Carbon quantum dots:Synthesis and correlation of luminescence behavior with microstructure
2021-06-18FANGLiyangZHENGJingtang
FANG Li-yang, ZHENG Jing-tang
(1.Shandong Chemical Engineering & Vocational College, Weifang 261100, China;2.State Key Laboratory of Heavy Oil Processing, School of Chemical Engineering, China University of Petroleum, Qingdao 266580, China)
Abstract:Two kinds of carbon quantum dots,C-dots-160 and C-dots-200 with different fluorescence luminescence behaviors were synthesized by a one-step hydrothermal method at 160 and 200 °C,respectively,using ammonium citrate as the raw material.The relationship between the microstructure of the C-dots and the fluorescence emission behavior was investigated.Results indicate that an increase of synthesis temperature introduces more oxygen and nitrogen atoms into the C-dots,increasing the total number of structural defects and altering their concentation ratio .It is this ratio difference in the two C-dots that causes their different luminescence behaviors.The proportion of several types of defects in the C-dots-200 are relatively balanced,leading to excitation wavelength-dependent fluorescence while the most abundant defects in C-dots-160 are in the form of C=O,which is the main reason for its excitation independent luminescence behavior.The number of structural defects in C-dots-160 is less than in C-dots-200 and the latter has the stronger fluorescence emission.
Key words:Carbon quantum dots;Structural defects;Excitation-independent fluorescence;Excitation-dependent fluorescence
1 Introduction
Carbon quantum dots (C-dots) have been a research hotspot as a member of the carbon nanomaterial family in recent years.Because of their low or no toxicity,good biocompatibility,strong stability,measurability,fluorescence tunability,and excellent photoelectric properties[1,2],C-dots are widely used in biological imaging,biochemical analysis,fluorescence sensing and photocatalysis[3–7].However,the further applications have been impeded by lack of study on the luminescence mechanism of C-dots.At present,there is no unanimous view on the mechanism of fluorescence emission of C-dots,although it has been explained by some theories such as quantum effect[3],surface defects[1]and exciton recombination[8].Therefore,by studying the influence of various chemical states on the fluorescence emission of C-dots and deeply discussing their fluorescence emission characteristics,we can better understand the fluorescence emission mechanism and regulate the fluorescence performance of C-dots,which is very important to further develop their applications.
In general,the introduction of oxygen,nitrogen and other hetero elements into the sp2hybrid carbon skeleton of C-dots will form oxygen-containing,nitrogen-containing and other groups.The chemical states such as C—O,C—N,C=O and N—H all belong to the sp3hybrid structure,so these sp3defects can also be called structural defects.Li et al.[9]believed that different chemical states would introduce different energy levels into C-dots,and different electronic transitions would be generated during photoexcitation,which could cause C-dots to emit light that changed with excitation.C-dots have two types of fluorescence emission characteristics.One is that under different excitation wavelengths,the position of its maximum emission wavelength is red-shifted,which is called excitation wavelength-dependent fluorescence.The excitation wavelength-dependent fluorescence is a common phenomenon that can be observed in fluorescent carbon-based nanomaterials[10].Another type is that under different excitation wavelengths,the position of its maximum emission wavelength is basically unchanged,which is called excitation wavelength-independent fluorescence.The excitation wavelength dependence or independence of C-dots is one of the most attractive characteristics of its fluorescence emission.As for the characteristics of the excitation dependence of C-dots,Zhao et al.[11]explained that it was caused by the different sizes of C-dots.Baker et al.[12]believed that the main reason for the wavelenth-dependent fluorescence was the excitation of different emission sites under different wavelengths.However,there are very few reports aiming at the reasons for the generation of excitation wavelength-independent fluorescence,especially the phenomenon that only changing the synthesis temperature of the C-dots with the same raw materials can cause different luminescence behavior of the C-dots.
