Kaempferol inhibits Pseudorabies virus replication in vitro through regulation of MAPKs and NF-κB signaling pathways
2021-06-24CHENXuCHENYaqinYlNZhongqiongWANGRuiHUHuaiyueLIANGXiaoxiaHEChangliangYINLiziYEGangZOUYuanfengLILixiaTANGHuaqiaoJIARenyongSONGXu
CHEN Xu,CHEN Ya-qin,YlN Zhong-qiong,WANG Rui,HU Huai-yue,LIANG Xiao-xia,HE Changliang,YIN Li-zi,YE Gang,ZOU Yuan-feng,LI Li-xia,TANG Hua-qiao,JIA Ren-yong,SONG Xu
Natural Medicine Research Center,College of Veterinary Medicine,Sichuan Agricultural University,Chengdu 611100,P.R.China
Abstract Pseudorabies virus (PRV),in the family Herpesviridae,is a pathogen of Aujeszky’s disease,which causes great economic losses to the pig industry. Recent outbreaks of Pseudorabies imply that new control measures are urgently needed. The present study shows that kaempferol is a candidate drug for controlling PRV infection,as it possesses the ability to inhibit PRV replication in a dose-dependent manner in vitro. Kaempferol at a concentration of 52.40 μmol L-1 could decrease PRV-induced cell death by 90%. With an 50% inhibitory concentration (IC50) value of 25.57 μmol L-1,kaempferol was more effective than acyclovir (positive control) which has an IC50 value of 54.97 μmol L-1. A mode of action study indicated that kaempferol inhibited viral penetration and replication stages,decreasing viral loads by 4-and 30-fold,respectively. Addition of kaempferol within 16 h post infection (hpi) could significantly inhibit virus replication,and viral genome copies were decreased by almost 15-fold when kaempferol was added at 2 hpi. Kaempferol regulated the NF-κB and MAPKs signaling pathways involved in PRV infection and changed the levels of the target genes of the MAPKs (ATF-2 and c-Jun) and NF-κB (IL-1α,IL-1β and IL-2) signaling pathways. The findings of the current study suggest that kaempferol could be an alternative measure to control PRV infection.
Keywords:kaempferol,antiviral activity,Pseudorabies virus
1.lntroduction
Pseudorabies virus (PRV) is a swine herpesvirus of theHerpesviridaefamily (Pomeranzet al.2005). The genome of PRV consists of double-stranded linear DNA of approximately 150 kb with a high content of G+C nucleotides (73%) (Mettenleiteret al.1991). PRV infection causes outbreaks of Aujeszky’s disease,one of the most important infectious diseases of swine (Masotet al.2017).PRV can infect most mammals and some avian species (Boadellaet al.2012). Pigs are the only natural host of PRV,and infections can induce different degrees of damage to the propagating system,respiratory system and nervous system (Kluppet al.2004;Luoet al.2014;Masotet al.2017). With the use of live-attenuated vaccines with the glycoprotein E gene deletion,Aujeszky’s disease was well controlled worldwide (Tong and Chen 1999),and has even been eradicated from pig farms in some developed countries such as the USA (Hahnet al.2010),Germany (Mülleret al.2003) and New Zealand (MacDiarmidet al.2000). However,Aujeszky’s disease remains a constant threat to the pig industry in developing countries. Since late 2011,outbreaks of Aujeszky’s disease have occurred in many vaccinated pig farms in China (Anet al.2012),which suggests that the live-attenuated vaccines cannot provide enough protection against PRV infection and new control measures for PRV infection are needed.
Kaempferol is a flavonoid that is widely distributed in tea,broccoli,apples and beans (Somerset and Johannot 2008). It exhibits antioxidant (Wanget al.2006;Kampket al.2007;Imranet al.2019a;Santoset al.2019),antiinflammatory (Limet al.2007;Medeiroset al.2009;Minet al.2009),anti-cancer (Garcia-Closaset al.1999;Gateset al.2007;Nöthlingset al.2007;Lamet al.2008;Imranet al.2019b) and antiviral activities. Previous studies indicated that kaempferol alleviated H9N2 swine influenza virus-induced acute lung injury by the inactivation of TLR4/MyD88-mediated NF-κB and MAPKs signaling pathways (Zhanget al.2017) and it inhibited the multiplication of herpes simplex virus and human immunodeficiency virus (HIV) (Debiaggiet al.1990;Amoroset al.1992;Mahmoodet al.1996;Minet al.2010). It also showed the ability to inhibit the genomic RNA synthesis of poliovirus (Robinet al.2001). However,there is no study on the antiviral activity of kaempferol against PRV. It is noteworthy that,viral infection always induces changes in signaling pathways. MAPKs signaling pathway was proven to be closely related to viral infection,and it was even considered as a target against influenza virus (Planz 2013). It also mediates apoptosis of host cells induced by PRV infection (Yehet al.2008).NF-κB signaling pathway regulates many cytokines and inflammatory factors (Pahl 1999),which mediate the whole process of viral infection.Thus,regulation of NF-κB could be a target for inhibiting viral infection (Hakimet al.2018).In the present study,the anti-PRV activity of kaempferol was evaluated for the purpose of providing a new alternative control measure for Aujeszky’s disease.
