Highly Moisture Resistant 5-Aminovaleric Acid Crosslinked CH3NH3PbBr3 Perovskite Film with ALD-Al2O3 Protection
2021-06-04TianWangTaiyangZhangYuetianChenYixinZhao
Tian Wang,Taiyang Zhang,Yuetian Chen ,Yixin Zhao
School of Environmental Science and Engineering,Shanghai Jiao Tong University,Shanghai 200240,China.
Abstract:In recent years,hybrid lead halide perovskites have attracted significant research interest in the optoelectronic fields owing to their exceptional physical and chemical properties.However,their commercialization process is limited largely because of the sensitive nature of perovskite materials towards external stresses,such as heat,UV irradiance,oxygen,and moisture.Among various perovskitestabilization methods,deposition of a protective layer over the vulnerable perovskite film via simple atomic layer deposition (ALD)technology is of great potential.However,the corrosive effect of H2O or O3 on perovskites,which is used as the oxygen source during ALD process,is one of the main obstacles in the application of regular ALD technology for coating compact and highly conformal layer directly onto the perovskite film.In this study,by introducing bifunctional 5-aminovaleric acid (AVA)crosslinking into the layers of CH3NH3PbBr3 (MAPbBr3)units,we propose a simple yet effective strategy to prevent the degradation of sensitive perovskite structure during the ALD process when H2O is used as the oxygen source.The formed crosslinked 2D/3D structure of AVA(MAPbBr3)2 perovskite film was extremely dense and ultra-smooth compared to the coarse MAPbBr3 film.With the passivation and protection of AVA,the AVA(MAPbBr3)2 perovskite film exhibited high moisture resistance,thereby leading to the successful deposition of dense and conformal Al2O3 protective layer onto the perovskite surface.The deposition of Al2O3 layer with different thicknesses had a negligible effect on the crystalline phase and morphology of AVA(MAPbBr3)2 film,as confirmed by X-ray diffraction,UV-Vis absorption spectroscopy,and scanning electron microscopy characterizations.The steady-state photoluminescence (PL)intensity and time-resolved PL lifetime of AVA(MAPbBr3)2 film was kept almost unchanged before and after the coating of Al2O3 layer,suggesting that the thin Al2O3 layer did not significantly alter the optical properties of the perovskite material,thereby enabling the potential usages in optical and optoelectronic devices.The thermal stability and water resistance ability of Al2O3-coated AVA(MAPbBr3)2 film was proven to have greatly improved in accelerated circumstances.No impurities or decomposition were detected for Al2O3-coated AVA(MAPbBr3)2 film after the long-time annealing at high temperature (150 °C for 2 h),whereas the crosslinked 2D/3D structure of bare MAPbBr3 film quickly broke down at the elevated temperature.Intriguingly,the AVA(MAPbBr3)2 film with 15-nm-thick Al2O3 coating layer could endure strong water corrosion for at least 10 min when immersed in water.Overall,the proposed strategy could not only give a good reference for successfully depositing metal oxides onto the perovskite films with preservation of the materials’ intrinsic properties,but also provide a method of introducing amino acid to passivate and protect the perovskite materials from H2O corrosion during the ALD process.Therefore,the proposed work has practical potential in improving the device stability against various external stresses under different operating conditions,thereby paving way for various applicational advances.
Key Words:Lead halide perovskite; Atomic layer deposition; Crosslinked 2D/3D structure; Thermal stability;Water resistance
1 Introduction

In recent years,the application of atomic layer deposition(ALD)technology to deposit ultrathin pinhole-free,conformal and compatible Al2O3protective film has gained increased attention22,23,29–33.Due to the self-terminating surface chemistry,the coating thickness of Al2O3film by ALD can be precisely Angstrom-scale-controlled by the number of ALD cycles23,32,34–38.For now,ALD has not only been proven feasible for surface passivation and encapsulation in lab settings,but also facile for commercial large-scale manufacturing39.Despite all the advantages,there still exists one obstacle for ALD application in perovskite protection.In most of ALD processes,water or ozone(H2O or O3)is used as the oxygen source,which could be destructive to the sensitive organic-inorganic lead halide perovskite structure37,39–43.Dong et al.37used O3as oxygen source to deposit Al2O3as capping layer onto the perovskite at 70 °C and found that just one cycle of ALD was adequate to destroy the perovskite.Kim et al.23investigated the effect of H2O and O3on the degradation of perovskite and proposed a non-hydrolytic ALD method using acetic acid as the oxygen source.Koushik et al.44found that the MA+cations got etched from the perovskite lattice when using trimethylaluminum(TMA)and H2O as precursor sources.These damages would cause the decrease of device efficiencies.Therefore,it is of great significance to find out a way to improve the intrinsic stability of perovskite against water to avoid the destruction of perovskite structure during ALD process.
