Fluorescent Sensor for Fluoride Ions Based on Perylene Diimide Tethered with Siloxane Moieties
2021-06-16ZHANGDanLIUJieSHIDongZHANGXinyiGAOBaoxiangFENGYakaiRENXiangkui
ZHANG Dan, LIU Jie, SHI Dong, ZHANG Xin-yi, GAO Bao-xiang, FENG Ya-kai, REN Xiang-kui*
(1. State Key Laboratory of Chemical Engineering, School of Chemical Engineering and Technology, Tianjin University, Tianjin 300350, China;2. Global Station for Soft Matter GI-CoRE, Hokkaido University, Kita-ku, Sapporo 001-0021, Japan;3. College of Chemistry and Environmental Science, Hebei University, Baoding 071002, China)
Abstract: A novel perylene diimide(PDI) derivative bearing siloxane moieties(PDI-TES) was synthesized as fluorescent sensor by simple one-step reaction with high yield. The crystal structure, self-assembly behavior and detection property of the compound were then elucidated via a combination of different experimental techniques such as small-angle X-Ray scattering, UV-Vis absorption spectra, photoluminescence spectra and dynamic light scattering experiments. The experimental results reveal that PDI-TES has good thermal stability and ordered crystal structure. Moreover, due to the cleavage of Si—O bonds, PDI-TES exhibits high selectivity and sensitivity to F-, with a detection limit as low as 1.58×10-6 mol/L. These excellent detection performances, in combination with the simple and low-cost synthesis method, make PDI-TES a practical fluorescent sensor for detection of F-.
Key words: perylene diimide; fluorescent sensor; siloxane; fluoride ions
1 Introduction
Perylene diimide derivatives(PDIs) are a kind of polycyclic aromatic hydrocarbon derivatives with strong π-π conjugated interaction. Due to the excellent thermal stability, optical and electronic properties, these n-type materials get more attention in a lot of fields during several decades, such as electron transport materials(ETMs)[1-2], singlet fission-based light harvesting applications[3], organic electron-transport materials[4-5]or fluorescent chemosensors[6]and imaging[7-8]. Meanwhile, the rigid planar geometry of molecules makes the self-assemble of liquid crystal[9-10]or crystal[11]structure possible. However, many of them show poor solubility, which restrict their further applications. The universal methods are to modify the imide position or the bay position. The incoming groups not only improve the solubility, but also modify the optical properties and self-assemble behaviors. Meanwhile, the specific modified groups can make a response to some molecules or ions according to different mechanisms, such as the detection of Fe3+or H+based on fluorescence resonance energy transfer[12], Hg2+based on the selective binding with thymine[13], CN-or F-based on the anion-π interactions[14].

Many works have been reported about the PDIs based on the cleavage of Si—O bond. For example, Baietal. fabricated a fluorescent sensor for F-by incorporating polyhedral oligosilsesquioxane(POSS) nanocage and PEO chain at amide positions of PDI[20]. Our group also synthesized a kind of S-heterocyclic annulated PDI with POSS groups attaching at the imide positions. In THF solution, the POSS units can suppress the aggregation of molecules[9]. Once adding TBAF, the F-can trigger the POSS groups cleavage, which causes the π-π stacking of PDI cores and the fluorescence quenched. Further, we synthesized a PDI derivative with special acetamide group at the bay position, which can response to the F-by dual mechanisms of POSS collapse and intermolecular proton[21]. Another work[25]about the detection of F-is to design an “off-on” luminescent polymer containing POSS and PDI. The degradation of POSS can block the process of photoinduced electron transfer, causing the fluorescent emission.
Although many F-sensor molecules have been reported, their synthesis methods require complex and harsh conditions, and often the yield is relatively low. Moreover, using POSS nanocage as triggering group may lead to a problem. The cleavage of Si—O bond is due to the nucleophilic attacking and coordination of F-with Si, and there are numerous Si—O bonds within a POSS nanocage. As a result, many F-are needed to trigger the POSS collapse, leading to higher detection limit. Therefore, it is of great interest to know whether it is possible to fabricate a PDI-based fluorescent sensor with low detection limit by simple and low-cost method.
Herein, a new PDI derivative(PDI-TES, Scheme 1) was designed and synthesized by introducing two siloxane substituents to PDI imide positions by simple one-step reaction with high yield. The synthetic route is shown in Scheme 1, all the reagents are cheap and easily available. The cleavage of Si—O bonds triggered by F-can induce the aggregation of PDI luminescence, endowing PDI-TES with turn-off fluorescent response and high selectivity to F-, with a detection limit as low as 1.58×10-6mol/L.

