Nitrogen acquisition,fixation and transfer in maize/alfalfa intercrops are increased through root contact and morphological responses to interspecies competition
2021-06-24SHAOZeqiangZHENGCongcongJohannesPOSTMALUWenlongGAOQiangGAOYingzhiZHANGJinjing
SHAO Ze-qiang,ZHENG Cong-cong,Johannes A.POSTMA,LU Wen-long,GAO QiangGAO Ying-zhi,ZHANG Jin-jing
1 College of Resources and Environmental Sciences,Jilin Agricultural University/Key Laboratory of Sustainable Utilization of Soil Resources in the Commodity Grain Bases in Jilin Province,Changchun 130118,P.R.China
2 College of Resource and Environment Engineering,Jilin Institute of Chemical Technology,Jilin 132022,P.R.China
3 Forschungszentrum Jülich GmbH,Institute of Bio-and Geosciences -Plant Sciences (IBG-2),Jülich 52428,Germany
4 Key Laboratory of Vegetation Ecology,Northeast Normal University,Changchun 130024,P.R.China
Abstract Nitrogen (N) fixation by legumes and nitrogen transfer to cereals have been considered as important pathways for overyielding and higher N use efficiency in cereal/legume intercropping systems. However,the extent to which root morphology contributes to N fixation and transfer is unclear. A two-factorial greenhouse experiment was conducted to quantify the N fixation,transfer and root morphology characteristics of the maize/alfalfa intercropping system in two consecutive years using the 15N-urea leaf labeling method,and combining two N levels with three root separation techniques. N application could inhibit N fixation and transfer in a maize/alfalfa intercropping system. Irrespective of the N application level,compared with plastic sheet separation (PSS),no separation (NS) and nylon mesh separation (NNS) significantly increased the total biomass (36%) and total N content (28%),while the N fixation rate also sharply increased by 75 to 134%,and the amount of N transferred with no root barrier was 1.24-1.42 times greater than that with a mesh barrier. Redundancy analysis (RDA) showed that the crown root dry weight (CRDW) of maize and lateral root number (LRN) of alfalfa showed the strongest associations with N fixation and transfer. Our results highlight the importance of root contact for the enhancement of N fixation and transfer via changes in root morphology in maize/alfalfa intercropping systems,and the overyielding system was achieved via increases in maize growth,at the cost of smaller decreases in alfalfa biomass production.
Keywords:maize/alfalfa intercropping,nitrogen fixation and transfer,root morphology,nitrogen utilization
1.Introduction
Jilin Province is located in the northeastern part of China,which is an important area for grain commodity production and animal husbandry. However,long-term maize monocropping and excessive or unreasonable chemical fertilizer usage,especially N fertilizer,cause severe problems for food production,animal husbandry and the ecological environment in this region (Zhanget al.2013; Liet al.2019). In addition,the low economic value of maize in recent years has reduced the incentives for maize growers (Sunet al.2018). To address food security and ecological security issues,research on biological N fixation and transfer should be adopted to promote agricultural sustainability and crop productivity (Muset al.2016).
Cereal/legume intercropping is considered a sustainable agricultural production system (Liet al.2011;Huanget al.2019) and has significant advantages over other systems in terms of yield and N uptake (Zhanget al.2020),mainly due to its efficient utilization of natural resources (i.e.,light,water and fertilizer) (Sunet al.2014). The N-fixing ability of legume crops reduces N competition between the cereal and legume crops in intercropping systems (Jensenet al.2010) and diversifies the niches of N absorption and utilization (Andersenet al.2014),while legumes also transfer a certain amount of N to the associated cereal crops (Wanget al.2019),providing an important N source for the cereal crops (Menget al.2015). This can effectively reduce the amount of N fertilizer applied and thereby protect the ecological environment (Schipanskiet al.2010).
Root architecture refers to the shape (mainly including the morphological structure and topological structure) and spatial distribution of plant roots in the growth medium. Morphological structure refers to the phenotypic characteristics of the root system,mainly including the root thickness,length,root hairs and number and length of lateral roots (Lynch 1995). Studies have shown that interspecific competition in wheat/faba bean intercropping systems increases root nodulation,N fixation and growth of faba bean,which in turn significantly increases N fixation (Xiaoet al.2004). Fanet al.(2006) found that the amount of N fixed by faba bean in the maize/faba bean intercropping system was significantly greater than that in faba bean monocropping (Fanet al.2006). Moreover,N transfer has also been detected in most intercropping systems,for example,maize/bean (Gilleret al.1991),barley/pea (Jensen 1996),faba bean/wheat (Xiaoet al.2004),peanut/rice (Chuet al.2004),lettuce/manure (Sakaiet al.2011),pea/maize (Olujobi and Oyun 2012),soybean/maize (Zhanget al.2017) and garlic/bean (Tanget al.2018). Studies have shown that N fixation by leguminous plants is strongly correlated with N transfer (Thilakarathnaet al.2016). However,the results of studies on N fixation and N transfer in cereal/legume intercropping systems are inconsistent. Dansoet al.(1987) pointed out that barley/faba bean intercropping increased the N fixation rate of faba bean but reduced its biomass,and that the proportion of biomass decrease was larger than the proportion of N fixation. Therefore,N fixation was lower than that observed for monocropping. Depending on species and the environment,the proportion of N derived from air (%Ndfa) in legumes can vary from 0 to more than 90% in intercropping systems (Herridgeet al.2008). At the same time,the N transferred from leguminous plants to nonleguminous plants ranges from 0 to 80% (Chalket al.2014). According to Jensen (1996),on the basis of foliar15N labeling,19% of the total N in barley was transferred from pea plants,while barley did not transfer any N to peas. In contrast,foliar15N labeling revealed bidirectional N transfer in a peanut/rice intercropping system,and the net transfer direction was from peanut to rice (Shen and Chu 2004). However,there have been no attempts to explore the effect of root contact on N fixation and transfer under maize/alfalfa intercropping.
The mechanisms promoting N fixation and transfer in cereal/legume intercropping systems mainly involve the following four factors:(1) root exudates,such as citric acid (Wanget al.2019),flavonoids (Liet al.2016),soluble sugars and organic acids (Badri and Vivanco 2009);(2) mycorrhizae (Thilakarathnaet al.2016);(3) root and nodule decomposition (Fustecet al.2010);and (4) rhizosphere soil NO3-/NH4+ratio decreases (Wanget al.2019). Studies have found that short-term N transfer from alfalfa to maize is dependent more on arbuscular mycorrhizal fungi than on root exudates in N-deficient soil (Zhanget al.2020).However,the relationships between root morphology and N fixation and transfer in maize/alfalfa intercropping systems are unclear. Therefore,a two-year,two-factorial greenhouse root box test experiment involving maize/alfalfa intercropping was performed. The research objectives were as follows:(1) to investigate how root contact affects crop yield,N uptake,N fixation and N transfer by changing root morphology under different N levels and (2) to determine whether root morphology contributes to N fixation and transfer in a maize/alfalfa intercropping system.
