新型琥珀酸脱氢酶抑制剂类杀菌剂氟唑菌酰羟胺对水稻恶苗病的防治研究 (
2021-06-11侯毅平曲香蒲蔡小威武洛宇陈长军王建新田宝华周明国
侯毅平, 曲香蒲, 蔡小威, 武洛宇, 陈长军,王建新, 田宝华, 周明国*,
(1. 南京农业大学 植物保护学院,南京 210095;2. 先正达 (中国) 投资有限公司,上海 200120)
0 Introduction
Rice bakanae disease mainly caused by the filamentous fungusFusarium fujikuroiis the most vital seed-borne disease in rice planting[1-3].F. fujikuroiis a necrotrophic pathogen in theGibberella fujikuroispecies complex (GFSC)[4-5]. The pathogen can infect roots, stems, crowns, leaf sheaths and panicles in older plants[3]. This destructive disease can trigger symptoms including seedlings elongated, stunted and yellow with crown rot[1]. Losses of rice yield caused by rice bakanae disease could be up to 40 % in an epidemic or outbreak[6]. During recent decades, chemical fungicides of seed dressing have been extensively applied in China, which has been regarded as the main management strategy. Due to the resistance problem ofF. fujikuroito common fungicides, the disease incidence has increased in recent years.
In the past, benzimidazole fungicides had been used as the main fungicide against most fungi includingF. fujikuroisince the 1970s. The resistance of this kind of fungicide come out soon[7-8].Subsequently, carbendazim resistance inF. fujikuroiemerged in China and benzimidazole-resistanceF.fujikuroipopulations rose in scale[9]. The efficacy of benzimidazole fungicide had greatly diminished because of resistance. Then the imidazole fungicides especially prochloraz was put in use for controlling the disease[10]. But the emergency of fungal resistance to prochloraz had been reported afterwards[11-14].Phenamacril, a fungicide that was considered to be very effective againstF. fujikuroiby seed dressing,had been applied to bring rice bakanae disease under control. However in 2016, resistant strains ofF. fujikuroiwere identified in the field of Zhejiang Province of China[15]. The resistance ofF. fujikuroistrains has posed a serious threat to rice cultivation, so it is vital to make the early warning and management of resistance as soon as possible.
Screening new fungicides is a common method for controlling plant disease. The chemiacal pydiflumetofen is a new succinate dehydrogenase inhibitor(SDHI) which was created by Syngenta recently[16].Commonly, SDHI fungicides are effective against many different pathogens because the respiration of pathogens could be interrupted by this kind of fungicides which could inhibit the activity of succinate dehydrogenase[17-18]. Until now, the novel SDHI fungicide pydiflumetofen has been registered in Argentina for controlling end-of-cycle diseases and in China for controlling wheat scab caused byFusariumgraminearum and rape sclerotinia stem rot caused bySclerotinia sclerotiorum. However, the effects of pydiflumetofen onF. fujikuroiand its control efficacy on rice bakanae disease remain unknown.
In this study, the above scientific problem will be solved by achieving following objectives: ① figure out baseline sensitivityF. fujikuroito pydiflumetofen by analysing EC50(half effective concentration)values of the fungicide on 100 strains ofF. fujikuroiisolated from different regions of China; ② reveal the influence of pydiflumetofen onF. fujikuroiin morphology and physiology; ③ find out whether pydiflumetofen is beneficial for the safety of rice growth; ④ test the efficacy of pydiflumetofen againstF. fujikuroion rice plant. Our study will yield novel data for reference to better manage rice bakanae disease by pydiflumetofen in the future, and deepen our understanding about how pydiflumetofen take effect onF. fujikuroiand other pathogens.
1 Materials and Methods
1.1 Fungicides, media and strains
Pydiflumetofen (technical grade; 99.0%) was supplied by Syngenta (China) Investment Co., Ltd. and dissolved in methanol as a stock solution at concentration of 10 mg/mL. pydiflumetofen 200 g/L suspension concentrate (SC) was provided by Syngenta (China)Investment Co. Ltd. Prochloraz 250 emulsion in water(EW) was purchased from Nanjing market and its manufacturer was Shaanxi Biaozheng Crop Science Co., Ltd.
Media of potato dextrose agar (PDA), water-agar(WA) and yeast extract peptone dextrose (YEPD) was prepared as previously reported[19-20]. Strains ofF.fujikuroiwere isolated from young rice with bakanae disease from the fields of Zhejiang and Jiangsu Province of China according to a previous method[21].ThenF. fujikuroistrains were confirmed by a molecular biology identification of TEF-1 maker[22]. Finally, 100 strains ofF. fujikuroiwere obtained for following test.