To improve the intrinsic stabilities of perovskite structures,a series of approaches have been proposed,such as tuning the composition of perovskite (mixed cations or mixed halide)to obtain a more stable perovskite layer7,45–51,substituting of small cations with long chain or bigger organic molecules to convert the three dimensional (3D)hybrid perovskites to two dimensional(2D)structures (or mixed 2D and 3D structure)46,52–55,adding functional scaffolds or additives47,56and so on.For example,the introduction of AVA (5-aminovaleric acid)or AVAI additives into the perovskite structure can form crosslinked 2D/3D structure or mixed 2D and 3D structure,which could greatly improve the moisture stability57,58.Therefore,we propose that the combination of intrinsic structural-stability enhancement and extrinsic Al2O3protective layer would enable a double protection for the vulnerable perovskite towards external stimuli.
Here in this study,we introduce the bifunctional 5-aminovaleric acid to passivate and protect MAPbBr3perovskite film from damaging by H2O in the regular ALD method when using TMA and H2O as sources.AVA was inserted into the layer of MAPbBr3units to form AVA(MAPbBr3)2film with crosslinked 2D/3D structure.The AVA(MAPbBr3)2film was extremely stable during the ALD process.And the Al2O3-coated AVA(MAPbBr3)2film exhibited remarkably improved thermal stability and water resistance in accelerated circumstances.
2 Experimental
2.1 Materials
Methylamine ethanol solution (MA,33%,mass fraction (w))and HBr were purchased from Sigma-Aldrich Co.,Ltd.5-Aminovaleric acid (AVA),PbBr2and dimethyl formamide(DMF)were purchased from Aladdin Industrial Inc.Diethyl ether and ethanol were bought from Sinopharm Chemical Reagent Co.,Ltd.All the chemicals were used as-received without further purification.
2.2 Synthesis of MABr and AVABr
MABr and AVABr were synthesized according to previous reports57,59.Briefly,MA and HBr with a molar ratio of 1.2 :1 were reacted in an ice bath for 2 h followed by vacuum drying.The product was dissolved in hot ethanol and then injected into diethyl ether for recrystallization.The process was repeated for three times.The washed white powder was dried in a vacuum oven overnight to obtain purified MABr.AVABr was synthesized using the same method as MABr except with AVA as precursor.
2.3 Film preparation
The glass substrate was cleaned by soaking in 5% (w)NaOH ethanol solution for hours,then rinsed with deionized water and cleaned under 10 min of plasma.MAPbBr3film was prepared via solvent engineering method by spin coating the precursor solution of MABr and PbBr2(molar ratio of 1 :1)in DMF at 3500 r·min−1for 20 s,and annealed at 100 °C for 10 min.AVA(MAPbBr3)2film was prepared by an in situ gas/solid method previously reported by our group57,60.AVABr and PbBr2with the molar ratio of 1 :2 were dissolved in DMF to form a precursor solution.A drop of 80 μL precursor solution was spin coated on glass substrate at a speed of 4000 r·min−1for 20 s.The film was dried at room temperature for 10 min to evaporate DMF.Then the film was placed upside-down over MA ethanol solution for 3 s and the obtained colorless film were annealed at 100 °C for 10 min to remove the residual vapor and form the AVA(MAPbBr3)2perovskite.
2.4 Deposition of Al2O3 by ALD process
The Al2O3layers were deposited directly on perovskite films by the ALD system (ALD f-100-4,MNT)using trimethylaluminum (TMA)vapor as aluminum source and H2O vapor as oxygen source.High purity N2(99.999%)was used as purge gas.The pressure in the ALD reaction chamber was around 27 Pa,and the temperature was set at 85 °C.In each complete ALD cycle,~0.09 nm thickness Al2O3layer was acquired when both TMA and H2O were dosed into the chamber for 15 ms and waited for 5 s before a 20 s purge.