2.Materials and methods
2.1.Study area
Two pot experiments were carried out from 2015 to 2016 in a rain shelter at Jilin Agricultural University,which is located in Changchun City,Jilin Province,China (125°24´50.38´´E and 43°48´28.59´´N,248.5 m a.s.l). The climate in this area belongs to the north temperate continental monsoon category,with four distinct seasons and moderate semiarid characteristics. The annual frost-free period includes 120-160 days,and the growing degree days (≥10°C) are characterized by a temperature of 2 200-3 000°C. The final frost and first frost occur in April and September,respectively. The temperature,light and air conditions in the half-opened rain shelter are almost the same as those in nature,while the water conditions are controlled.The mean annual air temperature and precipitation in 2015 and 2016 were 19°C and 494 mm,respectively. We used evaporative cooling and shade cloth to control the temperature on sunny days.
The experimental soil was collected near the rain shelter (125°24´44.69´´E and 43°48´36.49´´N,231.7 m a.s.l) and classified as a black soil,equivalent to typical Hapludoll in the USDA Soil Taxonomy System (Comaset al.2014). The physical and chemical properties of the experimental soil were as follows:organic C,21.85 g kg-1;total N,1.48 g kg-1;alkali hydrolyzable N,80.36 mg kg-1;rapidly available P,14.82 mg kg-1;rapidly available K,115.42 mg kg-1;and soil pH (soil:water=1:2.5),6.46.
2.2.Experimental materials and design
The alfalfa (MedicagosativaL.) cultivar used in this experiment was Dongmu 1,which was bred by Northeast Normal University,China. The maize (ZeamaysL.) cultivar Zhengdan 958 was chosen as the maize test variety because of its strong resistance to pests and diseases and its short period to reach maturity of only 128 days. These two cultivars are widely grown in the farming and pastoral area of Northeast China due to their high and stable yields and high resistance to lodging and disease.
The experiment was conducted from June 1st,2015 to May 23rd,2017. The experiment followed a randomized complete block design and included two factors (A×B):factor A included two N levels (N0 and N1),where N0 involved no N fertilizer application and N1 involved 225 kg ha-1N fertilizer application,and factor B included three methods of maize and alfalfa root separation:no separation,nylon mesh separation and complete root separation. Maize was fertilized with 225 kg N ha-1(N1 only),120 kg P ha-1,and 60 kg K ha-1,and alfalfa with 53 kg N ha-1,135 kg P ha-1and 90 kg K ha-1. The pots in the pot experiment were fertilized and mixed thoroughly through half of the pots during sowing of the N1 treatments (May 29,2015 and May 28,2016). The experimental details are shown in Appendix A. Each treatment was replicated four times. Maize and alfalfa were sown on June 1st,2015,and May 23rd,2016,respectively. The plants were irrigated after sowing and thinned after crop emergence.Weeds were regularly controlled using a hand hoe,and pests and diseases of maize and alfalfa were controlled separately and promptly.
Air-dried soil (51 kg;passed through a 2-mm sieve) was placed into plastic pots (length:45 cm,width:30 cm,height:50 cm). The NPK fertilizer contained the following components:urea (46% N content),superphosphate (46% P2O5content) and potassium chloride (60% K2O content). The alfalfa in all pots was inoculated with 20 mL of rhizobial liquid medium at the seedling stage (provided by the College of Resources and Environment of Jilin Agricultural University). The plants were watered with deionized water to maintain the soil moisture at 60-70% of the field waterholding capacity throughout the growth stage,and the soil moisture in each pot was monitored by microtensiometers (Nanjing Canglang Technology Development Co.,Ltd.,China).
Alfalfa leaves were labeled at the second harvest (August 15th,2015 and August 18th,2016). First,the surface of the potting was covered with two layers of plastic film and two layers of filter paper,which were located above the plastic film. A polyvinyl chloride cylinder (25 cm×50 cm) was used to enclose the canopy of alfalfa. A 1.5% (w/w) solution of15N-labeled urea (5.14 atom%) was sprayed on the surface of alfalfa leaves as described by Shen and Chu (2004). After spraying 10 mL of solution,the alfalfa leaves were covered with a white plastic bag. The procedure was repeated five times for each plant. After the foliar15N-labeling was completed,the leaves were washed three times with deionized water. The same amount of urea solution (not labeled) was used as a control to determine the natural15N abundance given these test conditions.
2.3.Sample collection and measurement
Maize was harvested on October 15th,2015,and October 18th,2016,and alfalfa was harvested twice a year,specifically,on August 2nd and October 7th in 2015 and on August 2nd and October 3rd in 2016. Fresh weight was recorded before maize grains were oven-dried at 65°C to a constant weight,at which point their dry weight was recorded. Water content and grain yield were calculated based on the dry weight. After the final harvest of maize and alfalfa,the intact roots were destructively excavated with shovels. The complete root system was rinsed with tap water at low pressure,maize root crowns were placed under the camera on a matte black background for imaging,and the nodal root growth angle from the horizontal was measured with the image-processing software ImageJ 1.50 Software (National Institutes of Health,Bethesda,USA).The maize root angle (RA) (the root angle here refers to the angle between the root and the horizontal) was determined with ImageJ. Root characteristics of alfalfa were studied according to the Marquez-Qrtiz and Johnson methods. The root and stem characteristics were analyzed (Johnsonet al.1998). For maize,all nodal roots emerging belowground were classified as crown roots,and the crown root number in each nodal whorl was counted manually (Sunet al.2018).For alfalfa,morphological traits of the crown (depth,branch number,and diameter),taproot (diameter) and lateral roots (number and diameter) were measured manually. All roots were then preserved in 75% ethanol,scanned with an Epson Perfection V800 scanner (Epson America,Inc.,USA) at a resolution of 23.6 pixels mm-1(600 dpi),and analyzed using the image-processing software WinRhizo Pro (Regent Instruments,Québec,Canada) to obtain root length,root surface area,and root volume. The roots of the two crops were mainly differentiated based on tissue structure and color. The roots of maize are white,smooth and fibrous,while the roots of alfalfa are linear,with obvious main and lateral roots,and brown when exposed to air. All the roots were washed to remove the attached soil,and the remaining soil was poured onto a 2-mm soil screen and soaked in a basin for 1 h. Then,the soil was rinsed,and the roots were separated with tweezers. The subsamples were ground and homogenized into a fine powder using a bead mill (Retsch,Germany),and then passed through a 100-mesh sieve.Theδ15N and total N contents of the plants were measured by a K-05 automatic N analyzer (Shanghai Shengsheng Automation Instrument Co.,Ltd.,China).
2.4.Data calculation
Total N accumulated by the plant (g/pot) was calculated by the following equation (Zhanget al.2015):

where M is the amount of dry matter (g/pot) and C is the N concentration in the plant (%).
%Ndfa,based on the15N natural abundance method,was calculated according to Shearer and Kohl (1986):

where theδ15N values of the reference plant and N2-fixing plant are theδ15N values of monoculture maize and alfalfa,respectively,and the B value is derived fromδ15N analyses of shoots of plants grown with a nutrient solution free of N. In this study,the B value was -0.92‰ for alfalfa shoots (Wanget al.2010).