1.2 Baseline sensitivity to pydiflumetofen
Mycelial growth and spore germination were two important life stage ofF. fujikuroi. In order to know the effect of pydiflumetofen toF. fujikuroiin vitro,the sensitivity of mycelial growth or spore germination to pydiflumetofen was determined. Through analyzing the distribution of 100 strains’ EC50values,the baseline sensitivity of mycelial growth as well as spore germination ofF. fujikuroito pydiflumetofen was established.
For the sensitivity test of mycelial growth, PDA plates (9 cm in diameter) containing 0.015 625, 0.031 25,0.062 5, 0.125 and 0.25 μg/mL pydiflumetofen were prepared. Mycelial plugs (5 mm in diameter) growing 3 days on PDA medium without fungicides were transferred to above PDA plates containing different concentrations of pydiflumetofen and the PDA plates without fungicide (control). After cultured at 25 °C for 7 days in a growth chamber, the colony diameters of each PDA plate were measured. The EC50values were calculated according to a previous study[23].Similarly, for the sensitivity test of spore germination,WA medium plates containing 0.001 95, 0.003 9, 0.007 8,0.016 and 0.003 2 μg/mL pydiflumetofen were prepared.The spores of each strain were produced in CMC(carboxymethylcellulose) medium and its sensitivity to pydiflumetofen was tested according to a previous method[21]. The EC50values were calculated according to a previous study[23]. There were three replicates in each treatment and the experiments was repeated two times.
1.3 Effect of pydiflumetofen on mycelial morphology of F. fujikuroi
The strains ofF. fujikuroiXY11, XY22 and XY96 were used for morphology assay. 1 mL melted PDA medium containing 0.05 μg/mL (EC50value) or 1.3 μg/mL (EC90value) pydiflumetofen was dripped on slide glass. After it was curdled, a mycelial plug growing 5 days on PDA plate was transferred to the slide glass. The slide glass only covered with 1 mL PDA medium and without fungicide was performed as control. After cultured for 24 h at 25 °C in a growth chamber, the morphology of mycelial top was observed by a microscope (Olympus IX-71, Japan). There were three replicates for each treatment and the experiment was repeated three times.
1.4 Effect of pydiflumetofen on sporulation ability of F. fujikuroi
The strains ofF. fujikuroiXY11, XY22 and XY96 were used for sporulation ability assay. Five mycelial plugs growing 3 days on PDA plate were transferred into 250 mL flask which contained 100 mL carboxymethylcellulose (CMC) medium. Pydiflumetofen was added at the ultimate concentration of 0.05 μg/mL(EC50value) or 1.3 μg/mL (EC90value) into partial flasks which had been shaken at 25 °C for 36 h in a shaker (175 r/min, 12 h illumination every day). After the flasks finished an additional 36 h-shaking, spore concentration of each strain treated with different concentrations of pydiflumetofen was counted under a microscope with a hemacytometer[24]. There were three replicates in each treatment and the experiment was repeated three times.
1.5 Effect of pydiflumetofen on mycelial cell membrane permeability of F. fujikuroi
The strains ofF. fujikuroiXY11, XY22 and XY66 were used for this study. Ten mycelia plugs (5 mm in diameter) from a colony growing 5 days on PDA plate of each strain were put into 250 mL flasks which contained 100 mL YEPD medium. Pydiflumetofen was added at the ultimate concentration of 0.05 μg/mL(EC50value) into partial flasks which had been shaken at 25 °C for 36 h in a shaker (175 r/min). Then shaken for another 36 h in the same condition,mycelia were collected and washed with sterile water[25]. Filtrated in vacuum for 20 min, 0.3 g fresh mycelia of each sample was cultured with liquid suspension way in 20 mL of double-distilled water.Flasks without pydiflumetofen were used as control.After different times of 0, 5, 10, 20, 40, 60, 80, 100,120, 140, 160 and 180 min, the conductivity of the distilled water of each sample was tested by a conductivity meter (CON510 Eutech/Oakton,Singapore). Before measuring the ultimate conductivity, the water with mycelia in it was boiled for 5 min after 180 min. The relative conductivity of mycelia was determined according to a previous study[21]. There were three replicates in each treatment and the experiment was repeated three times.