2.5 Characterizations
The crystal structure of films with or without ALD deposited Al2O3were analyzed using X-ray diffraction (Shimadzu XRD-6100,Cu Kαradiation).UV-Vis spectroscopy analysis was carried on Cary 60 UV-Vis spectrometer.Steady state photoluminescence (PL)spectra was measured by a LS55 luminescence spectrometer (Perkin Elmer Inc.,USA).Timeresolved photoluminescence was measured by QM/TM/IM fluorescence spectrofluorometer (PTI,USA).The morphologies of the perovskite films were characterized by scanning electron microscope (SEM,FEI Sirion 200).The atomic force microscope (AFM)images were obtained by a Bruker fast scan scanning probe microscope.
3 Results and discussion
We firstly investigated the effect of Al2O3deposition on MAPbBr3perovskite film by ALD.The XRD patterns and UVVis spectra of the MAPbBr3perovskite films with and without deposition of 10 nm thickness Al2O3were compared.The strong diffraction peaks (Fig.1a)at 14.84° and 30.08° assigning to the(100)and (200)planes (blue dashed lines)of MAPbBr3disappeared,indicating the decomposition of MAPbBr3by reacting with H2O vapor during the ALD process61,62.The comparison in the UV-Vis spectra before and after the Al2O3deposition as shown in Fig.1b also confirmed the destruction of MAPbBr3after ALD.It is obvious that MAPbBr3perovskite film could be damaged when depositing Al2O3directly onto it,let alone providing the originally expected surface protection23,63.On the contrary,after directly depositing 10 nm Al2O3onto the surface of AVA-passivated AVA(MAPbBr3)2films,the XRD patterns remained unchanged in comparison with the pristine AVA(MAPbBr3)2perovskite (Fig.1c),suggesting the remarkable resistance of AVA(MAPbBr3)2film against H2O in the process of ALD.We also investigated the influence of ALD processing time (which is proportional to the thickness of Al2O3)on the structure of AVA(MAPbBr3)2films.Prolonging ALD time means longer exposure to TMA,H2O and longer annealing time at the operation temperature (85 °C).Intriguingly,no change that reflects the degradation of perovskite films were observed when 5 and 15 nm thicknesses of Al2O3layers were coated onto the AVA(MAPbBr3)2films.Fig.1d displayed the UV-Vis spectra of AVA(MAPbBr3)2films with different thickness of Al2O3layers.The Al2O3-coated films exhibited the same characteristic perovskite absorption as the pristine one.All these results strongly indicate that,the introduction of bifunctional AVA groups into the MAPbBr3structure would effectively passivate and protect MAPbBr3from moisture-induced decomposition during the ALD process.
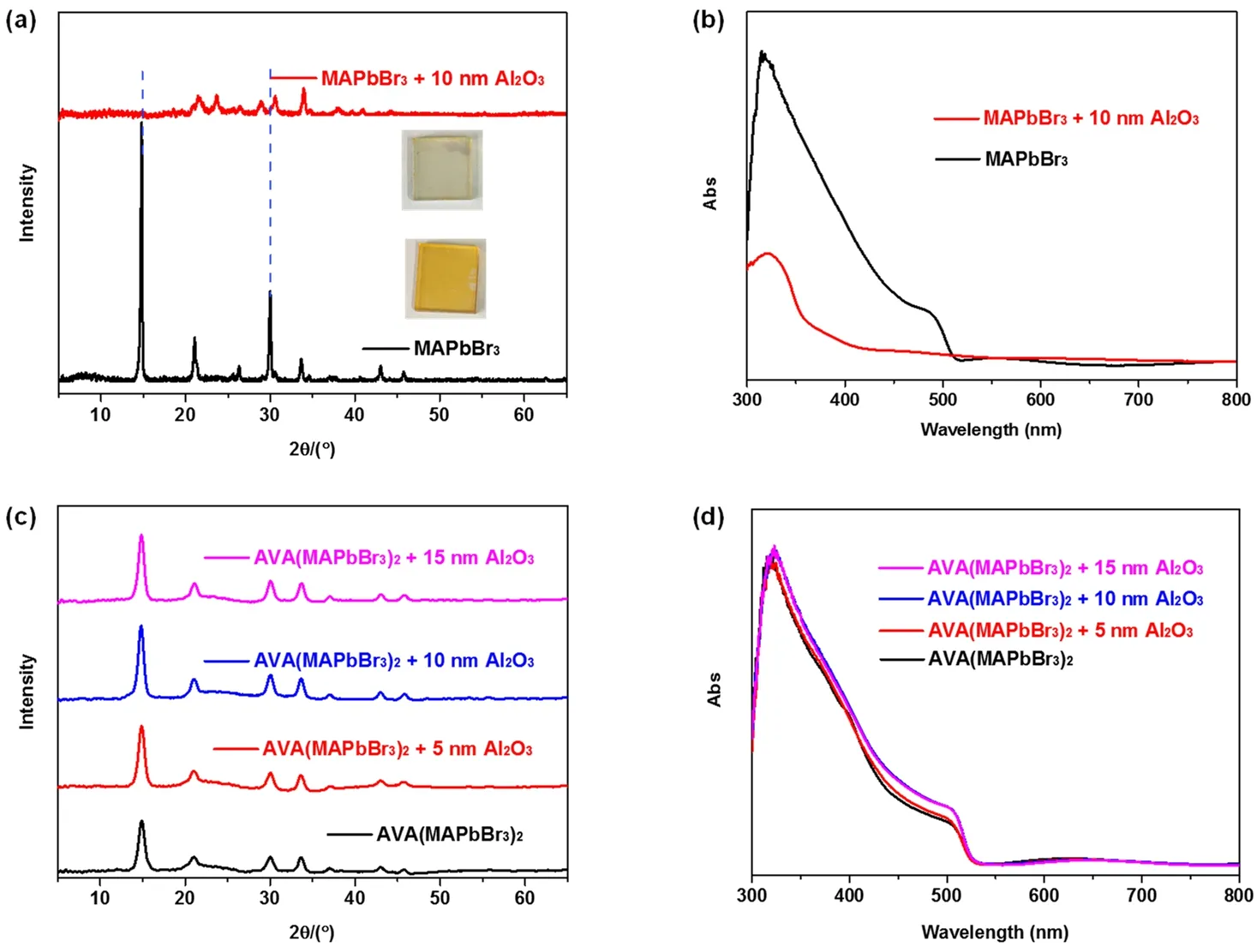
Fig.1 (a)XRD patterns and (b)UV-Vis spectra of the MAPbBr3 perovskite films before and after ALD of Al2O3; (c)XRD patterns and(d)UV-Vis spectra of the AVA(MAPbBr3)2 perovskite films before and after ALD of Al2O3.