The amount of N2fixed (Ndfa) was calculated as the product of %Ndfa,alfafa dry mass (DM),and shoot N concentration. The equation is as follows:

The N transfer rate (%Ntransfer) represents the amount of total N transferred from alfalfa (15N donor plant) to maize (15N receiver plant),it was estimated by the following equation (Thilakarathnaet al.2016):
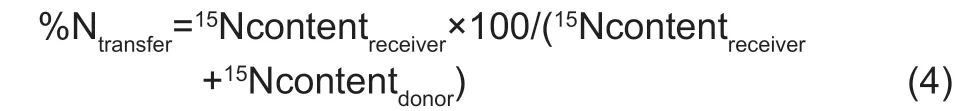
The amount of N (mg/pot) transferred from alfalfa was calculated as follows:

2.5.Statistical analysis
Statistical analyses were performed using SAS 9.2 (SAS Institute,Inc.,Cary,North Carolina,USA). Normal distribution and homogeneous variances were tested for all data with the Shapiro-Wilk test and Levene’s test. Two-way analysis of variance (ANOVA) was used to evaluate the effects of N addition levels,root separation patterns and their interactions on the yield,N content,N fixation,N transfer,and the root morphological traits of maize and alfalfa. To determine significant differences among separation treatments,the significance level was set atP<0.05. Ordination techniques were applied using the international standard software CANOCO 4.5 and CanoDraw (Microcomputer Power,Ithaca,USA),and a linear model was selected for redundancy analysis (RDA).
3.Results
3.1.Maize and alfalfa yield and N content
The crop yields and N content under maize/alfalfa intercropping were significantly affected by N application,root separation pattern and their interactions (Appendix B). On average,maize yield increased by 23% with no root barrier,15% with the mesh barrier and 71% with the plastic sheet barrier,and alfalfa yield increased by 11% with no root barrier,7% with the mesh barrier and 3% with the plastic sheet barrier (Fig.1-A and C) with N application,respectively. Similarly,maize N content increased significantly by 99% with no root barrier,89% with the mesh barrier and 152% with the plastic sheet barrier,and alfalfa N content increased slightly,by 18% with no root barrier,12% with the mesh barrier and 19% with the plastic sheet barrier with N application,respectively (Fig.1-B and D). The total yield and total N content under the three root separation patterns with two N levels showed different trends (Fig.1-E and F). In response to N application,total yield increased by 21% with no root barrier,14% with the mesh barrier,and 47% with the plastic sheet barrier,and total N content increased by 75% with no root barrier,60% with the mesh barrier,and 73% with the plastic sheet barrier,respectively.
Under the N0 treatment,compared with those observed with the plastic sheet barrier,the maize yield and N content increased by 82% with no root barrier and 67% with the mesh barrier and by 123% with no root barrier and 96% with the mesh barrier,respectively (Fig.1-A and B). However,the yield and N content of alfalfa decreased by 44% with no root barrier and 23% with the mesh barrier and by 38% with no root barrier and 19% with the mesh barrier,respectively (Fig.1-C and D),and the total yield and total N content increased by 37% with no root barrier and 35% with the mesh barrier and by 27% with no root barrier and 28% with the mesh barrier,respectively. Maize yield and N content with no root barrier exceeded those with the mesh barrier by 9 and 14%,respectively;however,alfalfa yield and N content decreased by 27 and 23%,respectively,and the total yield and N content with no root barrier and mesh barrier had no significant differences (Fig.1-E and F).
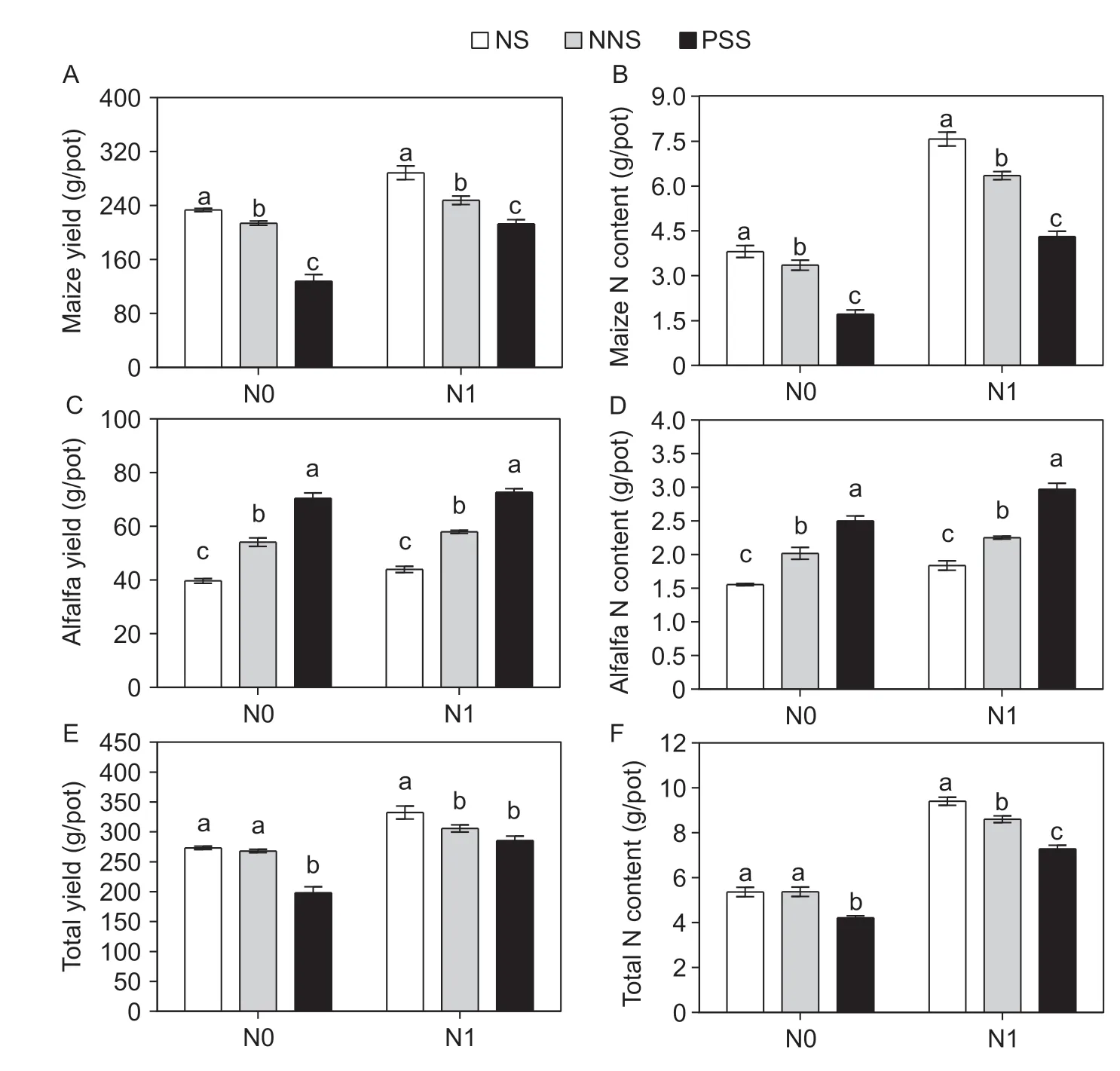
Fig.1 Comparisons of average crop yield and N content in two consecutive years under different root separation patterns and N application levels. A,maize yield. B,maize N content in the shoot. C,alfalfa yield. D,alfalfa N content in the shoot. E,total yield. F,total N content in the shoot. N0,no N application;N1,N application (225 kg ha-1);NS,no separation;NNS,nylon net separation;PSS,plastic sheet separation. Different lower-case letters indicate a significant difference of the three root separation patterns under the same N application. Values are mean±SE (n=4) across two years of experiments.