In this work,the C-dots were synthesized by a one-step hydrothermal method from ammonium citrate at 160 and 200 ºC.The physical and chemical structures and optical properties of the as-prepared Cdots were investigated by means of transmission electron microscopy (TEM),Fourier transform infrared spectroscopy (FTIR),X-ray photoelectron spectroscopy (XPS),ultraviolet visible (UV-Vis) absorption spectroscopy and fluorescence spectroscopy,so as to correlate the microstructures of C-dots to the fluorescence emission behavior.The experimental results show that the as-synthesized C-dots at the lower temperature (160 ºC) exhibit excitation wavelength-independent fluorescence,while the as-synthesized C-dots at the higher temperature (200 ºC) possess excitation wavelength-dependent fluorescence.By comparing the influence of the contents of different chemical states on the luminescence behavior of the C-dots,the reason of different luminescence behaviors is analyzed,which can provide a theoretical basis for controllable synthesis of highly efficient fluorescent Cdots.Due to their different contents and proportions of the chemical states,the as-synthesized C-dots under the two temperatures have different luminescence behaviors.At the same time,the as-prepared C-dots at the higher temperature have strong fluorescence because of their more structural defects,especially nitrogen-containing defects.Through further investigation,it is also found that the as-synthesized C-dots at the higher temperature have very good stability.Fig.1 shows a schematic diagram of the research route of this article.
2 Experimental
Ammonium citrate was used without further purification.Ammonium citrate (3 g) was dissolved in 60 mL deionized water to form a transparent solution.The solution was transferred to an 80 mL Teflon-lined stainless steel autoclave and heated at 160 ºC for 4 h,and then cooled down to room temperature.The sample was purified by centrifugation and dialysis.The resulting sample was then concentrated and dried.Another sample was synthesized following the same procedure above except that the hydrothermal treatment temperature was 200 ºC.The two nitrogendoped carbon quantum dots were recorded as CDs-160 and CDs-200.
3 Results and discussion
3.1 Structure characterization of C-dots