1.6 Effect of pydiflumetofen on mycelial exopolysaccharide (EPS) production of F. fujikuroi
The strains ofF. fujikuroiXY11, XY15 and XY22 were used for EPS production assay. Ten mycelia plugs (5 mm in diameter) from a colony growing 5 days on PDA plate of each strain were put into 250 mL flasks which contained 100 mL YEPD medium. Pydiflumetofen was added at the ultimate concentration of 1.3 μg/mL (EC90value) into partial flasks which had been shaken at 25 °C for 36 h in a shaker (175 r/min).Then shaken for another 36 h in the same condition,the supernatants were collected and EPS was extracted according to a previous study[21]. EPS was tested by phenol-sulfuric acid method of previous studies[21,26]. There were three replicates in each treatment and the experiment was repeated three times.
1.7 Effect of pydiflumetofen on peroxidase(POD) activity of F. fujikuroi
The strains ofF. fujikuroiXY11, XY15 and XY22 were used for POD activity test. Mycelia was treated as mentioned in last segment. The extractions of 0.5 g of freeze-dried mycelia treated with 10 mL of 100 mmol/L sodium phosphate buffer with 0.1 g of polyvinyl polypyrrolidone (PVPP) were centrifuged with high speed centrifuge. POD activity was measured as mentioned[27]. There were three replicates in each treatment and the experiment was repeated three times.
1.8 Effect of pydiflumetofen on cell ultrastructure of F. fujikuroi
F. fujikuroiXY11 was selected as the tested strain in this experiment. Three mycelial plugs (5 mm in diameter) were cut from the margin of a colony which grew 5 days and added into 50 mL flask which contained 20 mL of CMC medium. Spores were produced and prepared as section 2.5. 100 μL spore suspension with concentration of 1×107/mL was inoculated into 50 mL flask with 20 mL YEPD medium. Pydiflumetofen was added at the ultimate concentration of 1.3 μg/mL (EC90value) into partial flasks which had been shaken at 25 °C for 12 h in a shaker (175 r/min, 12 h illumination every day). Then continue to be cultured for 8 h, mycelia was fixed and observed by an electron microscope (Hitachi-7 650,Tokyo, Japan).
1.9 Control efficacy of pydiflumetofen against rice bakanae disease in vivo
The spores ofF. fujikuroistrain XY11 was prepared as above section 2.5. Rice seeds (cultivar: Nanjing 46)were immersed in suspension liquid ofF. fujekuroistrain XY11spores (106spores/mL) for 12 h. The volume rice seeds was equal to that of the spore suspension. The rice seeds were spread out on filter paper. Dried for one day at room temperature, 50 g of the rice seeds was respectively immersed in 50 mL of water containing different concentrations of pydiflumetofen (10, 15 and 20 g a.i./100 kg seeds).The regular fungicde was prochloraz and its concentration was 20 g a.i./100 kg seeds. The control was the rice seeds mixed with water only. After steeped for 24 h, seeds treated with pydiflumetofen were steeped in water for another 24 h and then spread out on a damp filter paper in a plastic box.After a 2-day incubation at room temperature, the germinated seeds were planted in the soil in a plastic box (100 seeds per box). When the seedings growing in the greenhouse reached the third leaf stage, the incidence of disease was expressed in the percentage of dampened or elongation seedlings. 300 seedlings were treated with the same fungicide of every treatment as replicates.
1.10 Data analysis
The statistical variances among repeated experiments were analyzed by the software of SPSS14.0 (SPSS Inc. Chicago, IL). Whether there were significantly differences among different treatment were evaluated by Fisher’s LSD test (P= 0.05).
2 Result and analysis
2.1 Baseline sensitivity of mycelial growth of F.fujikuroi to pydiflumetofen
A total of 100 strains ofF. fujikuroiconfirmed by identification of molecular biology were used for establishing baseline sensitivity ofF. fujikuroito pydiflumetofen. The effect of pydiflumetofen to inhibit mycelia from growing ofF. fujikuroistrain XY60 at different concentrations of pydiflumetofen was showed in Fig. 1A. The inhibition rate of 0.25 μg/mL pydiflumetofen against mycelial growth of strain XY60 was more than 80%. EC50values distribution of mycelial growth of 100F. fujikuroistrains to pydiflumetofen was established through the test on PDA plates containing different concentrations of pydiflumetofen by mycelial growth rate method.The minimum EC50value was 0.012 5 μg/mL while the maximum EC50value was 0.111 8 μg/mL, and the mean EC50value of 100 strains was 0.050 3 (± 0.002 9) μg/mL.The variation factor (maximum EC50value/minimum EC50value) was 8.944. The baseline sensitivity of mycelial growth of 100F. fujikuroistrains to pydiflumetofen was unimodal curve (Fig. 1B).
