Evaluation of a povidone-iodine and chitosan-based barrier teat dip in the prevention of mastitis in dairy cows
2021-05-23ZHANGHuiminJIANGHongruiCHENDaijieSHENZiliangMAOYongjiangLIANGYushengJuanLOORYANGZhangping
ZHANG Hui-min ,JIANG Hong-rui ,CHEN Dai-jie ,SHEN Zi-liang ,MAO Yong-jiang ,LIANG Yusheng,Juan J.LOOR,YANG Zhang-ping
1 Key Laboratory for Animal Genetics,Breeding,Reproduction and Molecular Design of Jiangsu Province,College of Animal Science and Technology,Yangzhou University,Yangzhou 225009,P.R.China
2 Joint International Research Laboratory of Agriculture &Agri-Product Safety,Ministry of Education/Yangzhou University,Yangzhou 225009,P.R.China
3 Department of Animal Sciences &Division of Nutritional Sciences,University of Illinois,Urbana,IL 61801,USA
Abstract Postmilking teat dip is an important tool used to prevent mastitis in the modern dairy industry. In this study,we evaluated the in vitro and in vivo efficacies of a barrier teat dip containing povidone-iodine and chitosan for the prevention of mastitis. In experiment 1,we evaluated the antibacterial effects of chitosans with different molecular weights against six mastitis-causing bacteria based on the minimal inhibitory concentration test. The results showed that 50 kDa chitosan had the maximum antibacterial activity compared with 5,150 and 350 kDa chitosans. In experiment 2,the inhibition zone test indicated that the barrier teat dip with 4.0% povidone-iodine and 1.0% chitosan had higher (P<0.05) in vitro antibacterial efficacy against most tested mastitis-causing bacteria than the barrier teat dip with 4.0% povidone-iodine and no chitosan. In experiments 3 and 4,we evaluated the efficacies of two postmilking teat dips,1) a barrier teat dip containing 1.0% chitosan and 4.0%povidone-iodine and 2) a conventional nonbarrier product containing 10% povidone-iodine in a field trial at two commercial dairy herds (1 and 2). A 56-d split-udder experiment (experiment 3) was conducted using 47 lactating Chinese Holstein cows in herd 1. Both left teats were immersed in barrier postmilking dip,and both right teats were dipped with nonbarrier postmilking dip. During a 56-d split-herd experiment (experiment 4),a total of 139 lactating Chinese Holstein cows from herd 2 were allocated to two groups:1) all teats of 67 cows were dipped in the nonbarrier teat dip,and 2) all teats of 72 cows were dipped in the barrier teat dip. Milk samples were collected and analyzed for somatic cell count (SCC),fat content,protein content,and fat-to-protein ratio prior to the start of sampling (0 d),and at 28 and 56 d after initiation. Bacteriological analysis was only performed on milk samples with SCC≥200 000 cells mL–1. In experiment 3,no differences (P>0.05)in SCC,somatic cell score (SCS) or other milk quality indicators were observed between nonbarrier and barrier teat dip treatment teats throughout the experiment. At the end of experiment 4,compared with nonbarrier teat dip group,a reduction(P<0.05) of 29% was observed for subclinical mastitis infection prevalence in the barrier teat dip group. In the barrier teat dip group,the subclinical mastitis infection prevalence on 56 d was lower (P<0.05) than 0 d. No differences (P>0.05) in milk qualities or clinical mastitis incidence were detected between groups. Bacteriological analysis demonstrated that the barrier product containing povidone-iodine and chitosan reduced the subclinical mastitis infection prevalence induced by mastitis pathogens. This effect was mainly due to the reductions in Klebsiella pneumoniae,Pseudomonas aeruginosa,and Escherichia fergusonii infections. Overall,the data indicated that a barrier teat dip containing 4% povidone-iodine and 1%chitosan was more effective than 10% povidone-iodine in preventing subclinical mastitis.