2.Materials and methods
2.1.Compounds
Kaempferol (no.520-18-3) and acyclovir (no.59277-89-3) with a purity of 98% were bought from Meilun Biotechnology Co.,Ltd.(Dalian,China) and kept at 4°C away from light. For antiviral assays,kaempferol was dissolved in 95% ethanol and acyclovir was dissolved in dimethyl sulfoxide (DMSO). The stored solutions were diluted by at least 100-fold to different final concentrations in Dulbecco’s modified eagle medium (DMEM,HyClone,USA).
2.2.Cells and virus
PK-15 cells were preserved in the Natural Medicine Research Center Sichuan Agricultural University (Chengdu,China) and grown in DMEM (high glucose) supplemented with 10% (v/v) fetal calf serum (HyClone),100 U mL-1penicillin and 100 μg mL-1streptomycin. For maintenance medium (MM),the serum concentration was reduced to 2%.
PRV (Ra strain) was bought from China Veterinary Culture Collection Center (Beijing,China) and propagated in PK-15 cells. The 50% tissue culture infective dose (TCID50) was determined as 10-7.1mL-1.
2.3.50% cytotoxic concentration assay
The cytotoxicity of kaempferol and acyclovir was evaluated by Determination of Cell Viability with Cell Counting Kit-8 (CCK-8;Dojindo,Kumamoto,Japan) assay according to the manufacturer’s instructions. PK-15 monolayers grown in 96-well plates were incubated with MM containing 2-fold dilutions of either kaempferol ranging from 416 to 26.2 μmol L-1or acyclovir ranging from 1 410 to 88 μmol L-1. After incubation at 37°C for 48 h,10 μL of the CCK-8 solution was added to each well of the plate. The plates were reincubated at 37°C for 30 min,followed by measurement of absorbance values at 450 nm in a microplate reader (Bio-Rad,USA). The 50% cytotoxic concentration (CC50),i.e.,the concentration of kaempferol or acyclovir required to reduce cell viability by 50%,was calculated by the Reed-Muench method (Reedet al.1938).
2.4.50% inhibition concentration assay
The PK-15 monolayer cells in 96-well plates were infected with 200 TCID50PRV per well with or without a series of two-fold dilutions of either kaempferol ranging from 52.40 to 3.28 μmol L-1or acyclovir ranging from 113.23 to 7.08 μmol L-1. After incubation at 37°C for 1 h,the medium was removed and MM containing a corresponding dose of kaempferol or acyclovir was added. The virus-infected cells treated with ethanol (1,0.5 and 0.25%) or DMSO (1,0.5 and 0.25%) were used as solvent controls. When the infected cells without any treatment showed approximately 80% cytopathic effect (CPE),CCK-8 assays were performed as described above. The 50% inhibitory concentration (IC50),i.e.,the concentration of kaempferol or acyclovir required to inhibit 50% cell death caused by viral infection (Xuet al.2013),was calculated by the Reed-Muench method (Reedet al.1938).
2.5.Multiplicity of infection (MOI) assay
The PK-15 cells in 6-well plates were infected with MOI=1 PRV in the presence or absence of different concentrations of kaempferol or acyclovir at 37°C for 1 h,then the medium was removed and the plates were washed thrice with PBS. The MM containing the same concentrations of kaempferol or acyclovir was added again. When the infected-untreated cells showed approximately 80% CPE,the samples were subjected to freezing and thawing twice,and total DNA was extracted from each well by using DNAiso Reagent (D305;TaKaRa,China) according to the manufacturer’s instructions. For determination of viral gene copies,fluorescent quantitative PCR (FQ-PCR) was performed using a Bio-Rad CFX96 ConnectTMReal-Time PCR Detection System (CA,USA) according to the method described by Zhaoet al.(2017).