Scheme 1 Chemical structure of PDI-TES
2 Experiments
2.1 Chemicals
3,4,9,10-perylenetetracarboxylic dianhydride(Heowns,98%),3-aminopropyltriethoxysilane (Aladdin, 98%). Toluene is dried through molecular sieves and activated alumina and distilled through a standard procedure.
2.2 Instruments
1H NMR and13C NMR spectra were recorded using a Bruker Avance spectrophotometer(400 MHz for1H NMR, 100 MHz for13C NMR, 25 ℃). A trace amount of tetramethylsilane(TMS) was used as the internal standard for chemical shifts(δ). High resolution mass spectra(HRMS) were determined on an IonSpec 4.7 Tesla Fourier Transform Mass Spectrometer.
The X-ray scattering experiment was performed with a high-flux small-angle X-ray scattering instrument(SAXSess, Anton Paar) equipped with a Kratky block-collimation system. An imaging plate was used as the detector, which can measure the small-angle and wide-angle X-ray scattering of the sample simultaneously, covering theq-range from 0.06 to 29 nm-1(q=4πsinθ/λ, whereλis the wavelength of 0.154 2 nm and 2θis the scattering angle).
UV-Vis absorption spectra were performed with an Agilent Technologies Cary 300 UV-Vis spectrophotometer. Photoluminescence spectra were obtained on a Hitachi FL-2500 Fluorescence Spectrometer. The dynamic light scattering(DLS) experiments were performed by a zetasizer using a Malvern Nano ZS instrument. The TEM images were obtained on a JEOL JEM-1400 Flash instrument.
2.3 Synthesis of PDI-TES
15.00 g imidazole was added in a 100 mL round bottom flask, and was heated to 120 ℃ to melt imidazole into liquid. Then 0.50 g 3,4,9,10- perylenetetracarboxylic dianhydride(1.27 mmol) and 0.71 g 3-aminopropyltriethoxysilane(3.19 mmol) were added and was heated to 130 ℃, and continue to react for 3 h with reflux. When the reaction was over, stop stirring and cool to room temperature. The reaction solution was poured into 300 mL methanol and stirred for a period of time. Then, it was placed for laying so that imidazole could be fully dissolved in methanol to remove imidazole and the product could be precipitated. The precipitate was obtained by vacuum filtration and dried in a vacuum drying oven at 40 ℃ to remove methanol. Then perform column chromatography. The polarity of the eluent is dichloromethane: ethyl acetate =3∶1(v/v), the obtained product was dried overnight in a vacuum drying oven with yield 75% and was named PDI-TES. Using CDCl3as the solvent, the obtained product PDI-TES was characterized by1H NMR,13C NMR and high resolution mass spectra(HRMS), and the results were shown in Fig.1 and Fig.S1-S2.1H NMR(400 MHz, CDCl3),δ: (TMS, 10-6):8.55(4H, Ar—H), 8.44(4H, Ar—H), 4.25(4H, N—CH2), 3.85(12H, SiO—CH2), 1.90(4H, N—CH2CH2), 1.23(18H, O—CH2CH3), 0.78(4H, Si—CH2).13C NMR(100 MHz, CDCl3),δ: (TMS, 10-6): 163.23, 134.51, 131.27, 129.26, 126.30, 123.30, 122.96, 58.44, 43.08, 21.56, 18.31, 8.08. HRMS(MALDI(N), 100%)m/zcalcd for C42H50N2O10Si2: 798.30, found 798.30.