Under the N1 treatment,maize yield and N content increased by 31% with no root barrier and 13% with the mesh barrier,and by 76% with no root barrier and 47% with the mesh barrier compared with those observed with the plastic sheet barrier,respectively (Fig.1-A and B),but the yield and N content of alfalfa decreased by 40% with no root barrier and 20% with the mesh barrier and by 38% with no root barrier and 24% with the mesh barrier,respectively (Fig.1-C and D). Total yield and total N content increased by 13% with no root barrier and 5% with the mesh barrier and by 29% with no root barrier and 18% with the mesh barrier,respectively,compared with those observed with the plastic sheet barrier. Maize yield and N content with no root barrier exceeded those with the mesh barrier by 16 and 19%,respectively,however,alfalfa yield and N content decreased by 24 and 19% and total yield and total N content increased by 8 and 9%,respectively (Fig.1-E and F).
3.2.The rate of N fixation,the amount of N fixed by alfalfa,the rate of N transfer and the amount of N transferred
N fixation and transfer in the maize/alfalfa intercropping system were significantly affected by N application,root separation pattern and their interactions,except that the difference in N transfer rate was not significant (Appendix C). Compared with the N1 treatment,the N0 treatment significantly increased the rate of N fixation and the amount of N fixed by alfalfa under the three root separation patterns (Fig.2). On average,the rate of N fixation and amount of N fixed were increased by 53% with no root barrier,51% with the mesh barrier,31% with the plastic sheet barrier and 32% with no root barrier,38% with the mesh barrier,and 13% with the plastic sheet barrier,respectively (Fig.2-A and B);the rate of N transfer and the amount of N transferred were increased by 21% with no root barrier and 33% with the mesh barrier and by 4% with no root barrier and 19% with the mesh barrier,respectively,compared with those observed with the plastic sheet barrier (Fig.2-C and D).
Fig.2 Comparison of average N fixation among the three root separation patterns of alfalfa and N transfer between two root separation patterns. A,rate of N fixation. B,amount of N fixation. C,rate of N transfer. D,amount of N transfer. N0,no N application;N1,N application (225 kg ha-1);NS,no separation;NNS,nylon net separation;PSS,plastic sheet separation. Different lower-case letters indicate a significant difference of the three root separation patterns under the same N applications. Values are mean±SE (n=4) across two years of experiments.
Under the N0 treatment,the rate of N fixation and the amount of N fixed by alfalfa were increased by 152% with no root barrier and 88% with the mesh barrier and by 58% with no root barrier and 52% with the mesh barrier compared with those observed with the plastic sheet barrier,respectively. The rate of N fixation,the amount of N fixed by alfalfa,the rate of N transfer and the amount of N transferred with no root barrier exceeded those with the mesh barrier by 34,35,62 and 24%,respectively. Under the N1 treatment,the rate of N fixation and the amount of N fixed by alfalfa were increased by 115% with no root barrier and 62% with the mesh barrier and by 36% with no root barrier and 25% with the mesh barrier,respectively,compared with those observed with the plastic sheet barrier. The rate of N fixation,the amount of N fixed by alfalfa,the rate of N transfer and the amount of N transferred with no root barrier exceeded those with the mesh barrier by 33,8,78 and 42%,respectively.
3.3.Morphological traits of maize crown roots
Significant differences in root morphology of maize were observed with different N levels and root separation methods (Fig.3-A,C,G and I). The crown root dry weight (CRDW),crown root length (CRL),crown root surface area (CRS),crown root volume (CRV),crown root average diameter (CRAD),lateral root branch density (LRBD),crown root angle (CRA) and crown root number (CRN) of maize roots were all affected by N application and root separation pattern (Appendix D). Compared to no N application,on average,the CRDW,CRL,CRS,CRV,CRAD,LRBD and CRN increased by 24,19,14,14,28,14 and 24% with no root barrier,19,8,27,7,21,13,and 31% with the mesh barrier,and 48,15,22,13,14,20 and 18% with the plastic sheet barrier under N1 treatment,respectively,but the maize CRA decreased by 14% with no root barrier,12% with the mesh barrier and 19% with the plastic sheet barrier.
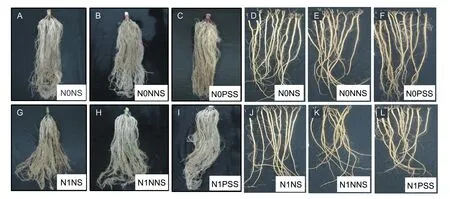
Fig.3 Root morphology of maize and alfalfa when grown with different root separation patterns and N application levels.A-C,maize root morphology under N0 treatment. G-I,maize root morphology under N1 treatment. D-F,alfalfa root morphology under N0 treatment. J-L,alfalfa root morphology under N1 treatment. N0,no N application;N1,N application (225 kg ha-1);NS,no separation;NNS,nylon net separation;PSS,plastic sheet separation.
Under the N0 treatment,compared with those observed with the plastic sheet barrier,the CRDW,CRL,CRS,CRV,CRAD,LRBD and CRN of maize increased by 79,26,43,33,30,19 and 25% with no root barrier and 59,16,13,16,14,11 and 12% with the mesh barrier,but the CRA was reduced by 15% with no root barrier and 7% with the mesh barrier,respectively. The CRDW,CRL,CRS,CRV,CRAD,LRBD and CRN with no root barrier exceeded those with the mesh barrier by 12,8,26,15,14,7,and 12%,but the CRA decreased by 9%,respectively (Fig.4).
Under the N1 treatment,compared with those observed with the plastic sheet barrier,the CRDW,CRL,CRS,CRV,CRAD,LRBD and CRN increased by 50,30,33,35,46,13 and 31% with no root barrier and by 29,9,18,10,20,5,and 25% with the mesh barrier,respectively,but the CRA was decreased by 20% with no root barrier and 10% with the mesh barrier. The CRDW,CRL,CRS,CRV,CRAD,LRBD and CRN with no root barrier exceeded those with the mesh barrier by 17,19,13,23,21,8 and 5%,but the CRA was decreased by 11%,respectively (Fig.4).