2.6.Inhibitory action assay
Pre-treatment assayKaempferol and acyclovir were added to cell monolayers and the plates were incubated at 37°C for 1 h. Then the medium was removed and the monolayers were incubated with 200 TCID50PRV at 37°C for 1 h. After incubation for 48 h,total DNA was extracted from PRV-infected cells and the virus titers in each well were measured by the FQ-PCR method as described above.
Adsorption assayA PK-15 monolayer grown in 6-well plates was infected with 200 TCID50PRV per well in the presence of dilutions of kaempferol or acyclovir. After incubation at 4°C for 1 h,the cells were washed thrice with cold PBS to remove unadsorbed viruses and then incubated with MM at 37°C. After incubation for 48 h,the virus titers in each well were measured by FQ-PCR method as described above.
Penetration assayA PK-15 monolayer grown in 6-well plates was infected with 200 TCID50PRV per well and incubated at 4°C for 1 h. The medium was aspirated and the monolayer was washed twice with cold PBS to remove unabsorbed viruses. Various dilutions of kaempferol or acyclovir were then added to each well,and plates were incubated at 37°C for 1 h to allow penetration. After removal of the medium,the monolayer was washed with a citrate buffer (pH=3.0) to inactivate virions that had not penetrated the cells. MM was added and the plates were incubated at 37°C for 48 h. The virus titers in each well were measured by the FQ-PCR method.
Replication assayPK-15 monolayers grown in 6-well plates were infected with 200 TCID50PRV per well and incubated at 37°C for 1 h to allow absorption and penetration. After washing,MM containing various dilutions of kaempferol or acyclovir were then added to each well and plates were incubated at 37°C. After incubation for 48 h,the virus titers in each well were measured by the FQ-PCR method.
Inactivation assayMM containing various dilutions of kaempferol or acyclovir were incubated with an equal volume of concentrated virus suspension (2×106TCID50PRV) at 37°C for 1 h. The solutions were then diluted 104times and added into PK-15 monolayers to allow infection of the residual viruses. After incubation for 48 h,the virus titers in each well were measured by the FQ-PCR method.
Time of addition assayThe PK-15 monolayers in 6-well plates were infected with 200 TCID50PRV per well at 37°C for 1 h,then the plates were washed three times with PBS. The MM was added and the plates were re-incubated.Kaempferol was added at 2,4,8,and 16 h post infection (hpi),each at a final concentration of 52.40 μmol L-1.After incubation for 48 h,the virus titers in each well were measured by the FQ-PCR method.
2.7.Western blotting
NF-κB (P65) and MAPKs (P38,p-P38,ERK1/2,p-ERK1/2) signaling pathways were examined by Western blotting.The PK-15 cells were infected with or without PRV (MOI=1) for 1 h. Then the cells were washed three times with PBS and MM with or without kaempferol at a concentration of 52.40 μmol L-1was added. Total proteins were extracted with a commercial kit (BOSTER,Wuhan,China) at 4,8 and 12 hpi. Total cell lysates were run on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels for 100 min and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore,USA). Then the membranes were blocked with 5% BSA at room temperature for 90 min (Sigma,USA) and proteins were stained using primary antibodies directed against P65 (CST,USA,1:1 000),p-P65 (CST,USA,1:1 000),P38 (CST,USA,1:1 000),p-P38 (CST,USA,1:1000),ERK1/2 (CST,USA,1:1 000),p-ERK1/2 (CST,USA,1:1 000),β-actin (Boster,Wuhan,1:1 000) at 4°C overnight. The membranes were washed four times with Tris-buffered saline containing 0.1% Tween 20 (TBST) and incubated with horseradish peroxidaseconjugated secondary antibody (CST,USA,1:5 000) at room temperature for 1 h and washed four times,then the proteins were visualized using enzymatic chemiluminescence (ECL) reagents (Bio-Rad). The expressions of total proteins were normalized according to the expression of β-actin and ratios of protein band intensities were obtained with ImageJ Software (Version 1.47;NIH,USA).