Keywords:mastitis,povidone-iodine,chitosan,barrier postmilking dip
1.Introduction
Bovine mastitis is the main cause of economic losses in dairy farming worldwide (Ruegg 2017). Postmilking teat dip is an effective procedure for preventing mastitis during lactation (Hillertonet al.2007). Therefore,postmilking teat dip products are widely recommended by dairy advisors throughout the world. Over the past 20 years,more than ten different active compounds have been tested in teat disinfectants including chlorhexidine,iodine compounds and quaternary ammonium salts (El Behiryet al.2012;Doreet al.2019).
Recently,due to the low toxicity and excellent antibacterial effect,polyvinylpyrrolidone-iodine complex (povidone-iodine)has become the most commonly used teat disinfectant in dairy farms in China. However,as an external-use medicine,povidone-iodine has weak antibacterial persistence (Chenet al.2016). The development of a barrier teat dip based on film-forming ingredients and disinfectants could be one solution to achieve a long-term antibacterial effect against mastitis-causing bacteria.
The efficacies of iodine-based barrier teat dip and conventional iodine postmilking teat dip were compared,and a 24% reduction in clinical and subclinical mastitis was detected in cows dipped with barrier product compared with a nonbarrier teat dip (Foretet al.2006). Another study compared a barrier teat dip with a high level of free iodinevs.a nonbarrier product with a low level of free iodine and the results indicated that the barrier teat dip product reduced clinical mastitis by 46% compared with the nonbarrier product (Martinset al.2017). Therefore,the presence of a film-forming ingredient that can remain on the teat surface combined with an effective,persistent,and active antibacterial agent is important for a barrier product.
Direct application of high concentrations of povidoneiodine onto the skin can cause teat irritation (redness or chapping). Thus,it is necessary to find alternative antimicrobials that are effective at lower concentrations of povidone-iodine in the teat dip. Chitosan is a non-toxic and biodegradable biopolymer derived from the deacetylation of chitin. Due to its excellent antimicrobial activity against several bacteria,fungi,and yeasts,chitosan has been successfully used in the food,pharmaceutical,cosmetics,and agricultural industries (Konget al.2010). Besides its antimicrobial action,chitosan has other excellent properties including good film-forming capacity,biodegradability,and a hydrophilic/moisturizing effect (Zemljicet al.2018).Recent studies have demonstrated that chitosan has been widely used in food,feed and medicine industries,but only limited studies have investigated the effect of chitosan on the prevention of mastitis in dairy cows. Chitosan could be used in internal teat sealants to prevent mastitis in dairy cows during the drying-off period (Lanctotet al.2017). In addition,a chitosan (50–90 kDa) and cloxacillin combination could improve antibiotic efficacy against coagulase-negativeStaphylococcusisolates from chronic bovine mastitis(Breseret al.2018). It was found that 2.6 kDa chitosan was able to prevent biofilm production byS.aureus2117 and a bovine methicillin-resistantS.aureus(Asliet al.2017). So,povidone-iodine and chitosan have shown excellent antibacterial effects on mastitis-causing bacteria separately,however,the effect of a povidone-iodine and chitosan combination in the prevention of dairy cow mastitis remains unclear.
Various studies with chitosan have reported a correlation between its antibacterial activity and molecular weight (Silva-Diaset al.2014;Asliet al.2017). Based on the previous studies,it was hypothesized that chitosan with a specific molecular weight combined with lower concentrations of povidone-iodine in the teat dip might be more effective than higher concentrations of povidone-iodine in preventing mastitis. To test this hypothesis,we explored thein vitroantibacterial effects of chitosan with different molecular weights on six mastitis-causing bacteria. In addition,we compared thein vivoefficacy of a barrier teat dip containing chitosan and a low concentration of povidone-iodine with a conventional nonbarrier product containing a high level of povidone-iodine using split-udder and split-herd experimental designs.