2.2 Baseline sensitivity of spore germination of F. fujikuroi to pydiflumetofen
EC50values distribution of spore germination of 100F. fujikuroistrains to pydiflumetofen was established through the test on WA plates containing different concentrations of pydiflumetofen by spore germination assay. The minimum EC50value was 0.000 1 μg/mL while the maximum EC50value was 0.024 5 μg/mL,and the mean EC50value of 100 strains was 0.003 8 (±0.000 5) μg/mL. The variation factor was 245. The baseline sensitivity of spore germination of 100F.fujikuroistrains to pydiflumetofen was half unimodal curve (Fig. 2).
2.3 Effect of pydiflumetofen on mycelial morphology of F. fujikuroi
After treated with 0.05 μg/mL and 1.3 μg/mL pydiflumetofen, we can see the offshoot increase of mycelial top ofF. fujikuroistrains XY11, XY22 and XY96 (Fig. 3).
2.4 Effect of pydiflumetofen on sporulation ability of F. fujikuroi
Treated with 0.05 μg/mL (EC50value) or 1.3 μg/mL(EC90value) pydiflumetofen, strains XY11, XY22 and XY96 ofF. fujikuroishowed a decrease in the spore production (Table 1). The result indicated that pydiflumetofen could retard spore production ofF. fujikuroi.

Table 1 Effect of pydiflumetofen on spore production of Fusarium fujikuroi 1)
2.5 Effect of pydiflumetofen on cell membrane permeability of F. fujikuroi
The curves of relative conductivity of strains XY11,XY22 and XY66 ofF. fujikuroiwith the treatment of 1.3 μg/mL pydiflumetofen were all higher than those untreated with the fungicide (Fig. 4). The result indicated that pydiflumetofen could break mycelial cell membrane ofF. fujikuroiwhich resulted in increaseof mycelial electrolyte leakage.
2.6 Effect of pydiflumetofen on mycelial EPS production of F. fujikuroi
The mycelial exopolysaccharides (EPS) production ofF. fujikuroiwas evaluated by absorbance at 490 nm and calculated according to established standard curves (Fig. 5A). There was no remarkable difference on mycelial EPS content ofF. fujikuroistrains XY11,XY15 and XY22 between the treatment with 1.3 μg/mL pydiflumetofen for 36 h and the control (Fig. 5B).
2.7 Effect of pydiflumetofen on POD activity of F. fujikuroi
POD activity ofF. fujikuroistrains XY11, XY15 and XY22 with the treatment of 1.3 μg/mL pydiflumetofen for 36 h was all significantly lower than that untreated with the fungicide (Fig. 6). The result indicated that 1.3 μg/mL pydiflumetofen could inhibit POD activity ofF. fujikuroi.
2.8 Effect of pydiflumetofen on mycelial cell ultrastructure of F. fujikuroi
Mycelial cell wall of the strain XY11 with the treatment of 1.3 μg/mL pydiflumetofen for 8 h was thinner than that untreated with the fungicide. In addition, treated with 1.3 μg/mL pydiflumetofen, cell membrane of mycelia was incomplete because of being damaged(the red arrows in Fig. 7) and organelles (eg. mitochondria, the white arrows in Fig. 7) were broken in the cell.
2.9 Control efficacy of pydiflumetofen against rice bakanae disease in vivo
The disease incidence of cultivar Nanjing 46 was 31.47% untreated with any fungicide. Control efficacy of rice bakanae disease with different concentrations of pydiflumetofen 10, 15, 20 g a.i./100 kg seeds was 94.77%, 98.60 and 100%, respectively(Table 2). Control efficacy of 15 g a.i./100 kg pydiflumetofen was higher than that of prochloraz 20 g a.i./100 kg seeds.