2.8.Transcriptional levels of target genes assay
Real-time PCR (RT-PCR) was performed to reveal the effect of kaempferol on the expression of target genes of the proteins in PRV-infected cells. Briefly,the PK-15 cells in 6-well plates were infected with or without PRV in the presence or absence of kaempferol (52.40 μmol L-1). Total RNA was extracted by TRIzol reagent (Invitrogen,USA) according to the manufacturer’s protocol and the quality was determined by measuring the A260/A280 ratio. The RNA of each sample was reverse-transcribed by Revert Aid First-Strand cDNA Synthesis Kit (Thermo Scientific,USA). The cDNA of each sample was used for RT-PCR with SYBR Green Supermix Kit (Bio-Rad,USA) and the primers used are listed in Table 1. The PCR cycling was performed at 95°C for 3 min,followed by 40 cycles of cycling at 95°C for 10 s,59.8°C for 30 s,and 55°C for 5 s. At the end of the cycling a melt curve analysis was conducted. Expression of β-actin was used to normalize the differences in total mRNA expression in each sample.Data analysis was performed using the Bio-Rad CFX Manger software (Bio-Rad,USA).
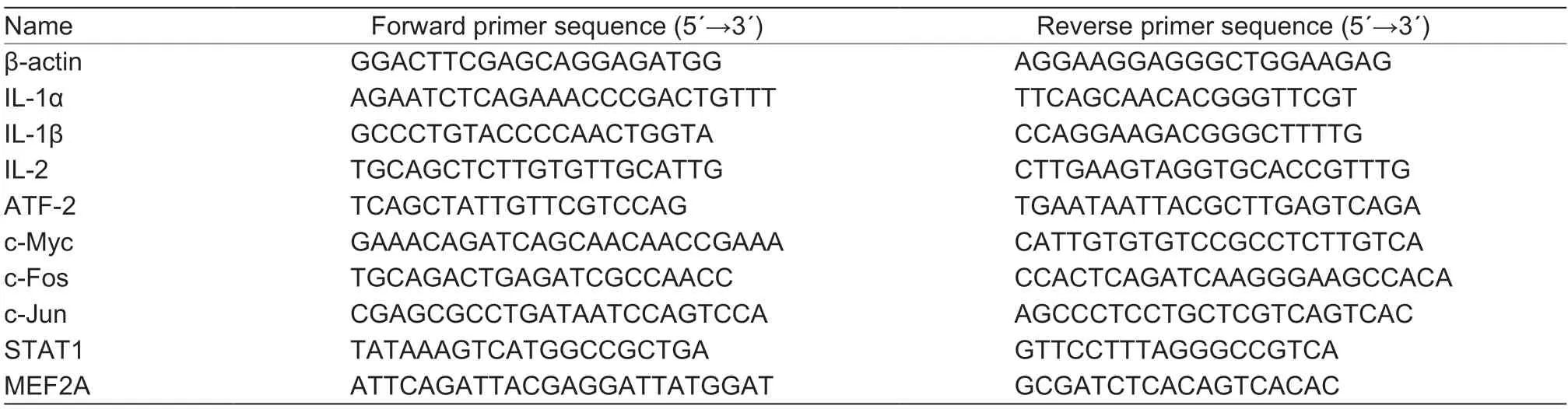
Table 1 Primer sequences used in real-time PCR1)
2.9.Statistical analysis
The data are presented as the mean±standard deviation and analyzed using SPSS 22.0 Statistical Software (IBM,NY,USA). Statistical significance of the data was compared by one way analysis of variance (ANOVA),withP<0.05 considered statistically significant.
3.Results
3.1.Cytotoxicity and antiviral activities of kaempferol and acyclovir
The cytotoxicity of different concentrations of ethanol and DMSO on the PK-15 cells was tested,since kaempferol was dissolved in ethanol and acyclovir was dissolved in DMSO. The results indicated that when the final concentrations of ethanol and DMSO were below 1%,there was no cytotoxicity or anti-PRV activity on the PK-15 cells (data not shown). Therefore,throughout the tests,the concentrations of ethanol or DMSO were no more than 1%. There was no toxicity to the PK-15 cells when the concentration of kaempferol was less than 104.8 μmol L-1and the CC50was 254.97 μmol L-1. The CC50of acyclovir was 348.85 μmol L-1. Kaempferol at the concentration of 52.4 μmol L-1could inhibit PRV-induced cell death by 89.7% (Fig.1). In contrast,the inhibition rate of acyclovir could reach 90% when the concentration was 113.23 μmol L-1(Fig.1). The IC50of kaempferol was 25.57 μmol L-1,which had a selectivity index (SI) of 9.97. The IC50of acyclovir was 54.97 μmol L-1,with an SI of 6.34 (Table 2).