The morphology and size of C-dots were investigated by TEM and HRTEM.The TEM images of CDs-160 and CDs-200 (Fig.2a,b) show that the sizes of C-dots are narrowly distributed.CDs-160 (Fig.2c)has a particle size distribution of 1.5−3.5 nm and an average diameter of 2.5 nm and CDs-200 (Fig.2d) has a particle size distribution of 2−4 nm and an average diameter of 3 nm.The HRTEM images of CDs-160 and CDs-200 (insets in Fig.2a and 2b) both show relatively blurred lattice fringes,indicating that their structure may be mainly amorphous with a small amount of the crystalline graphite structure in the amorphous structure,which is a hybrid structure.The lattice fringes of CDs-200 are slightly clearer than those of CDs-160,which may be caused by a larger fraction of the graphite structure.
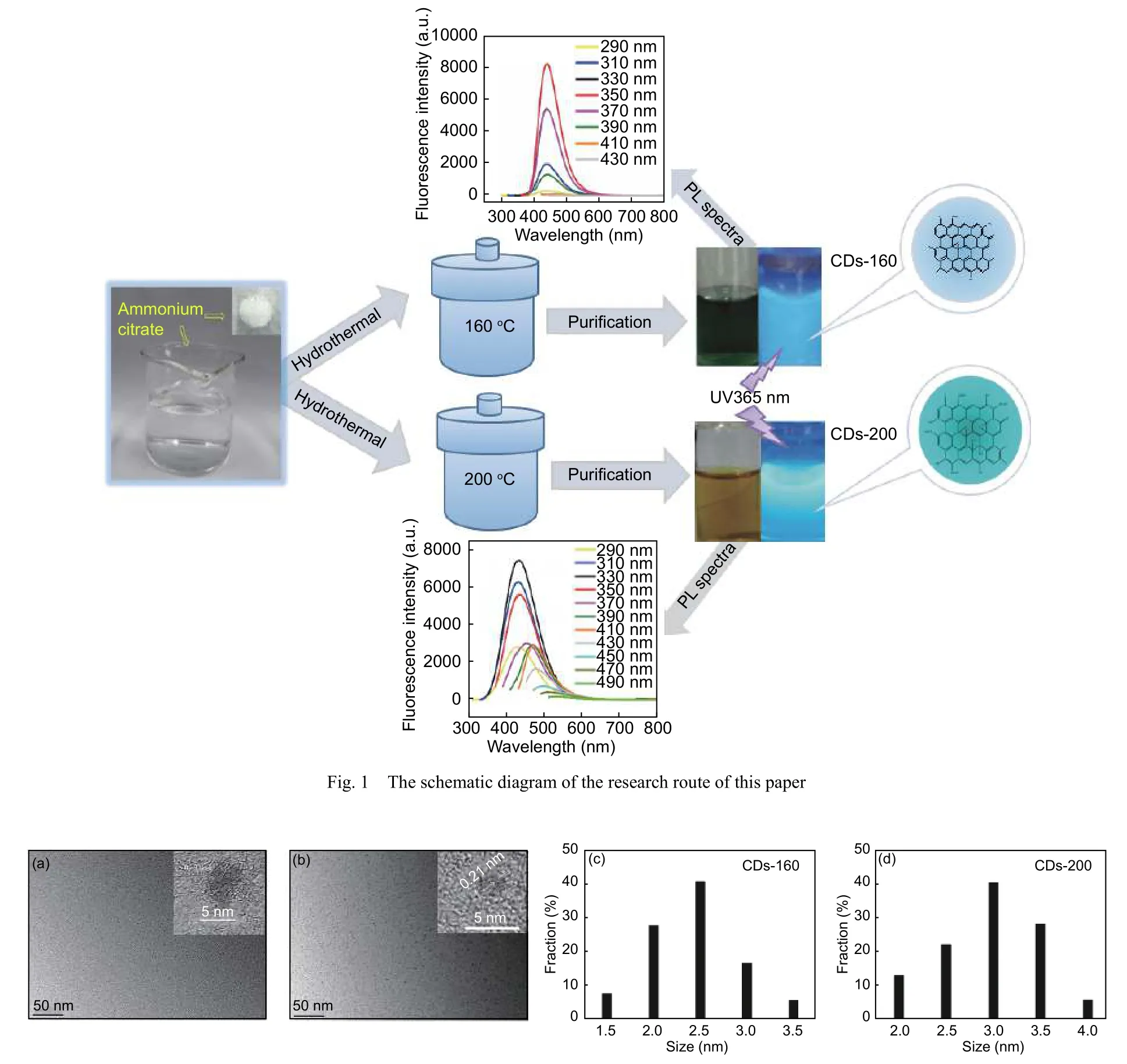
Fig.2 (a,b) TEM images,insert:HRTEM images of CDs-160 and CDs-200.The particle size distributions of (c) CDs-160 and (d) CDs-200.
The surface structure and composition of C-dots were characterized by FT-IR and XPS.The FT-IR spectra of CDs-160 and CDs-200 are shown in Fig.3a.It can be found that the infrared characteristic absorption peaks[13]are obviously stronger in CDs-200 than in CDs-160,including the stretching vibration peak of —OH at 3 218 cm−1,the stretching vibration peak of C=O of the amide I at 1 732 cm−1,and the inplane bending vibration peak of the N—H of the amide II band at 1 560 cm−1,stretching vibration peak of C—N of amide III band at 1 410 cm−1,and vibration peak of C=N/C—N at 1 201 cm−1and so on.It indicates that it is easier to form more nitrogen functional groups and oxygen functional groups in the Cdots synthesized at the higher temperature because the higher temperature makes it easier to introduce N,O and other elements to form more sp3hybrid structures in the sp2hybrid structure carbon skeleton during the formation process of the C-dots.The element composition and the content of various chemical states in the as-synthesized C-dots were further investigated by XPS characterization.Both XPS survey spectra of CDs-160 and CDs-200 (Fig.3b) show that they contain three main peaks:C 1s (around 284.8 eV),N 1s(around 400.4 eV) and O 1s (around 532.1 eV).It is obvious that the contents of N and O elements of the latter are higher than those of the former,especially the N content,indicating that more heteroatoms can be introduced at the higher temperature.This is consistent with the FT-IR characterization results.Fig.3c and 3d are the high-resolution C 1s spectra of CDs-160 and CDs-200,respectively.It can be seen that there are four chemical states of C element at different peak positions:C—C/C=C near 284.4 eV,C—N near 285.2 eV,C—O near 286.3 eV and C=O near 288.4 eV[8].The contents of different chemical states calculated by the peak area are as follows.CDs-160 has a C—C/C=C ratio of 56.12%,a C—N content of 5.75%,a C—O content of 6.77% and a C=O content of 31.36%.CDs-200 has a C—C/C=C ratio of 32.22%,a C—N content of 31.23%,a C—O content of 15.12% and a C=O content of 21.43%.