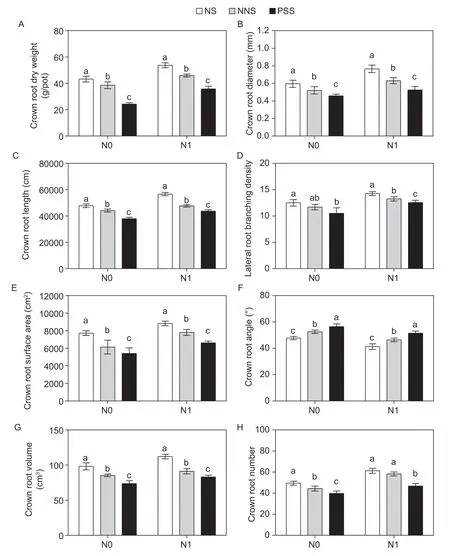
Fig.4 Comparisons of crown root dry weight (A),crown root average diameter (B),crown root length (C),lateral root branching density (D),crown root surface area (E),crown root angle (F),crown root volume (G) and crown root number (H) of maize in different root separation patterns at two N application levels. N0,no N application;N1,N application (225 kg ha-1);NS,no separation;NNS,nylon net separation;PSS,plastic sheet separation. Different lower-case letters indicate a significant difference of the three root separation patterns under the same N application. Values are mean±SE (n=4) across two years of experiments.
3.4.Morphological traits of alfalfa axial and lateral roots
The root dry weight (RDW),root length (RL),root surface area (RS),root volume (RV),root average diameter (RAD),root collar diameter (RCD),taproot length (TL) and lateral root number (LRN) of alfalfa roots were all significantly affected by N application,root separation pattern and their interactions (Appendix E). Compared to no N application,the RDW,RS and RAD of alfalfa increased by 17,13 and 12% with no root barrier,by 11,1 and 4% with the mesh barrier,and by 8,2 and 11% with the plastic sheet barrier,respectively,but there were no significant differences in RL,RV,RCD,ARD and LRN of alfalfa,which only slightly decreased.
Under the N0 treatment,compared with those observed with the plastic sheet barrier,the RDW,RL,RS,RV,RAD,RCD,TL and LRN of alfalfa decreased by 42,33,32,39,39,25,27 and 52% with no root barrier and by 19,18,12,18,15,7,10 and 26% with the mesh barrier,respectively,and the RDW,RL,RS,RV,RAD,RCD,TL and LRN with no root barrier abated those with the mesh barrier by 29,18,23,26,28,19,19,and 34%,respectively (Fig.5).
Under the N1 treatment,compared with those observed with the plastic sheet barrier,the RDW,RL,RS,RV,RAD,RCD,TL and LRN of alfalfa decreased by 37,38,24,34,38,27,28 and 45% with no root barrier and by 16,25,12,22,21,11,12 and 24% with the mesh barrier,respectively,and the RDW,RL,RS,RV,RAD,RCD,TL and LRN with no root barrier dwindled those with the mesh barrier by 25,18,13,15,22,18,18 and 28%,respectively (Fig.5).
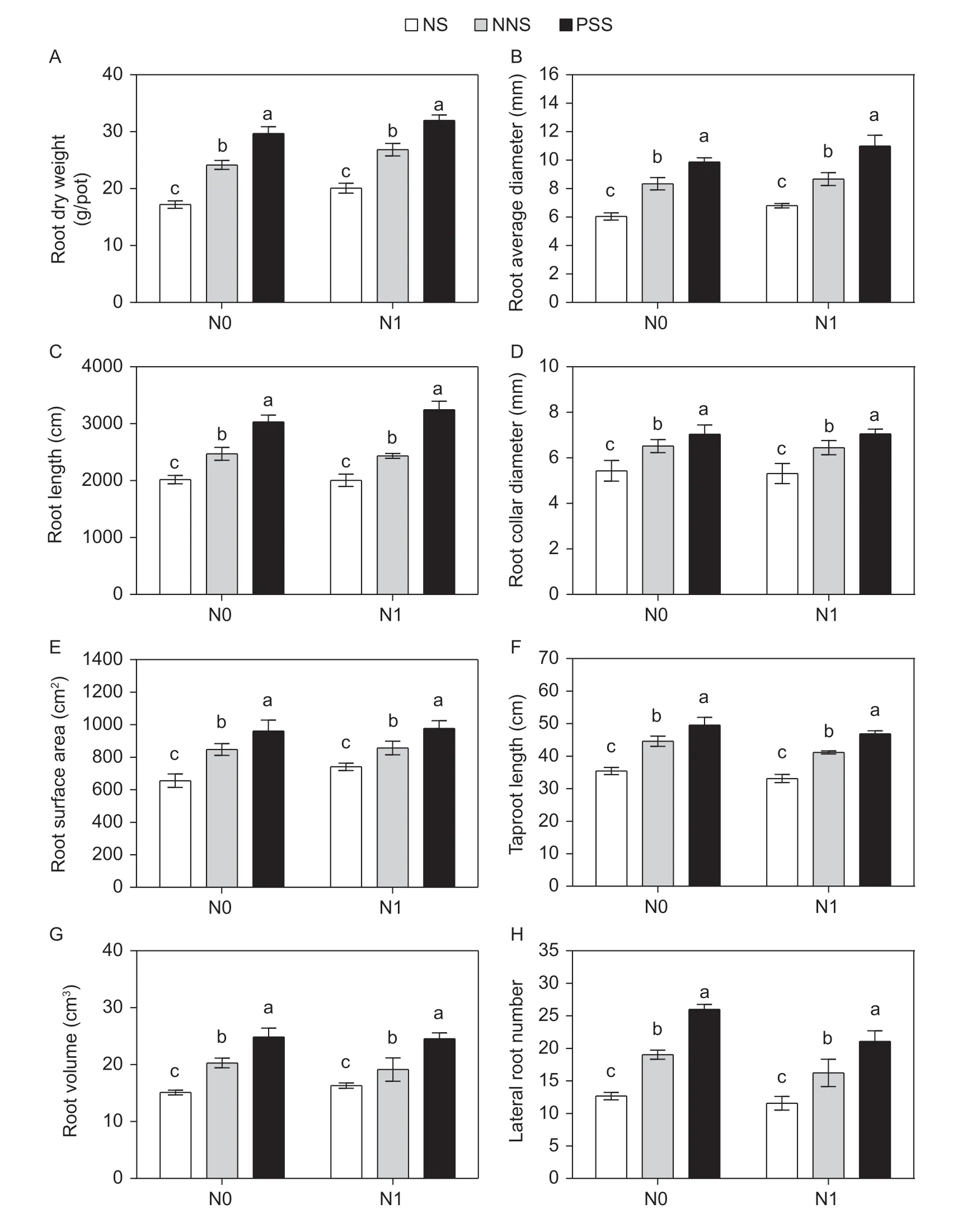
Fig.5 Comparisons of root dry weight (A),root average diameter (B),root length (C),root collar diameter (D),root surface area (E),taproot length (F),root volume (G) and lateral root number (H) of alfalfa in different root separation patterns at two N application levels. N0,no N application;N1,N application (225 kg ha-1);NS,no separation;NNS,nylon net separation;PSS,plastic sheet separation. Different lower-case letters indicate a significant difference of the three root separation patterns under the same N application. Values are mean±SE (n=4) across two years of experiments.