Fig.1 The inhibition rates of different concentrations of kaempferol and acyclovir against Pseudorabies virus (PRV). The PK-15 monolayers in 96-well plates were infected with 200 TCID50 (50% tissue culture infective dose) PRV per well,followed by the treatment with kaempferol or acyclovir for 48 h. The cell viability was determined by the CCK-8 method. The inhibition rate was determined as percent of cell viability in treated cells compared with untreated cells. Data are mean±SD. The different letters on the columns show those which differ significantly (P<0.05).

Table 2 Antiviral activities of kaempferol and acyclovir against PRV in the PK-15 cells
In order to test whether kaempferol could also show anti-PRV activity when the initial amount of infectious virus was increased,the PK-15 cells were infected with PRV at the MOI of 1 in each well in the presence or absence of kaempferol or acyclovir. Kaempferol of concentrations ranging from 52.40 to 13.10 μmol L-1could significantly inhibit PRV replication. When the kaempferol concentration was 52.40 μmol L-1,the viral DNA copies were inhibited by above 50-fold in comparison with the untreated group. In contrast,acyclovir could reduce the viral DNA copies by about 6-fold at a concentration of 113.23 μmol L-1(Fig.2). Kaempferol exhibited a higher level of anti-PRV activity than acyclovir when the input virus was increased to MOI=1.

Fig.2 The DNA copies of Pseudorabies virus (PRV)-infected cells treated with different concentrations of kaemepferol and acyclovir.The PK-15 cells were infected with MOI=1 PRV,followed by the treatment with kaemepferol or acyclovir for 24 h. The viral DNA copies were determined by fluorescent quantitative PCR. Control,the PRV-infected group without any treatment. Data are mean±SD. ***,P<0.001;**,P<0.01;*,P<0.05 vs. control.
3.2.Mode of action
In order to elucidate the action mode of kaempferol,five independent experiments were performed at different phases during one viral life cycle. In antiviral tests,kaempferol and acyclovir were sometimes directly incubated with the virus during infection. Inactivation was conducted to evaluate whether the observed antiviral effects were due to their inactivation,but no activity was detected (Fig.3-A and F). In pretreatment assays,the drug was incubated with cells prior to virus infection,which was designed to determine whether the kaempferol could bind to virus receptors on the cell surface involved in the initial virus entry. However,neither kaempferol nor acyclovir exhibited inhibitory activity (Fig.3-B and G). During PRV adsorption,kaempferol and acyclovir were simultaneously incubated with PRV. The results showed that kaempferol and acyclovir could not inhibit virus adsorption (Fig.3-C and H). After virus attachment to the cell surface,penetration is triggered. Kaempferol could inhibit PRV penetration and the viral DNA copies were reduced by almost 4-fold (Fig.3-I),while acyclovir exhibited no activity (Fig.3-D). When the virus achieved penetration into cells,virus replication begins by using host resources. The results showed that both kaempferol (Fig.3-J) and acyclovir (Fig.3-E) could significantly suppress PRV replication in a dose-dependent manner. After treatment with kaempferol (52.40 μmol L-1) or acyclovir (113.23 μmol L-1),the virus copies were reduced by almost 30-and 3-fold,respectively,in comparison with the untreated group.

Fig.3 The number of DNA copies of Pseudorabies virus (PRV) at different stages of infection in cells treated with kaempferol and acyclovir,respectively. A,inhibitory effects of acyclovir on PRV inactivation. B,pre-treatment effects of acyclovir on PRV infection. C,inhibitory effects of acyclovir on PRV absorption. D,inhibitory effects of acyclovir on PRV penetration. E,inhibitory effects of acyclovir on PRV replication. F,inhibitory effects of kaempferol on PRV inactivation. G,pre-treatment effects of kaempferol on PRV infection. H,inhibitory effects of kaempferol on PRV absorption. I,inhibitory effects of kaempferol on PRV penetration. J,inhibitory effects of kaempferol on PRV replication. Control,the PRV-infected group without any treatment. Data are mean±SD. ***,P<0.001;*,P<0.05 vs. control.
3.3.Time of addition
Kaempferol was added at different times post infection in order to test the anti-PRV potency of kaempferol after PRV entry into cells. As shown in Fig.4,compared with the control group,kaempferol added within 16 hpi could significantly inhibit PRV reproduction and the number of viral DNA copies increased gradually from 2 to 16 hpi.Compared with the control group,the number of virus copies was decreased by almost 15-fold when kaempferol was added at 2 hpi.