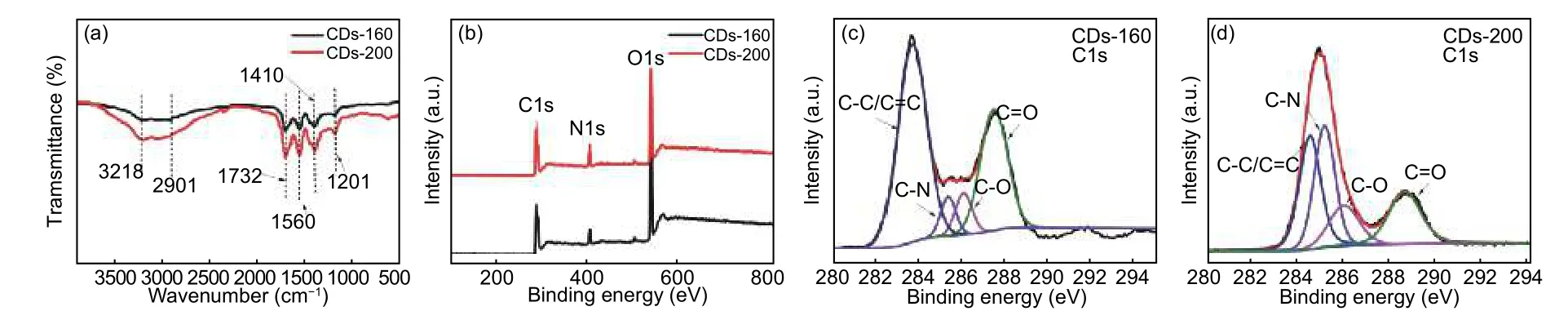
Fig.3 (a) FT-IR spectra,(b) XPS survey spectra and C1s spectra of (c) CDs-160 and (d) CDs-200.
By comparing the above data,it can be found that for the ratio of C—C/C=C,CDs-200 is significantly lower than CDs-160.In addition to the ratio of C—C/C=C,the sum of C—O,C—N and C=O contents,the remaining three chemical states (structural defects),is higher in CDs-200 than in CDs-160,which indicates that the amount of heteroatoms introduced into CDs-160 is relatively lower,and more sp3hybrid structure can be formed at a higher temperature.The changing trends of these three sp3hybrid structure defects are obviously different,among which the content of C-N in CDs-200 is much higher than that in CDs-160,demonstrating that a higher temperature is more conducive to the formation of nitrogen-containing groups.