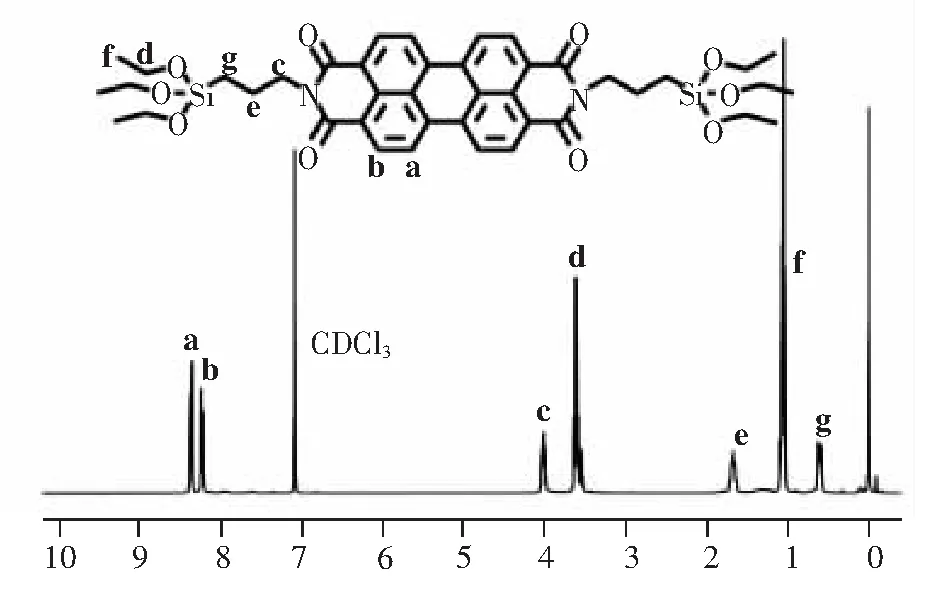
Fig.1 1H NMR of PDI-TES in CDCl3
3 Results and Discussion
3.1 Self-assembly and Photophysical Property
PDI-TES showed high solubility in many common organic solvents such as tetrahydrofuran (THF), dichloromethane(DCM) and toluene, but poor solubility in water. Therefore, the photophysical properties of PDI-TES in H2O/THF mixtures with different water fractions were performed to study its self-assembly behavior. As shown in Fig.2(a), the UV-Vis absorption spectra of PDI-TES exhibited a well-resolved vibration absorption band of 400-600 nm in THF solution. The maximum absorption occurred at about 515, 484, 426 nm, corresponding to S0-0, S0-1, and S0-2double electron transitions respectively, which was the characteristic of monomeric PDI chromophore. With addition of water, the absorption intensity decreased gradually, and a new peak appeared at 550 nm. These results indicated that PDI-TES began to aggregate with the increase of poor solvent content. The effect of poor solvent on molecular self-assembly can also be seen from the photoluminescence spectra. As shown in Fig.2(b), PDI-TES presented monomolecular luminescence in THF solution. When the water content increased, the fluorescence intensity decreased significantly as well as the slight red-shift which was attributed to the aggregation of PDI-TES.
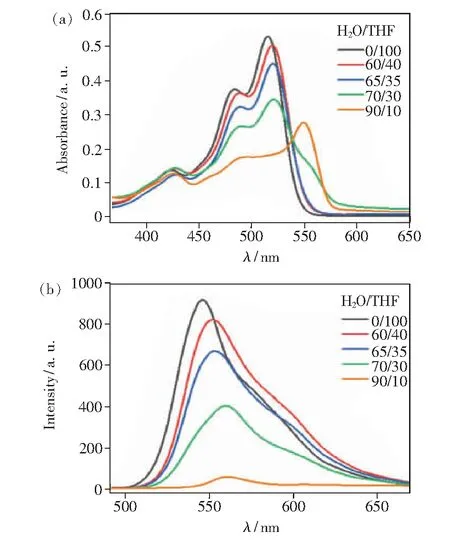
Fig.2 Solvent-dependent UV-Vis spectra(a) and Fluorescence spectra(b) of PDI-TES in H2O/THF mixed solvent systems at a concentration of 1×10-5 mol/L
3.2 Thermal Property and Crystal Structure
The PDI-TES sample was subjected to thermal analysis to check its stability. As shown in Fig.S3, the initial decomposition temperature (5% decomposition temperature) was as high as 412 ℃, indicating that PDI-TES had good thermal stability.
To obtain the aggregation behavior of PDI-TES in the bulk state, small-angle X-ray scattering(SAXS) experiment of PDI-TES crystals was performed. As shown in Fig.3, the two low-angle diffractions possessqratio of 1∶2 with correspondingd-spacings of 2.24 nm and 1.12 nm, respectively. This indicated the presence of a long-range ordered lamellar structure along the molecule’s long axes. Thed-spacing of the third peak can be attributed to the lateral spacing between two neighboring PDI units. In addition, there is a high-angle diffraction withd-spacing of 0.35 nm, which corresponds to the π-π stacking between adjacent PDI cores. Thus, PDI-TES possesses an ordered crystal structure, as shown the inset schematic diagram in Fig.3.