Fig.4 Anti-Pseudorabies virus (PRV) activity of kaempferol at different addition times. PK-15 cells were infected with 200 TCID50 (50% tissue culture infective dose) PRV mL-1. Then,kaempferol (52.40 μmol L-1) was added at either 2,4,8 or 16 h post infection (hpi). The number of viral DNA copies of each sample was tested by FQ-PCR. Control,the PRV-infected group without any treatment. Data are mean±SD. ***,P<0.001 vs. control.
3.4.Effect of kaempferol on PRV-activated cell signaling pathways
Virus infection always induces changes in the cell signaling pathways to regulate immunity and apoptosis (Lee and Kleiboeker 2005;Ludwig 2010). NF-κB and MAPKs signaling pathways mediate many pathological and physiological processes including viral infection (Yehet al.2008;Gao 2014;Hakimet al.2018). Therefore,the effects of kaempferol on the classical signaling pathways (NF-κB and MAPKs) involved in virus infection in PRV-infected cells were test (Fig.5). At 4 dpi,compared with blank control,the expressions of P65,p-P65,ERK1/2,p-ERK1/2,P38,p-P38 and p-ERK1/2/ERK1/2 were significantly increased,and the expressions of p-P65/P65 and p-P38/P38 were significantly decreased in PRV-infected cells without treatment.In comparison with the infected-untreated group,expressions of P65,p-ERK1/2,P38,p-P38,p-P65/P65 and p-P38/P38 were significantly enhanced and the expression of p-ERK1/2/ERK1/2 was significantly reduced in PRV-infected cells with kaempferol treatment. At 8 hpi,PRV infection decreased the expressions of P65,p-P65,ERK1/2,p-ERK1/2,P38,p-P38,p-P65/P65,p-ERK1/2/ERK1/2 and p-P38/P38 when compared with the blank control. After kaempferol treatment,the expressions of P65,p-P65 and p-P65/P65 were decreased and the expressions of ERK1/2,p-ERK1/2,P38,p-P38,p-ERK1/2/ERK1/2 and p-P38/P38 were significantly increased in PRVinfected cells. At 12 hpi,compared with blank control,PRV infection significantly up-regulated the expressions of P38,p-P38 and p-P38/P38 and significantly down-regulated the expressions of P65,p-P65,ERK1/2,p-ERK1/2,p-P65/P65 and p-ERK1/2/ERK1/2,while kaempferol treatment significantly up-regulated the expressions of P65,p-P65,ERK1/2,p-ERK1/2,P38,p-P38,p-P65/P65,p-ERK1/2/ERK1/2 and p-P38/P38.
3.5.Effect of kaempferol on PRV-induced antiviral gene expression
Based on the results of the PRV-activated cell signaling pathways,the effects of kaempferol on the target genes of the signaling pathways were determined (Fig.6). PRV infection inhibited the activation of P65,while the activation of P65 was enhanced after kaempferol treatment. As the target gens of P65,the expressions of IL-1α,IL-1β and IL-2 were tested. The results indicated that PRV infection significantly up-regulated the expressions of IL-1α,IL-1β and IL-2. After kaempferol treatment,the levels of IL-1β and IL-2 were significantly decreased. However,the expression of IL-1α in PRV-infected cells was significantly increased by kaempferol treatment. PRV infection inhibited the activation of ERK1/2 and enhanced the activation of P38;and after kaempferol treatment,the activation of ERK1/2 and P38 were up-regulated. As the common target genes of ERK1/2 and P38,the expressions of ATF-2,c-Fos,c-Jun,c-Myc and STAT1 were determined. As shown in Fig.6,PRV infection significantly increased the expression levels of ATF-2,c-Fos,c-Jun and c-Myc,and the level of STAT1 was slightly increased. After kaempferol treatment,the expression levels of ATF-2 and c-Jun were decreased significantly,while the levels of c-Fos,c-Myc and STAT1 were augmented significantly. MEF2A is the target gene of P38. PRV infection significantly down-regulated the expression level of MEF2A;and after kaempferol treatment,the level of MEF2A was dramatically decreased.

Fig.6 Effect of kaempferol on gene expressions at 12 h post infection (hpi). The PK-15 cells were infected with or without MOI=1 PRV in the presence or absence of kaempferol (52.40 μmol L-1). The mRNA of each sample was extracted and was subjected to real-time PCR to test the expressions of the target genes of NF-κB (IL-1α,IL-1β and IL-2) and MAPKs (ATF-2,c-Fos,c-Jun,c-Myc,STAT1 and MEF2A) signaling pathways. Data are mean±SD. The different letters on the columns show those which differ significantly (P<0.05).