3.2 Optical properties of C-dots
The photographs of CDs-160 and CDs-200 aqueous solutions are shown in the insets of Fig.4c and 4d,respectively.Under natural light conditions,CDs-160 is a peacock green transparent liquid while CDs-200 is a yellow transparent liquid.Both of them show blue fluorescence under the irradiation of a 365 nm ultraviolet lamp,but CDs-200 looks brighter than CDs-160.In the UV-Vis absorption spectra of CDs-160 and CDs-200 (Fig.4a),they exhibit characteristic absorption around 200 nm due to the π→π*transition of the aromatic sp2domain (C=C).In addition,an absorption peak at about 330 nm can also be observed,which is attributed to the n→π* transition of the sp3hybrid domain (C=O)[14].Notably,the n→π* transition of the C=O peak of CDs-160 shows a very strong symmetrical absorption peak,but that of CDs-200 shows a very weak peak,which implies that in the as-prepared C-dots at two synthesis temperatures,the proportion of C=O in the total groups of CDs-160 is much higher than that of CDs-200,which is consistent with the XPS results.Fig.4b is the best emission of the photoluminescence (PL) spectra of CDs-160 and CDs-200.It can be seen from the spectra that the fluorescence intensity of CDs-200 is much higher than that of CDs-160,indicating that the fluorescence enhancement is realized by increasing the synthesis temperature.According to the FTIR and XPS characterization in the previous section,the total content of the three chemical states C—O,C=O and C—N as structural defects in CDs-200 (67.78%) is significantly higher than that in CDs-160 (43.88%),suggesting that the fluorescence enhancement is associated with the increase of the amount of structural defects.In particular,nitrogen-containing defects enhance the fluorescence emission more effectively than other structural defects[15].For the N defect content of the as-prepared C-dots,it increases from 5.75% of CDs-160 to 31.23% of CDs-200,resulting in the stronger fluorescence emission of the latter than that of the former.

Fig.4 (a) UV–Vis absorption spectra of CDs-160 and CDs-200; (b) Optimal emission of PL spectra of CDs-160 and CDs-200; PL spectra at different excitation wavelengths of (c) CDs-160 and (d) CDs-200.
Fig.4c and 4d show the PL emission spectra of CDs-160 and CDs-200,respectively.In Fig.4c,when the excitation wavelength of CDs-160 increases from 290 to 430 nm,the emission wavelength is always concentrated near 439 nm,and the maximum emission wavelength does not change with the excitation wavelength,showing the excitation independent luminescence behavior.In Fig.4d,the maximum emission wavelength of CDs-200 shifts from 420 to 490 nm as the excitation wavelength increases from 290 to 490 nm,and the fluorescence intensity first increases and then decreases,exhibiting the excitation-dependent luminescence behavior.
The reason for the different luminescence behaviors of the two kinds of the as-prepared C-dots may be related to the proportion of different types of luminescent groups in the carbon nanomaterials.In the process of the formation and growth of C-dots,the carbon nucleus generally begins to form when a certain temperature is reached,and then gradually grows up with the increase of temperature,along with a process that the heteroatom groups of C-dots first increase and then decrease again under the very high synthesis temperature[13].According to the previous FTIR and XPS characterization results,in the two kinds of the as-prepared C-dots,the total amount of the various chemical states of CDs-200 is significantly larger than that of CDs-160,and the content of each chemical state has its own characteristics.The specific data show that the ratio of C—C/C=C as a sp2structure,which can form the carbon skeleton in the C-dots,has decreased from 56.12% of CDs-160 to 32.22% of CDs-200,showing that the as-synthesized C-dots at lower temperature have a higher ratio of C—C/C=C to form carbon skeleton,and the amount of structural defects is very small.Accordingly,the total content of C—O,C=O and C—N increases from 43.88% of CDs-160 to 67.78% of CDs-200,indicating that the total number of structural defects increases obviously in the as-prepared C-dots with the increase of synthesis temperature.By calculation,the ratio of the three chemical states of C—N,C—O,C=O is approximately 2.1∶1∶1.4 for CDs-200,the ratio of C—N,C—O,C=O is about 1∶1.1∶5.5 for CDs-160.It can be seen that CDs-200 has a large amount of structural defects and its proportion of different types is relatively balanced,which leads to the superposition of various types of fluorescent substances,so that it has composite fluorescence.However,for CDs-160,due to the small amount of structural defects,and the content of a certain type of structural defects (such as C=O) in the total structural defects is very high (this can also be confirmed from the UV spectrum),the contribution of C=O to the fluorescence emission in CDs-160 is absolutely dominant,and the contribution of other structural defects to the emission is almost negligible,leading to the concentration of PL wavelength near 439 nm on the surface,thus showing a single fluorescence that does not change with the excitation wavelength.