Fig.2 shows the morphologies of prepared perovskite films before and after the ALD process.The bare MAPbBr3perovskite film has a rough surface scattered with different sized MAPbBr3crystals (Fig.2a).While the AVA(MAPbBr3)2film was ultrasmooth with no obvious sign of grain boundaries (Fig.2b).The extremely dense morphology was due to the introduction of bifunctional AVA groups into the precursor solution forming a special crosslinked 2D/3D structure,where H3+and COO−in AVA crosslinked the MAPbBr3units by occupying the MA+sites and Br−sites respectively on the surface of two nearby unit57.The deposition of compact and conformal Al2O3directly onto AVA(MAPbBr3)2film had no distinct impact on the perovskite morphology as confirmed by SEM and AFM images (Fig.2c,d).It was also revealed by AFM height profiles that the Al2O3-coated film was quite compact with less than 20 nm roughness on the surface.The enhanced stability of AVA(MAPbBr3)2film towards H2O during the ALD process was benefiting from its denser morphology and the crosslinked 2D/3D structure57.On one hand,researches claimed that the pinhole-free morphologies and 2D structure can improve the stability of perovskite when exposed to moisture46,52–55.On the other hand,the organic ammonium cation in AVA could modify the surface of MAPbBr3perovskite grains and hence block the approach of H2O to the perovskite,avoiding the decomposition of MAPbBr3units46,64.Therefore,the AVA inserting into the lattice structure of MAPbBr3can passivate and protect the MAPbBr3units from reacting with H2O and suppressing their degradation during the whole ALD process.

Fig.2 SEM images of bare (a)MAPbBr3 and (b)AVA(MAPbBr3)2 film before ALD; (c)SEM image and(d)AFM image of AVA(MAPbBr3)2 film after ALD of 10 nm conformal Al2O3 layer.The scale bar is 1 μm.
The steady-state photoluminescence (PL)spectra and timeresolved PL decays of AVA(MAPbBr3)2films before and after the coating of Al2O3layer were compared.The steady-state PL intensity of AVA(MAPbBr3)2film barely changed after the deposition of different thickness of Al2O3(Fig.3a).The average PL lifetime for AVA(MAPbBr3)2with or without 10 nm Al2O3coating was 10.55 and 10.63 ns,respectively (Fig.3b),suggesting that the thin Al2O3did not affect the optical dynamic properties,which holds promise for using this perovskite film for optical and optoelectronic devices.The photos of bare and coated AVA(MAPbBr3)2films in Fig.3b showed comparable brightness.The above measurements strongly suggest that the compact and compatible Al2O3layer had been successfully deposited onto the AVA(MAPbBr3)2perovskite film through ALD process without damaging the film or affecting the optical properties of the perovskite film.