3.5.Relationships between maize and alfalfa root morphological traits and N fixation and transfer
The CRDW,CRL,CRS,CRV,CRAD,LRBD and CRN of maize had significant positive correlations with the N fixation rate at the two N levels (Appendix F). The CRDW,CRS,CRV,LRBD and CRN of maize had significant positive correlations with the N transfer rate at the two N levels. The CRA of maize had significant negative correlations with the N fixation and transfer rates at the two N levels. RDA showed that the CRDW,CRL,CRS,CRV,CRAD,LRBD,CRA and CRN of maize roots could explain 70.8 and 0.2% of the variation for RDA1 and RDA2,respectively (Fig.6).Among all the constrained variables,the CRDW,CRL,CRS,CRV,CRAD,LRBD,CRA and CRN of maize had significant influences on N fixation and transfer and explained 66.7,47.2,30.9,50.3,38.2,28.1,46.5 and 41.6% of the variance,respectively (Table 1).
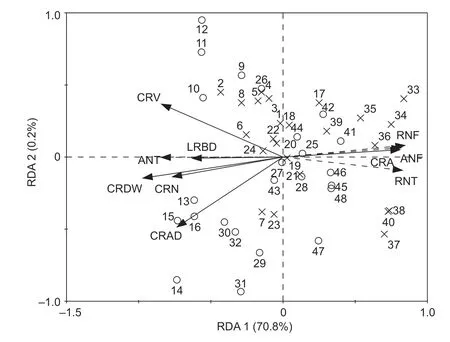
Fig.6 Redundancy analysis (RDA) on N fixation and transfer with the maize root morphology parameters. CRDW,crown root dry weight;CRL,crown root length;CRS,crown root surface area;CRV,crown root volume;CRAD,crown root average diameter;LRBD,lateral root branch density;CRA,crown root angle;CRN,crown root number of maize. RNF and ANF represent the rate of N fixation and the amount of N fixation in the maize/alfalfa intercropping system. RNT and ANT represent the rate of N fixation and the amount of N transfer in the maize/alfalfa intercropping system,respectively. The solid black line represents maize crown root morphological parameters (dependent variable),and the black dotted line represents the rate and amount of N fixation and transfer in the maize/alfalfa intercropping system (explanatory variables). The distribution of no N application (N0) is denoted by ×,and N application (N1) is denoted by ○ in 2015 and 2016,respectively.
The RDW,RL,RS,RV,RAD,RCD,TL and LRN of alfalfa had significant negative correlations with N fixation rate at the two N levels (Appendix F). The RDW,RS,RV,RAD,TL and LRN of alfalfa had significant positive correlations with N transfer rate under the N0 treatment. The RL,RS,RV,RAD,RCD and TL had significant negative correlations with N transfer rate under the N1 treatment. RDA showed that RDW,RL,RS,RV,RAD,RCD,TL and LRN of alfalfa roots could explain 79.7 and 0.3% of the variation for RDA1 and RDA2,respectively (Fig.7). Among all the constrained variables,the RDW,RL,RS,RV,RAD,RCD,ARD,and LRN of alfalfa had significant influences on N fixation and transfer and explained 35.4,50.1,42.2,47.3,46.3,17.6,54.3 and 55.5% of the variance,respectively (Table 1).
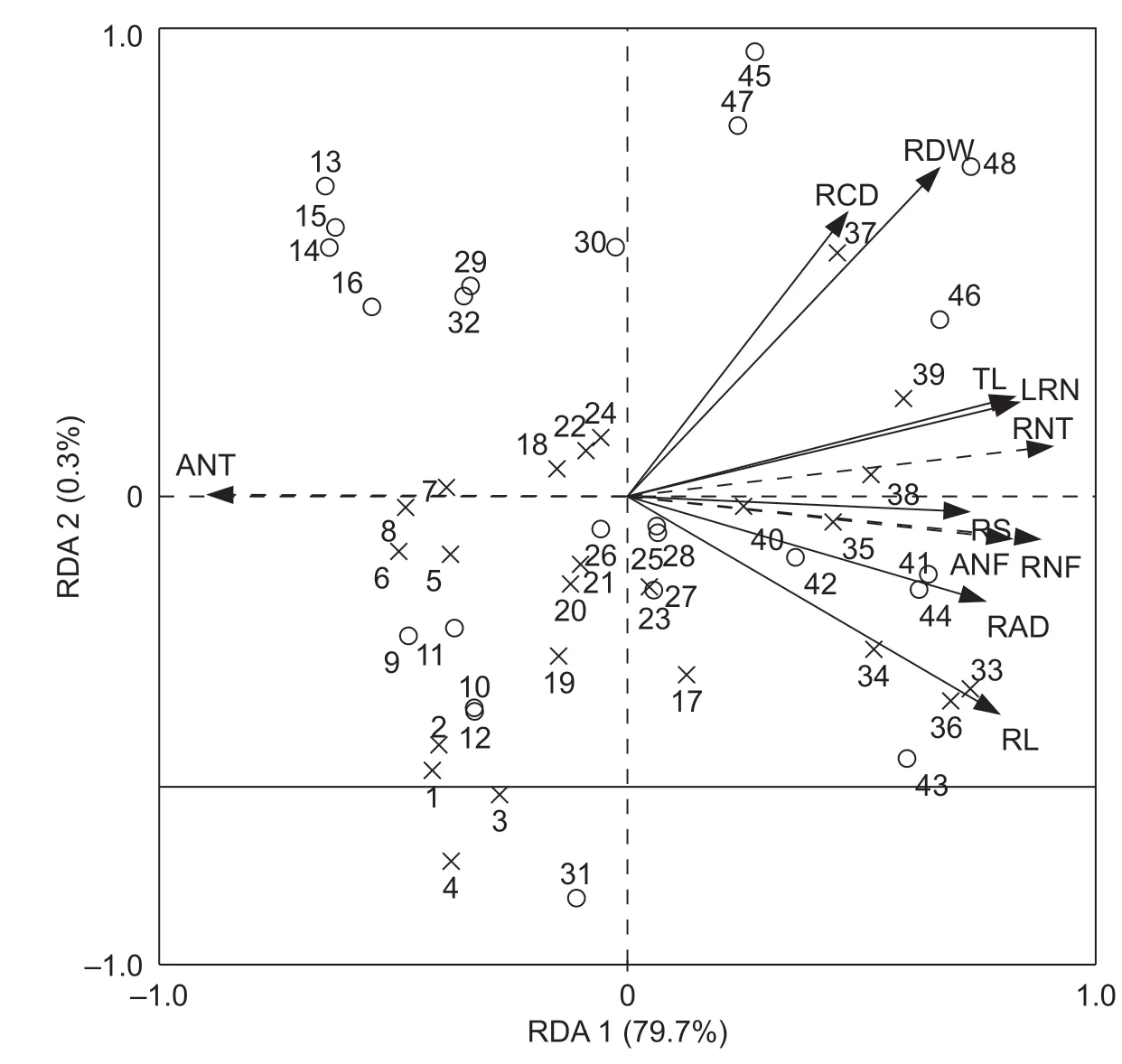
Fig.7 Redundancy analysis (RDA) on N fixation and transfer with the alfalfa root morphology parameters. RDW,root dry weight;RL,root length;RS,root surface area;RV,root volume;RAD,root average diameter;RCD,root collar diameter;TL,taproot length;LRN,lateral root number of alfalfa.RNF and ANF represent the rate of N fixation and the amount of N fixation in the maize/alfalfa intercropping system. RNT and ANT represent the rate of N fixation and the amount of N transfer in the maize/alfalfa intercropping system,respectively. The solid black line represents alfalfa root morphological parameters (dependent variable),and the black dotted line represents rate and amount of N fixation and transfer in maize/alfalfa intercropping system (explanatory variables). The distribution of no N application (N0) is denoted by ×,and N application (N1) is denoted by ○ in 2015 and 2016,respectively.