4.Discussion
PRV infection is a constant threat in many countries (Huet al.2015;Sunet al.2016),especially when emerging PRV variants occur. However,there is no specific medicine to control PRV infection and existing vaccines cannot provide full protection against the PRV variants (Anet al.2013). In this study,the natural compound kaempferol demonstrated potent anti-PRV activities though regulation of the NF-κB and MAPKs signaling pathways,exhibiting potential as a medicine for PRV infection control.
CC50and IC50,two indexes commonly used to evaluate the antiviral activity of compounds (Zhaoet al.2017),were tested in the current study. The CC50of kaempferol was lower than that of acyclovir,indicating that acyclovir had a concentration that was safer to PK-15 cells than kaempferol.The IC50and SI of kaempferol were higher than those of acyclovir,which suggested that kaempferol exhibited a higher anti-PRV activity on the PK-15 cells than acyclovir. Further confirmation of kaempferol’s anti-PRV efficacy was that it decreased virus reproduction by an order of magnitude after an intial increase in the amount of infectious virus.
The process of PRV infection is just like that of herpesvirus infection,which is initiated by the attachment of virions to the target cells and fusion of the virion envelope with the cellular cytoplasmic membrane (Mettenleiteret al.1994). The PRV attachment is made by the interaction of virion gC with heparin sulfate proteoglycans at the cell surface,which forms a primary,relatively labile interaction,followed by the interaction of gD with its cellular receptor which mediates the conversion of the labile interaction into the stable binding (Karger and Mettenleiter 1993). A tight contact is needed between the cellular cytoplasmic membranes and the viral envelop to complete their fusion,which requires at least four viral glycoproteins:gB,gH,gL and gD (Mettenleiteret al.1994). Nucleocapsid is translocated into the cytoplasm of the cell,then transported to the nuclear membrane and located next to the nuclear pores. Then a cascade-like process starts with the expression of the only one immediate-early gene to regulate PRV transcription (Cheunget al.1989). In the present study,the ability of kaempferol to reduce virus titer at different infection stages was tested. The results confirmed that kaempferol has the ability to decrease the virus titer,especially at the penetration and replication stages,which suggests that kaempferol could inhibit the functions of gB,gH,gL or gD (Mettenleiteret al.1994) and the cascade-like process of PRV transcription (Mettenleiteret al.1999). In contrast,acyclovir only inhibited the replication stage of PRV. Previous studies implied that acyclovir is targeted at thymidine kinase,whose activity could be activated by an HSV-or VZV-specified TK (Elionet al.1983). Besides,acyclovir is similar to deoxynucleoside triphosphate and has the ability to compete with deoxyguanosine triphosphate for the viral DNA polymerase (Elionet al.1983),which is why the anti-PRV activity of acyclovir mainly occurrs at the replication stage. The antiviral activity of kaempferol was dramatically higher than that of acyclovir in the inhibitory action study,which is consistent with the values of IC50and SI.
NF-κB and MAPKs signaling pathways take part in innate immunity (Riteauet al.2006;Gaoet al.2014),which plays an important role in virus infection (Xuet al.2019). The NF-κB signaling pathway was activated by PRV infection at 4 hpi by a luciferase reporter assay (Changet al.2013). In the present study,at the early stage of the PRV infectious cycle at 4 hpi (Changet al.2013),the expression levels of P65 and p-P65 were increased significantly. However,at 8 and 12 hpi,the levels of P65 and p-P65 were decreased significantly. Besides,after kaempferol treatment,the expression levels of P65 were increased significantly,which indicated that NF-κB mediates PRV-induced immunity,but kaempferol was not able to inhibit the activation of NF-κB. Virus infection changes the expression of ERK1/2. Previous studies have implied that Kaposi’s sarcoma-associated herpesvirus induced the expression of ERK1/2 during the early stage of infection and the activation probably allowed the virus to overcome the restriction on viral gene transcription imposed by the host cell and facilitated the viral gene expression (Sharmaet al.2005). It was reported that PRV infection activated ERK1/2 (Zhaoet al.2017). In the present study,ERK1/2 was activated by PRV infection at the early stage of infection (4 hpi) but inhibited at 8 and 12 hpi;kaempferol treatment was able to recover the level of ERK1/2 which was changed by PRV infection. The activation of P38 signaling takes part in PRV-induced apoptosis (Yehet al.2008). Compared with the uninfected control cells,the expression levels of P38 and p-P38 were enhanced after PRV infection at 4 and 12 hpi,while they decreased at 8 hpi. The levels of P38 and p-P38 which changed by PRV infection were recovered after kaempferol treatment. These results suggested that the anti-PRV activity of kaempferol on the PK-15 cells is generated by regulating MAPKs rather than NF-κB. Based on a previous study (Cheunget al.2000),the PRV-induced apoptosis was clarified. NF-κB and MAPKs signaling pathways also mediate cell apoptosis (Goodkinet al.2003;Changet al.2013;Sunet al.2015),which may be one of the reasons that PRV induced the changes of the NF-κB and MAPKs signaling pathways.