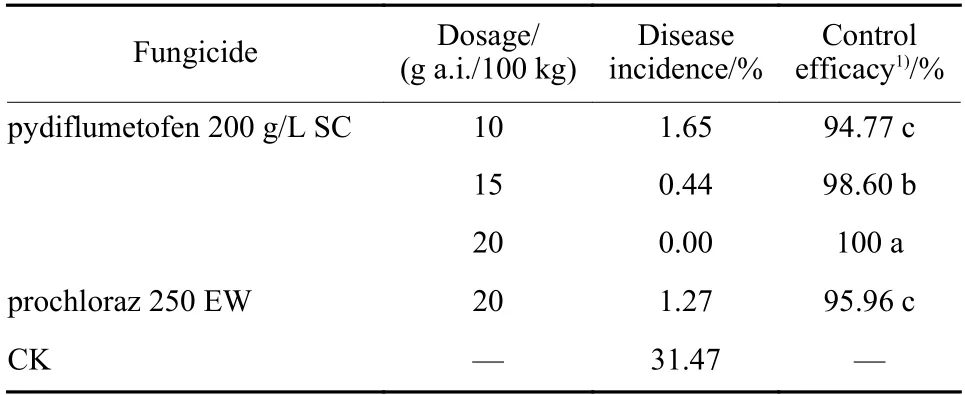
Table 2 Control efficacy of pydiflumetofen against rice bakanae disease by seeds dressing on the cultivar of Nanjing 46
3 Conclusion and discussion
F. fujikuroiis the main pathogen causing bakanae disease on rice. Seeds dressing with suitable fungicides is the most common method to control rice bakanae disease. But in recent years, theF. fujikuroistrains gradually showed resistance to the fungicides including carbendazim, prochloraz, phenamacril[9,11,15]. In order to manage common fungicides resistance,limiting the use of fungicides with the same mode of action, applications of mixtures with some different mode of action fungicides, replacement of common using fungicides that have not full control efficacy because of resistance with novel fungicides are the main methods. Screening novel fungicides for candidate are urgent for controlling rice bakanae disease.
Pydiflumetofen developed by Syngenta is a novel SDHI fungicide. In a previous study, we found that pydiflumetofen had high activityin vitroagainst one of Fusarium pathogens,F. asiaticumcausing head blight in wheat and had great control efficacy against Fusarium head blight of wheat in the field. In the research, the antifungal activity of pydiflumetofen againstF. fujikuroiwas determinedin vitro. We constructed the curve of sensitivity distribution of mycelial growth or spore germination ofF. fujikuroipopulations (n= 100). EC50values for mycelial growth and spore germination ranged from 0.012 5 to 0.111 8 μg/mL and from 0.000 1 to 0.024 5 μg/mL respectively, and the average EC50value was 0.050 3(± 0.002 9) μg/mL and 0.003 8 (± 0.000 5) μg/mL,respectively. The spore germination was more sensitivity than mycelial growth. The reason may be that spore germination ofF. fujikuroineed more energy than mycelial growth and pydiflumetofen strongly inhibited energy production because of its mode of action. The variation factor of 100 EC50values for spore germination was higher than that of 100 EC50values for mycelial growth. This may be caused bigger difference in spores vitality. The average EC50value of mycelial growth ofF. fujikuroiagainst pydiflumetofenin vitrowas lower than the fungicides thiram, carbendazim, phenamacril,tebuconazole, fludioxonil and prochloraz[10,28], which indicated that pydiflumetofen had more activity than above fungicidesin vitro. Futhermore,F. fujikuroihad shown resistance to the fungicides above according to reports[9,11,15], which suggested that pydiflumetofen could be put into use for the management of pathogen resistance against above common fungicides in the field.
The effect of pydiflumetofen on morphology and physiology ofF. fujikuroiwere also determined.Treated with 0.05 μg/mL (EC50value) or 1.3 μg/mL(EC90value) pydiflumetofen, the offshoot of mycelial top ofF. fujikuroiincreased (Fig. 3). The spore production ofF. fujikuroiwith the treatment of different concentrations of pydiflumetofen descreased(Table 1). The two results of relative conductivity and cell ultrastructure all indicated that pydiflumetofen could break mycelial cell membrane ofF. fujikuroiwhich results in increase of mycelial electrolyte leakage (Fig. 4; Fig. 7). The content of EPS remained almost unchanged but POD activity was lowered after treated with pydiflumetofen, which would undermine the effect of removing these toxic substances and may even result in their accumulation in cell ofF. fujikuroiwhose growth would be badly affected because H2O2,phenols, amine and hydrocarbon which was produced by fungal cells and exerted a negetive influence on fungal growth could react with POD[29].
Control efficacy of pydiflumetofen 15 g a.i./100 kg seeds and 20 g a.i./100 kg seeds against rice bakanae disease by seed dressing is 98.60 % and 100%respectively while control efficacy of prochloraz 20 g a.i./100 kg seeds against the disease is 95.96%, which indicates that fewer dose of pydiflumetofen can get better control efficacy compared with prochloraz. In addition, control efficacy of pydiflumetofen 20 g a.i./100 kg seeds is better than that of phenamacril 24 g a.i./100 kg seeds[30]. Pydiflumetofen is a great candidate for controlling rice bakanae disease caused byF. fujikuroiby seeds dressing.