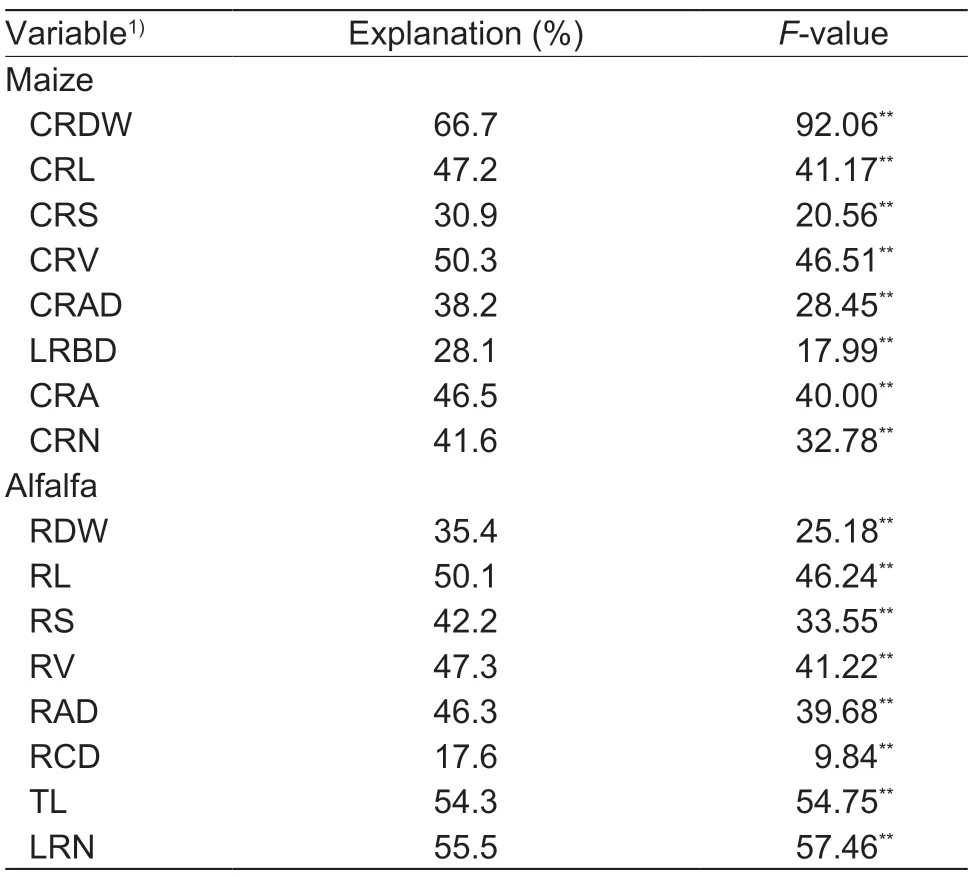
Table 1 Results of permutation testing of the redundancy analysis (RDA) on predictor variables for maize and alfalfa N fixation and transfer
4.Discussion
4.1.Yield and N content of maize and alfalfa
Cereal/legume intercropping systems have significant advantages over other systems in terms of crop yield and N uptake (Sunet al.2018). For example,several studies on rice/peanut intercropping in India (Khanet al.2006),maize/faba bean intercropping (Meiet al.2012),wheat/soybean intercropping (Liet al.2001),and maize/chickpea and maize/soybean intercropping (Xiaet al.2013) have demonstrated such advantageous effects. In this study,N application significantly increased total yield and N content. Compared with the plastic sheet barrier,no barrier and the nylon mesh barrier significantly decreased alfalfa yield and N content but significantly increased maize yield and N uptake.This finding shows that in the alfalfa intercrop,maize is the dominant species and stronger belowground competitor. The decreases in alfalfa biomass and N content were more than offset by the increases in maize. Consequently,the total biomass and total N content were increased by 25 and 28% (N0 and N1) with no root barrier and by 20 and 23% (N0 and N1) with the mesh barrier compared with the plastic sheet barrier.
The biomass of maize was increased by 32% with a mesh barrier compared with the plastic sheet barrier. The results showed that water and nutrients may move through the mesh barrierviamass flow and diffusion as well as through mycorrhizal hyphae,which can grow through the mesh barrier (Sierra and Daudin 2010;Thilakarathnaet al.2016). Besides,the biomass of maize with no root barrier was increased by 48.7 and 12.0% compared with the plastic sheet barrier and the mesh barrier,respectively,which could be due to the extra N from the alfalfa side or other available nutrients or soil. It has been well established that the rooting volume tends to limit biomass growth,even under wellfertilized conditions and with relatively large pots (Poorteret al.2012),and N application inhibited the intercropping advantage.
4.2.Root morphology responses of maize and alfalfa to barrier treatments and fertilization level
Previous studies have shown that root morphology variances are associated with soil N level (Ramirezgarciaet al.2015;Zhanget al.2020),root interaction intensity (Fagetet al.2013;Wanget al.2018) and their species in the intercropping system (Muhammadet al.2017). Similarly,different responses of maize and alfalfa root morphology to N application were observed in our experiment,as CRDW,CRL,CRS,CRV,CRAD,LRBD and CRN of maize were significantly increased,while CRA was significantly decreased by N application in the intercropping system. In contrast,RL,RV,RCD,ARD and LRN of alfalfa were slightly decreased,but the RDW,RS and RAD of alfalfa were significantly increased. This finding demonstrated that the application of N fertilizer could promote the growth of the maize root system,while the growth of the alfalfa root system was suppressed. This difference might be explained by the function of alfalfa in biological N fixation,and application of the N fertilizer caused“N suppression”(Ferguson and Mathesius 2003),which in turn affected carbon allocation and the growth of the alfalfa root system.
In addition to the N level,root separation methods can affect the root interaction intensity and further influence root morphology in the intercropping system (Wanget al.2018). Our results showed that regardless of N level,the CRDW,CRL,CRS,CRV,CRAD,LRBD and CRN of maize were significantly increased by no separation and nylon separation treatments when compared with plastic separation. In contrast,the RDW,RL,RS,RV,RAD,RCD,TL and LRN of alfalfa were significantly decreased by no separation and nylon separation treatments when compared with plastic separation. This difference can be explained by the interspecies competition between the maize and alfalfa symbiosis system,in which the competitiveness of maize is greater than that of alfalfa,consistent with Xiaoet al.(2004).The two plants reached different significance levels under the three root separation treatment conditions,which might be explained by the following reasons.