Pro-inflammatory cytokines are always increased by virus infection (Kidoet al.2015). In this study,the expressions of IL-1α (Mori and Prager 1996),IL-1β (Hiscottet al.1993) and IL-2 (Hoyoset al.1989;Serflinget al.1989),the target genes of NF-κB,were determined to further confirm whether the NF-κB signaling pathway was regulated by kaempferol. The results implied that the levels of IL-1α,IL-1β and IL-2 were up-regulated and kaempferol treatment significantly downregulated the levels of IL-1β and IL-2,which is consistent with a previous study (Zhaoet al.2017). Virus infection always changes the target genes of the MAPKs signaling pathway (Sharmaet al.2005). In this study,the expressions of c-Jun,c-Fos,ATF-2,MEF2A,c-Myc and STAT1,the target genes of MAPKs signaling pathway (Sharmaet al.2005),were changed by PRV infection,which is consistent with a previous study (Adamsonet al.2000). Kaempferol treatment significantly decreased the expressions of ATF-2 and c-Jun which were increased by PRV infection,but it did not recover the expressions of c-Fos,MEF2A,c-Myc or STAT1. The c-Jun,the most extensively studied protein of the activator protein-1 (AP-1) complex,takes part in various cellular processes,including proliferation,apoptosis,survival,tumorigenesis and tissue morphogenesis (Meng and Xia 2011). In this study,the expression of c-Jun was increased by PRV infection,which could possibly be due to c-Jun mediating the apoptosis of cells induced by PRV infection.
Studies on the anti-cancer activity of kaempferol revealed that it exerts protective effects for non-mutated cells and triggers apoptosis for mutated cells (Imranet al.2019b). In this study,the regulatory effects of kaempferol on normal cells and PRV-infected cells were also different. Based on the innate immunity and anti-apoptosis effects of the NF-κB and MAPKs signaling pathways,kaempferol exhibited anti-PRV activity by regulating innate immunity and protecting cells from PRV-induced apoptosis,which is probably attributed to its antioxidant effects on the inhibition of ROS generation and lipid peroxidation,and thus protection of the cells from damage (Salehiet al.2018).
5.Conclusion
Kaempferol exhibited anti-PRV activity in PK-15 cells,which is greater than that of acyclovir. Kaempferol mainly inhibited PRV replication through regulating the NF-κB and MAPKs signaling pathways and their target genes. Further studies should be conducted to evaluate the antiviral activity of kaempferol against PRVin vivo.
Acknowledgements
This work was supported by the Program of Sichuan Veterinary Medicine and Drug Innovation Group of China Agricultural Research System (SCCXTD-2020-18) and the Science and Technology Project of Sichuan Province,China (2018NZ0043,2018NZ0064 and 2018HH0076).
Declaration of competing interest
The authors declare that they have no conflict of interest.
杂志排行
Journal of Integrative Agriculture的其它文章
- Adoption of small-scale irrigation technologies and its impact on land productivity:Evidence from Rwanda
- African swine fever and meat prices fluctuation:An empirical study in China based on TVP-VAR model
- The impacts of oxytetracycline on humification during manure composting can be alleviated by adjusting initial moisture contents as illustrated by NMR
- Effects of long-term straw incorporation on nematode community composition and metabolic footprint in a rice-wheat cropping system
- Functional diversity of soil microbial communities in response to supplementing 50% of the mineral N fertilizer with organic fertilizer in an oat field
- Nitrogen acquisition,fixation and transfer in maize/alfalfa intercrops are increased through root contact and morphological responses to interspecies competition