Fig.3 Small-angle X-ray scattering(SAXS) pattern of PDI-TES
3.3 Fluoride Ions Sensing Property
It has been reported that F-have a strong affinity for silicon, and F-can promote the cleavage reaction of Si—O and Si—C bonds to form the Si—F bond. Moreover, the PDI derivatives are a kind of strongly electron deficient system[26-27], while F-are very electronegative, so the electron transfer between them can also be used to detect F-. Many works have studied the combination of two or three F-detective mechanisms to optimize the detection limit. Different with them, PDI-TES has a simpler structure and less Si—O bond. Simple structure means more easily application, and less Si—O bond means more serious destroy to PDI-TES by less F-.
Electron transfer effect between OH-and PDI molecules can cause the fluorescence intensity to decrease. In order to avoid the influence of pH on the detection system, the pH value of the detection system is 7. To study the optical response of PDI-TES to F-, tetra-n-butylammonium fluoride(TBAF) as the F-source was gradually added to a THF solution of the compounds from 0 to 3 equiv., then both the UV-Vis absorption spectra and fluorescence spectra were investigated. As shown in Fig.4(a), with the addition of F-, the absorption intensity decreased gradually, while two new peaks appeared at 672 nm and 752 nm. It can be considered that electron transfer occurs between PDI-TES and F-[28]. Furthermore, the ratio of peaks at 515 nm and 484 nm (A515/A484) decreased, indicating the formation of the new complex, it may be due to the aggregation of PDI cores once losing the bilateral TES units.
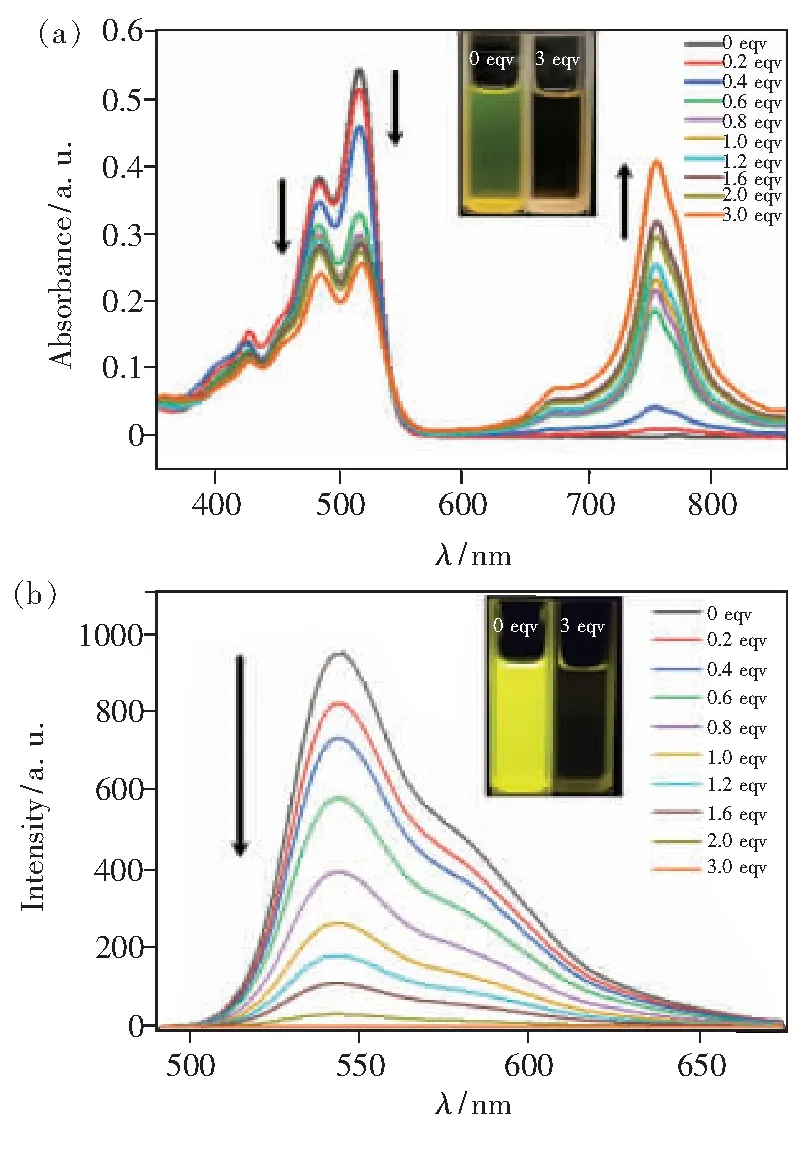
Fig.4 UV-Vis spectra(a) and Fluorescence spectra(b) of PDI-TES(1×10-5 mol/L in THF with different concentrations of fluoride ions). Inset: 0 equiv. and 3 equiv. in sunlight and under UV irradiation at 365 nm.
