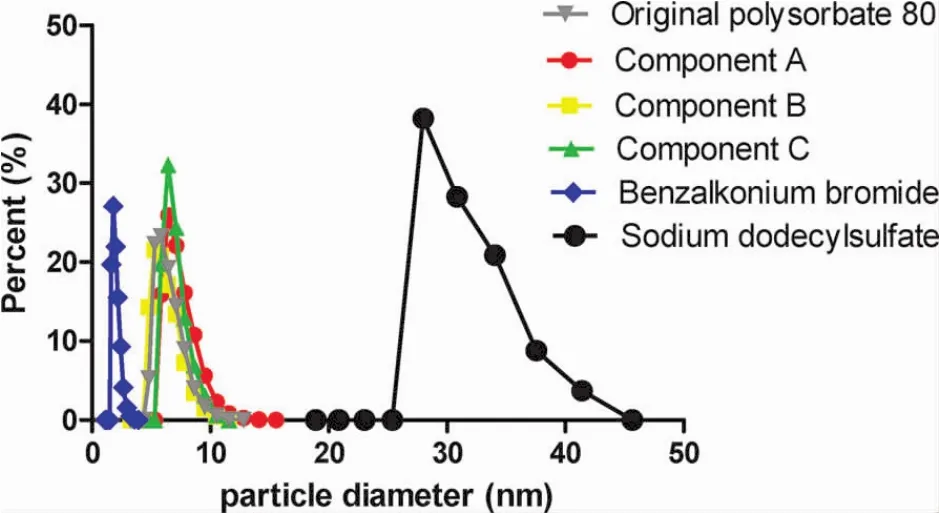
Figure 13 The particle size of the micelles formed by the original polysorbate 80 for injection and its components(5g/L)scanned by the laser particle analyzer.Component A,>100 kDa;component B,10-100 kDa;and component C,<10 kDa.
3.7 Infrared spectrograms of the original polysorbate 80 for injection and its components
Infrared spectrograms of the original polysorbate 80 for injection and its components were scanned and showed in Fig.14.There were three characteristic peaks at 2 930 and 1 750 cm-1that related to hydrocarbon (including methyl,methylene,and methylene)and carbonyl groups,respectively.As for component C,the peak at 2 930 cm-1was not split,which suggested that the hydrocarbons in component C had a better homogeneity,and the component contained fewer hydroxy groups.
4 DISCUSSION
Macromolecular substances in injections were one of the hot topics attracting biomedical scientists’eyes[10,17].Though some study put forward that unknown micromolecular substances could be dangerous by injecting,and modern immunology has been verified that macromolecules,as a pathogen-associated molecular pattern(PAMP)or danger-associated molecular pattern(DAMP),would trigger innate immune responses[18],the macromolecular impurities in injections caused nonspecific adverse reactions is still needed to be clarified.Excipients,like polysorbate 80,could be an important resource of macromolecular impurities in injections.Fortunately,the present study proved the hypothesis.
To our surprise,the main proportion (more than 85%)of polysorbate 80 for injection was macromolecular impurities according to its general formula with a molecular weight of 1 310 Da.In the present study,two components(components A and B)only containing macromolecular impurities were obtained.The two components and their original,different from component C,were proved to result in positive reactions in both anaphylactic and anaphylactoid models.However,the animal results do not mean that the two components and the original were able to cause both anaphylactic and anaphylactoid reactions.Theoretically,a substance caused anaphylactoid reactions may also result in positive reactions in an anaphylactic model.In the present study,the positive reactions caused by the original polysorbate 80 and its component A and B in the anaphylactoid model were similar to those in the anaphylactic model.It is more reasonable to accept that the positive reactions caused by the original and its component A and B in both models were anaphylactoid rather than anaphylactoid reactions.
Theoretically,as an antigen,the substance usually has a complex three-dimensional structure with a higher molecular weight and is usually composed of carbon,hydrogen,oxygen,nitrogen,and even other elements.The general formula of polysorbate 80 only contains three elements,including carbon,hydrogen,and oxygen,and the substance is polymerized from three substrates,sorbitol,ethylene oxide,and oleic acid.The three-dimensional structure of polysorbate 80 is complex but much simpler than that of a protein.Therefore,it is not likely for polysorbate 80 and its components with big molecular weight to become an antigen and trigger an anaphylactic reaction.However,it is possible for its components with big molecular weight,like components A and B in the present study,to cause anaphylactoid reactions.The present study not only verified that the original polysorbate 80 could cause anaphylactoid reactions[19]but also caught the impurities to cause the reactions.