Fig.3 (a)Steady-state PL spectra and (b)time-resolved PL decay curves of AVA(MAPbBr3)2 films with and without the deposition of different thickness Al2O3 by ALD.Insert photos in (b)are bare AVA(MAPbBr3)2 film (left)and AVA(MAPbBr3)2 film with 10 nm Al2O3 coating (right).
Thermal stability and water resistance of the Al2O3-coated AVA(MAPbBr3)2films were investigated under accelerated circumstances.Samples were annealed at 150 °C for 2 h in atmosphere with up to 90% relative humidity.Without Al2O3deposition,the crosslinked 2D/3D structure of AVA(MAPbBr3)2soon broke down to mixed 2D and 3D structure as indicated by the appearance of a strong peak at 8.5° related to 2D structure(Fig.4a)57.While the films with different thickness of Al2O3coatings exhibited excellent thermal stability as no impurities were formed after the long time annealing at high temperature(Fig.4b).The enhanced peak intensity was ascribed to the regrowth of AVA(MAPbBr3)2crystals65,66.The compact Al2O3layer can effectively prevent the escaping of organic cations(MA+)from the AVA(MAPbBr3)2perovskite structure during annealing,therefore leading to outstanding thermal stability.The water resistance of bare AVA(MAPbBr3)2and Al2O3deposited films were evaluated by directly immersing them in water (Fig.5).Not surprisingly,the uncovered AVA(MAPbBr3)2film rapidly decomposed within 3 s.As expected,the Al2O3-coated AVA(MAPbBr3)2films displayed remarkably improved tolerance of water erosion,and the water resistance abilities of those films exhibited positive relation with the thickness of coated protection layers.The results were easily understood as the thicker and compacter the layer was,the stronger protection it would provide.The films with 5 to 15 nm Al2O3coverage could endure the water corrosion from seconds to minutes.As can be seen from the pictures,the decomposition began from the edges as uneven points and then extended to areas.For the 15 nm Al2O3-coated film,no apparent damages can be observed within 3 min in water,and the film even could go through the water stimulation for at least 10 min before completely decomposing.This test result confirmed that,the Al2O3layer that armored onto the surface of AVA(MAPbBr3)2perovskite could separate the vulnerable perovskite from intimate contact with water,resulting in significantly improved water resistance.

Fig.4 XRD patterns of (a)bare AVA(MAPbBr3)2 film and(b)conformal Al2O3-coated AVA(MAPbBr3)2 films before and after annealing at 150 °C for 2 h as comparison of thermal stabilities.

Fig.5 Optical photos to compare the water resistance abilities of bare and Al2O3-coated AVA(MAPbBr3)2 films as a function of time in water.
4 Conclusions
In summary,the Al2O3protection layer was successfully deposited onto the perovskite film via regular ALD method using TMA and H2O as aluminum and oxygen source,respectively.This direct deposition method effectively protected the perovskite film with no damage or sacrifice to the film performances.For the perovskite film used in this study,the insertion of bifunctional AVA into MAPbBr3layer not only worked as a crosslinker to form ultra-dense AVA(MAPbBr3)2film with a more stable crosslinked 2D/3D structure,but also passivated the perovskite crystal boundaries and impeded the direct contact of MAPbBr3and H2O,leading to the successful Al2O3deposition.The Al2O3-coated films exhibited significant improvement in both thermal stability and water resistance under accelerated circumstances (i.e.annealed at 150 °C and immersed in water).The strategy of introducing amino acid to passivate and protect perovskite from H2O corrosion during ALD process has practical potential in improving device stability in the future.
杂志排行
物理化学学报的其它文章
- SCN-doped CsPbI3 for Improving Stability and Photodetection Performance of Colloidal Quantum Dots
- 两步互扩散法制备高性能CsPbCl3薄膜紫外光电探测器
- 电泳法制备的致密氧化锡薄膜及其在高稳定性钙钛矿太阳能电池中的应用
- Structural,Thermodynamical and Electronic Properties of All-Inorganic Lead Halide Perovskites
- 基于易升华添加剂辅助合成纯相富铯CH(NH2)2)xCs1−xPbI3钙钛矿
- 锡基钙钛矿太阳能电池研究进展