In agreement to UV-Vis results, as shown in Fig.4(b), the fluorescence intensity was gradually quenched after F-being added. The electron transfer should be in between of PDI core and anions since after adding F-, PDI-TES may transfer to other compounds. More interestingly, as shown the insets of Fig.4(a) and Fig.4(b), with the addition of F-, the color of the solution also changed significantly. The change from yellow to colorless can be observed by naked-eye before and after the addition of F-. When observed under UV irradiation at 365 nm, it can be found that the solution without F-is bright yellow, while the solution with F-is almost colorless. As reported[20], the detection limit is the fluoride ion concentration corresponds to the fluorescence intensity that fluoride ion is not added decreases by 10%. According to Fig.S4, we can calculate the detection limit is 1.58 μmol/L, which is better than that of PDI derivative with POSS substituent(10 μmol/L)[20].
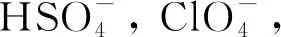
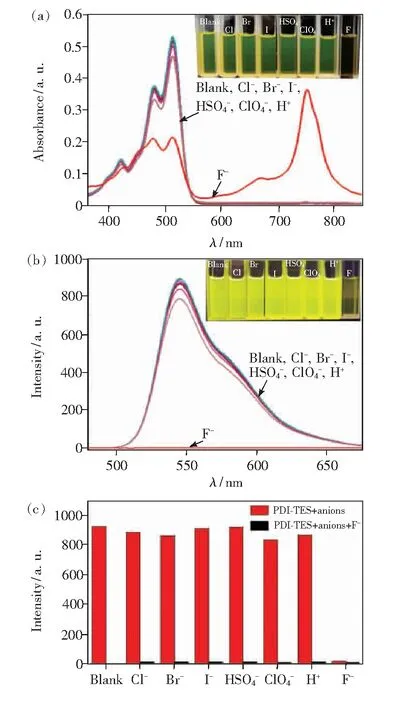
Fig.5 UV-Vis spectra(a) and fluorescence spectra(b) of PDI-TES. Inset: in sunlight and under UV irradiation at 365 nm(naked-eye detection). (c)Fluorescence spectra of PDI-TES in the presence of a single anion(red bars) and in the mixture of F- and other anions(black bars). Solvent: THF, [PDI-TES]=1×10-5 mol/L, [anions]=3 equiv.
To study the reaction mechanism between PDI-TES with F-, the1H NMR in CDCl3was acquired as Fig.S5. We can see that characteristic signals of PDI-TES at 8.55×10-6and 8.44×10-6disappear, which indicates the formation of paramagnetic PDI-TES•-radical anions. This is because PDI-TES is an electron-deficient system, F-is an electron-rich system, and F--induced electron transfer[19].
Fluoride ions can promote the cleavage reaction of Si—O and Si—C bonds to form the Si—F bond, so PDI-TES may aggregate with the addition of F-, leading to the final fluorescence quenching. In order to verify this mechanism, the DLS experiment was employed(Fig.6(a)). Before the addition of F-, the average particle size of the sample in the THF solution was about 40 nm. After the addition of F-, the particle size increased to around 80 nm. It indicated that with the addition of F-, the PDI molecules aggregate more severely. This result can also be verified by TEM. As shown in Fig.6(b), it can be clearly seen that the particle size increased significantly after adding F-.

Fig.6 (a)Particle size measurements of PDI-TES before and after reacting with F- in THF (1×10-5 mol/L, 3 equiv. F-). (b)TEM of PDI-TES before and after reacting with F- in THF(1×10-5 mol/L, left: Blank; right: add 3 equiv. F-).
4 Conclusion
In summary, we have synthesized a novel PDIderivative containing siloxane substituents(PDI-TES) as fluorescent sensor by simple one-step reaction with low cost and high yield. The TGA and SAXS results reveal that PDI-TES has good thermal stability and highly ordered crystal structure. The UV-Vis absorption spectra and photoluminescence spectra indicate that PDI-TES possesses high selectivity and sensitivity to fluoride ions due to the mechanism of Si—O bond cleavage, with a detection limit as low as 1.58×10-6mol/L.
Supplementaryinformationis available for this paper at http://cjl.lightpublishing.cn/thesisDetails#10.37188/CJL.20210068.