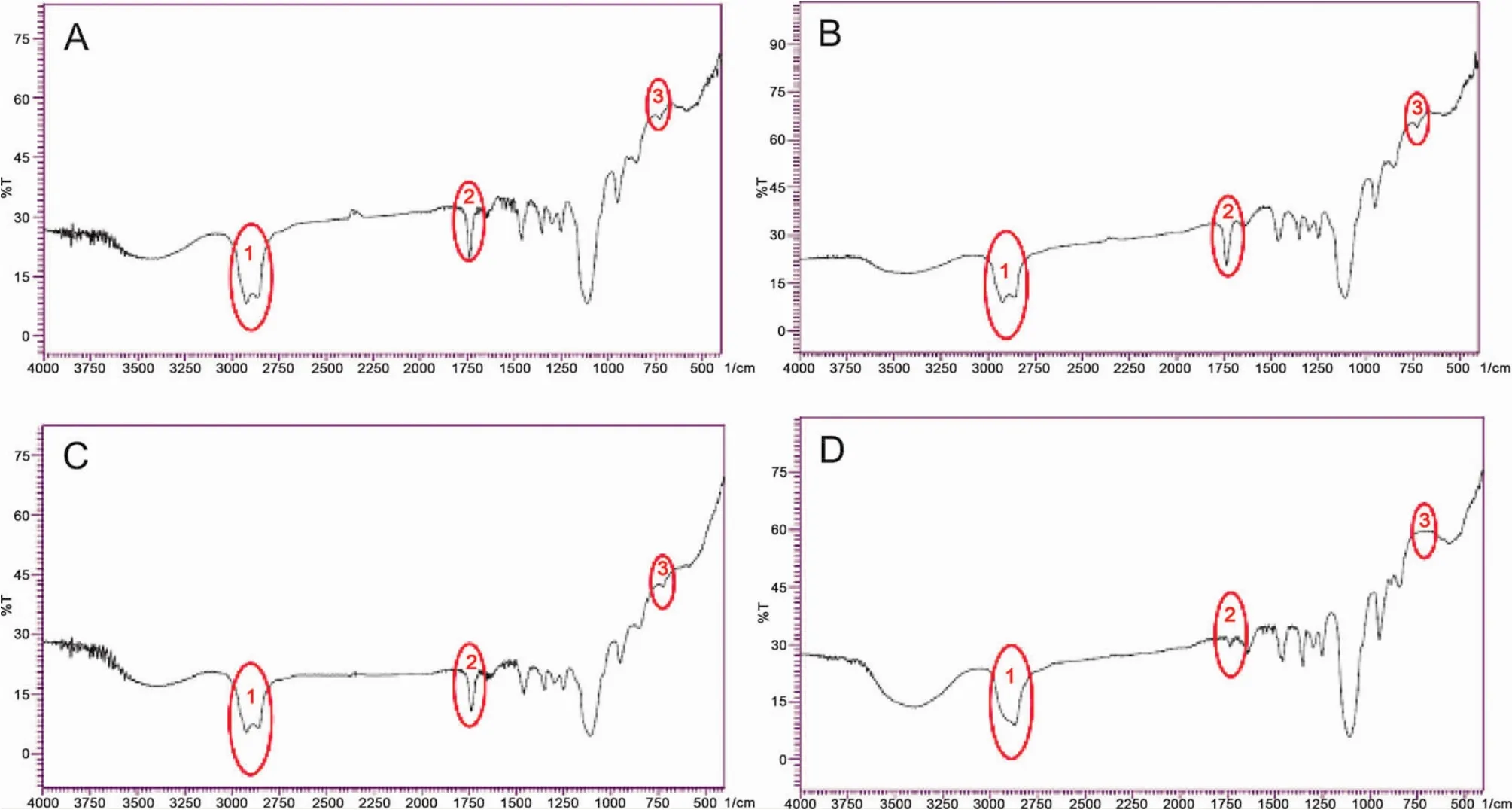
Figure 14 Infrared spectrogram of the original polysorbate 80 for injection and its components scanned by the infrared spectrometer.Red cycle 1 showed the peak at 2 930 cm-1that related to-CH3,-CH2-,and=CH-;Red cycle 2 showed the peak at 1 750 cm-1that related to C=O,and red cycle 3 showed the peak at 730 cm-1that related to-OH.A,the original polysorbate 80 for injection;B,Component A,> 100 kDa;C,component B,10-100 kDa;and D,component C,< 10 kDa.