2.Materials and methods
2.1.Bacterial strains
Escherichia coliNBRC102203,Pseudomonas aeruginosaDSM 50071,Staphylococcus aureusATCC12600,Streptococcus agalactiaeATCC 13813,Klebsiella pneumoniaeDSM 30104 andBacillus cereusATCC14579 were acquired from the culture collection at the Veterinary College of Yangzhou University,China. All strains were cultured at 37°C in LB broth (Hopebio,China). For the susceptibility study,the inocula were adjusted using DensiCHEK Plus (BioMérieux Inc.,France) according to McFarland scale values and corroborated by plate counting.
2.2.Chitosan solution
Firstly,91–92% N-deacetylated chitosans (Golden Shell Pharmaceutical Co.,China) of different molecular weights(5,50,150,and 350 kDa) were dissolved in deionized water.To increase solubility of the chitosan,acetic acid was added to the solution at a final concentration of 2% (v/v). Then,the pH of the chitosan solution was adjusted to 6.5 with 1%NaOH solution.
2.3.Experimental design and treatments
A completely randomized design was used in the four experiments. Experiment 1 was carried out to study thein vitroantibacterial effects of chitosans with different molecular weights against several mastitis-causing bacteria,and the four treatment groups included different molecular weights (5,50,150 and 350 kDa). Experiment 2 was implemented to study thein vitroantibacterial activity of the barrier teat dip with different chitosan contents (0.0,0.5,1.0,and 1.5%,w/v). Experiment 3 (split-udder design) and experiment 4 (split-herd design) were performed to evaluate thein vivoefficacy of barrier teat dip containing povidoneiodine and chitosan for the prevention of mastitis. There were two treatments in experiments 3 and 4,barrier teat dip (containing 4.0% (w/v) povidone-iodine and 1.0% (w/v)chitosan) and nonbarrier teat dip (10.0% povidone-iodine,Nanjing Risingsun Biotechnology Co.,China).
2.4.Minimal inhibitory concentration (experiment 1)
The double dilution technique was employed to determine the minimal inhibitory concentration (MIC) of chitosan (Zohriet al.2010). Bacteria were inoculated at 106CFU mL–1in 96-well plates. Next,dilution of the chitosan solution was prepared with LB medium at final concentrations of 1.25,2.5,5,10,20,40,and 80 mg mL–1. All samples were incubated at 37°C for 12 h. The MIC value represented the lowest concentration of chitosan that had an OD600nmidentical to the control well containing only LB medium (no visible growth). For each bacterium,five replicates of each chitosan concentration per treatment were conducted in experiment 1.
2.5.Teat dip preparation (experiment 2)
Polyvinyl alcohol-124 (PVA-124,Sinopharm Chemica Reagent Co.,China) water solution (6%,w/v) was obtained by dispersion of the polymer in distilled water for 24 h at room temperature,followed by heating at 95°C for 2 h for complete dissolution. After cooling,6% (v/v) glycerol,1%(w/v) Tween 80,and 0.5% (w/v) sodium carboxymethyl cellulose were added to the PVA-124 water solution.Lastly,4.0% (w/v) povidone-iodine and chitosan at different concentrations (0.0,0.5,1.0,and 1.5%,w/v) were added to the polymer solution. The resulting mixture (i.e.,the teat dip) was kept away from light at room temperature to avoid decomposition of the iodine.
2.6.Antibacterial test of teat dip (experiment 2)
The teat dip prepared in our lab was a viscous solution,so thein vitroantibacterial performance was evaluated by inhibition zone test. All six pathogenic bacteria were grown in LB medium at 37°C overnight. Subsequently,150 μL bacterial suspension (106CFU mL–1) was spread onto the tryptone soy agar medium (Hopebio,China) using a sterile swab. Then,two holes with a 7.58-mm diameter were made by a hole punch in the plating medium. To conduct the inhibition zone test,50-μL of teat dip was placed in the hole. The plates were then incubated at 37°C for 24 h and the antibacterial performance of the teat dip was evaluated based on the diameter of the inhibition zone. A larger zone of inhibition usually means that the bacteria are more sensitive to the teat dip. Four treatments with three replicates for each bacteria strain were conducted in experiment 2.