On the one hand,in the maize/alfalfa intercropping system,in addition to the contribution of nutrient flow in the biomass and N uptake improvement,root contact also plays an important role in the absorption and utilization of N in intercropping systems,which has been confirmed in Shaoet al.(2020). On the other hand,in the maize/alfalfa intercropping system,maize can absorb nutrients from the alfalfa side,thereby obtaining more nutrients for growth. Last but not least,the separation media of nylon mesh and plastic board limited the space for maize and alfalfa root growth,while maize roots could extend to the alfalfa side under conditions of no root separation treatment,expanding the root growth space.
4.3.N fixation and transfer between plants
One of the main drivers of the yield and N uptake advantages of cereal/legume intercropping is biological N2fixation in the symbiotic association,which broadens the niches of N absorption and utilization (Andersenet al.2014) and thereby provides an important N source for cereal crops (Fustec 2010) with minimal or no application of industrial N fertilizers (Carlsson and Huss-Danell 2003). Previous studies have shown that the proportion of %Ndfa in legumes varies from 0 to more than 90%,which mainly depends on species and the environment (Herridgeet al.2008;Wang and Gao 2020). In our study,the %Ndfa varied from 18 to 60%. The rate of N fixation by alfalfa was 62-152% higher with no root sheet barrier than with the mesh barrier or the plastic barrier,regardless of the N application level,but the yield and N content of alfalfa were decreased by 30 and 32%,respectively. This phenomenon indicated significant interspecific competition in the maize/alfalfa intercropping system for the soil-derived N,with maize being the stronger competitor (Zhanget al.2013). Moreover,it has been shown that N fixation by legumes consumes carbon from photosynthesis and thereby may reduce overall growth compared to N acquisition from the soil (Marschner 1995). We concluded that when alfalfa competes with maize for N,it will increase the N fixation of alfalfa and at the same time decrease the biomass of alfalfa. Furthermore,our study also revealed that the rate of N fixation by alfalfa with no root barrier exceeded that with the mesh barrier by 34%,regardless of the N application level,but the yield and N uptake of alfalfa were decreased by 25 and 20%,respectively. These results suggest that the potential mechanism increasing N fixation in maize/alfalfa intercropping can be strongly affected by the spread of the root system and the resulting proximity between the roots of the maize and alfalfa (Liet al.2016).
Another factor affecting the yield and N uptake of cereal/legume intercropping is N transfer in the symbiotic association. Previous studies found that N transfer occurs in most cereal/legume intercropping systems,and the rate of N transfer ranged from 3.1 to 15% (Menget al.2015). Consistent with these findings,in our study,the transfer rate ranged from 7 to 15% and thus fell within the previously reported range (Fig.3). The N transfer rate and amount with no root barrier exceeded those with the mesh barrier by 62-78% and 24-42%,respectively,regardless of the N application level. We also found that the amount of N transferred with no root barrier was 1.24-1.42 times greater than that with a mesh barrier,regardless of the N application level. These results suggest that the potential factor increasing N transfer in maize/alfalfa intercropping can be strongly affected by root contact (Zhanget al.2017),consistent with the findings of Unkovich (2013).Furthermore,we also found that N application significantly restricted N transfer (Fig.3),which may be due to the inhibition of alfalfa N fixation caused by N application (Wanget al.2019).
4.4.Relationship between N fixation and transfer and root morphological traits in a maize/alfalfa intercropping system
Plant roots have strong plasticity in the soil,and root morphology is an important factor determining plant N uptake and utilization (Fordeet al.2014). Interspecific interactions in cereal/legume intercropping systems alter root morphology (Liuet al.2020),which in turn increases nutrient availability by increasing the root area (Smetet al.2012). Studies have shown that root morphology is significantly positively correlated with crop N uptake (Miet al.2007). In our study,N application (N1) under the three root separation treatments significantly promoted the growth and development of maize and alfalfa roots and altered their morphologies (Figs.4 and 5). Previous studies have shown that root morphology is a key factor affecting N acquisition and content and utilization in cereal/legume intercropping systems (Postmaet al.2014;Liet al.2016). In our study,we found that the CRDW,CRL,CRS,CRV,CRAD,LRBD and CRN of maize roots were the major root architectural traits associated with the amount of N transfer (ANT),while the CRA of maize was the major factor associated with the amount of N fixation in the maize/alfalfa intercropping system.
Regarding the relative importance of alfalfa root morphology to N transfer and fixation,the RDA results showed that the increased RDW,RL,RS,RV,RAD,RCD,TL and LRN levels in alfalfa roots were associated with the amount of alfalfa N fixation in the polyculture system,but N fixation was negatively correlated with the amount of N transfer. These results indicate that the intercropping system of maize/alfalfa has advantages in N transfer by increasing the CRDW,CRL,CRS,CRV,CRAD,LRBD and CRN of maize roots and N fixation by promoting the CRA of maize,as well as RDW,RL,RS,RV,RAD,RCD,TL and LRN of alfalfa roots. This is consistent with previous results from a maize/chickpea intercropping system (Liet al.2004).We suggest that the observed plasticity responses in root morphology in the maize/alfalfa intercropping system alter the degree of competition for N,the degree of dominance of maize,the stimulation of N fixation by alfalfa,and rates of N transfer,ultimately leading to increased biomass production of the intercrop when soil exploration is not restricted by artificial barriers.
5.Conclusion
Maize/alfalfa intercropping significantly increased total yield and N uptake through root contact and morphological changes to interspecies competition. Among the root morphological traits,maize root CRDW and alfalfa root LRN showed the strongest associations with N fixation and transfer,respectively. This study demonstrates that changes in root morphologyviaroot contact and the promotion of N content,fixation and transfer in intercropping systems increase N utilization and provides a scientific basis for the optimal management of N fertilization in maize/alfalfa intercropping systems. The challenge of increasing the food supply,especially in nonfertile soils,could be met by maximizing the effects of root morphology on promoting N uptake and fixation.
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (31471945). Zheng Congcong was supported by the China Scholarship Council and J.A.Postma was institutionally funded by the Helmholtz Association (POF III Program -Research Field Key Technologies -Key Technologies for the Bioeconomy),Germany. We would also like to thank Associate Professor Cui Juntao,College of Resources and Environment,Jilin Agricultural University,China for providing the rhizobium strain ACCC177512.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Appendicesassociated with this paper are available on http://www.ChinaAgriSci.com/V2/En/appendix.htm
杂志排行
Journal of Integrative Agriculture的其它文章
- Adoption of small-scale irrigation technologies and its impact on land productivity:Evidence from Rwanda
- African swine fever and meat prices fluctuation:An empirical study in China based on TVP-VAR model
- The impacts of oxytetracycline on humification during manure composting can be alleviated by adjusting initial moisture contents as illustrated by NMR
- Effects of long-term straw incorporation on nematode community composition and metabolic footprint in a rice-wheat cropping system
- Functional diversity of soil microbial communities in response to supplementing 50% of the mineral N fertilizer with organic fertilizer in an oat field
- Kaempferol inhibits Pseudorabies virus replication in vitro through regulation of MAPKs and NF-κB signaling pathways