Based on all the above discussion,by the characterization of physical properties of the two types of asprepared C-dots,it is obvious that the morphology and size are not the main factors affecting the optical properties of the C-dots in a certain range because their shape and size are basically similar.At the same time,further study is also needed to determine whether the change of graphitization structure of C-dots affects its optical performance.However,from the characterization results of chemical properties of the two types of as-prepared C-dots,the content and type of structural defects obviously affect the fluorescence intensity and luminescence behavior.It can be concluded that a higher synthesis temperature can introduce more O,N heteroatoms into the C-dots to form more structural defects,especially nitrogen defects,resulting in stronger fluorescence intensity of the asprepared C-dots at a higher temperature.In particular,the C-dots at different synthesis temperatures have different PL behaviors due to the different ratios of structural defects.
3.3 Stability of CDs-200
CDs-200 with strong fluorescence was selected as the research object to further investigate its stability.The effects of external factors on the fluorescence performance of CDs-200 under different conditions,including room temperature holding,continuous ultraviolet light irradiation,pH value and ionic strength,were studied.Fig.5 exhibits that the fluorescence intensity of CDs-200 has no significant change under continuous UV irradiation for 6 h (Fig.5a),at room temperature for 3 months (Fig.5b),in NaCl aqueous solutions of different concentrations (Fig.5c)and the PL emission intensity does not change much in the pH value range from 2 to 11 (Fig.5d),which shows that CDs-200 has good stability under various conditions.

Fig.5 (a) Effect of time under UV irradiation of 365 nm,(b) effect of time at room temperature,(c) effect of ionic strength (ionic strength were controlled by various concentrations of NaCl aqueous solution) and (d) effect of pH (the pH was adjusted with hydrochloric acid and sodium hydroxide) on fluorescence intensity of CDs-200.
4 Conclusion
Using the same raw material,two different types of C-dots with different fluorescence intensities and luminous behaviors can be obtained at different synthesis temperatures.Increasing the synthesis temperature can introduce more sp3hybrid structures into the sp2domains of the as-prepared C-dots,resulting in an increase in the total amount of structural defects in the C-dots at a high synthesis temperature.At the same time,it also changes the ratio of various structural defects,which in turn leads to different luminous behaviors.The C-dots obtained under a low temperature exhibit the excitation wavelength-independent fluorescence because the content of a certain type of structural defects is absolutely dominant.The C-dots synthesized under a high temperature exhibit the excitation wavelength-dependent fluorescence due to the relatively balanced ratio of various structural defects.At the same time,the increase of temperature increases structural defects of the as-synthesized C-dots,especially the nitrogen defects,resulting in its increased fluorescence intensity.These results provide a theoretical basis for the controllable synthesis of efficient fluorescent C-dots.In addition,the C-dots synthesized at a higher temperature have good water solubility,uniform particle size,stronger fluorescence and good stability,which can better meet various applications.
Acknowledgements
This work is financially supported by the Science Development Plan Project of Weifang City,Shandong Province,China (No.2018GX106).
杂志排行
新型炭材料的其它文章
- 碳纳米管对炭纤维/聚碳酸酯复合材料界面结合性能的影响
- Near-infrared emission carbon dots for bio-imaging applications
- Synthesis of size-controlled carbon microspheres from resorcinol/formaldehyde for high electrochemical performance
- The electrochemical behavior of nitrogen-doped carbon nanofibers derived from a polyacrylonitrile precursor in lithium sulfur batteries
- An interfacial self-assembly strategy to fabricate graphitic hollow porous carbon spheres for supercapacitor electrodes
- Boron and nitrogen co-doped carbon dots for boosting electrocatalytic oxygen reduction