Because the polysorbate 80 and its component A and B caused anaphylactoid reactions,the function of the immune organ,spleen,was up-regulated by the signs of its micro-pathological sections(Fig.7)and its spleen index(Fig.4C and D).Unfortunately,the function of the spleen and the other immune organ,the thymus,were not examined in the present study.The present study also verified that the liver[5,20]and kidney[20]were sensitive target organs for the toxicity caused by macromolecular substances since the macromolecular impurities in polysorbate 80 for injection were almost non-degradable by the liver and thronged in the kidney.
As for component C, whose substances with molecular weight higher than 10 kDa were removed,the component almost caused no positive reactions both in anaphylactic and anaphylactoid models.The results suggested that component C was much safer than the original polysorbate 80.Theoretically, soluble molecules with molecular weight lower than 10 kDa can be filtered through and excreted by the kidney.
According to the general understanding,the physicochemical properties of component C could differ from those of component A,B,and the original polysorbate 80,and the present study supported the hypothesis.First,the emulsifying ability of component C decreased because it had a bigger CMC(Fig.9 and Table 2),suggesting that more of the“new”polysorbate 80(component C)should be used to make an emulsion for injection as usual.However,polysorbate 80 was more frequently used as a solubilizer rather than an emulsifier.
Second,the“new”polysorbate 80(component C)had an adequate solubilizing ability though much weaker than the original one(Fig.10).As for lipophilic substances with shorter fatty chain (usually ball-like molecules),the“new”polysorbate 80 had an adequate solubilizing ability though lightly weaker than the original;as for those with long fatty chain(usually thread-like molecules),the new excipient can also solubilizer them though much weaker than the original.
Third,when substances with molecular weight higher than 10 kDa were removed,apart from density,the physicochemical constants,including viscosity,absorption spectrum,pH,and particle size were still acceptable,even improved.In detail,compared with the viscosity of the original,that of component C decreased to some extent with significance,supporting that substances with higher molecular weight are usually more viscous.Component C had the biggest density,which suggested the substances in it were the most homogenous and can be piled most tightly.As for pH,theoretically,the polysorbate 80 and its component could be neutral because the general structure of polysorbate 80 contains no acidic groups.The acidity of their solution could result from the remaining oleic acid.As a micro-molecule,oleic acid can easily pass through the ultra-filter and enriched in the solution with smaller molecular weight;the enriched oleic acid in the solution of component C would cause the smallest value of pH.In the end,the particle sizes in component C solution were distributed in a narrower range than those in the original.
The changed physicochemical properties would be derived from the substances modified.The infrared spectrograms suggested that component C contained the lest macromolecular substances,with the lest hydroxy groups and the most homogenous hydrocarbons.According to the synthetic route,component C would have the highest proportion of oleic acid group,the functional group for solubilization,which facilitates solubilizing lipophilic substances.
Finally,according to the general molecular formula,substances with molecular weight bigger than 1 310 also should be removed.However,the removal of macromolecular substances with molecular weight less than 10 kDa could be less feasible than that with molecular weight bigger than 10 kDa at present.Even further,component C was proved to be much safer than the original.Of course,with the technology advancing,substances with molecular weight bigger than 5 kDa or 3 kDa in the polysorbate 80 for injection should be removed in the future,which would make the excipient safer and safer.
Taken together,there are many macromolecular impurities in the polysorbate 80 for injection,and the removal of impurities with molecular weight bigger than 10 kDa makes it safer.And the study also gives a strategy to improve the safety of other excipients for injection.