2.7.Cows and experimental design (experiments 3 and 4)
Thein vivoefficacy of the barrier teat dip containing povidone-iodine and chitosan was evaluated in a field trial at two commercial dairy herds (herd 1 and herd 2) in Jiangsu Province (China). In experiment 3,a split-udder design was conducted in herd 1 from November 5,2018 to December 30,2018 (barn temperature,(11.35±4.26)°C;relative humidity,(71.39±8.40)%). In experiment 4,a total of 139 lactating Chinese Holstein cows were used for a split-herd design in herd 2 from April 1,2019 to May 26,2019 (barn temperature,(15.58±3.59)°C;relative humidity,(69.59±10.59)%). Cows used in the experiment were in good clinical health at the time of recruitment,and had not been treated with antibiotics or anti-inflammatory products in the 30 d before recruitment. Cows were housed in free-stall barns and milked three times daily in a side-byside milking parlor. Forty-seven Chinese Holstein cows of 2.47±1.08 parities,(63.7±14.48) days in milk,and(46.74±9.95) kg d–1of milk yield (mean±SD) were used in experiment 3. Both left teats of all cows were immersed in the barrier teat dip,and both right teats of all cows were dipped with nonbarrier teat dip. In experiment 4,the animals were allocated into two groups:1) all teats of 67 cows were dipped with nonbarrier teat dip,and 2) all teats of 72 cows were dipped with barrier teat dip.Groups within the herd did not differ in feeding,housing,and management practices. Cows in both herds were fed the same total mixed rations (TMR) (Table 1),formulated to meet or exceed the NRC (2001) nutrient requirements for lactating Holstein cows.
2.8.Teat dipping (experiments 3 and 4)
The postmilking teat dip was placed in a clean,dry,teatdip cup. After milking,teats were dipped to at least 50%of the teat length as soon as possible following removal of the milking machine. Teats were allowed to dry naturally.
2.9.Milk sample collection (experiments 3 and 4)
At 0,28,and 56 days of the experiment,quarter milk samples (100 mL) were manually collected aseptically at morning milking and stored at 4°C for immediate analyses of milk quality and microbial populations.
2.10.Milk sample analyses (experiments 3 and 4)
Infrared scanning (Milkoscan 6000,Foss Electric,Denmark)was used to determine concentrations of fat,protein,and fatto-protein ratio. Somatic cell count (SCC) of each milk sample was analyzed by flow cytometry (Fossmatic 5000,Foss Electric,Denmark). In experiment 4,only mammary quarter milk samples with SCC≥200 000 cells mL–1were identified as subclinical mastitis and submitted for bacteriological analysis (Scheperset al.1997;Breenet al.2009). A total of 100 μL milk was spread onto a blood agar plate (5% sheep blood). All plates were incubated at 37°C under aerobic conditions for 24 h. After observation of colony morphology,hemolytic patterns and size,species identification of each isolate was confirmed further by 16S sequencing (Rahamanet al.2017). Each isolate was cultured in liquid medium with shaking (220 r min–1) at 37°C for 12 h. The resulting bacterial culture was then used for DNA extraction using the TIANamp Bacteria DNA Kit (Tiangen Biotech,China)according to the manufacturer’s instructions. The variable regions of the 16S rRNA gene were amplified with primers 27F (5´-AGAGTTTGATCCTGGCTCAG-3´) and 1492R(5´-TACGGCTACCTTGTTACGACTT-3´). PCR amplification was performed with the following conditions:denaturation at 98°C for 2 min;30 cycles of 98°C for 10 s,58°C for 30 s and 72°C for 45 s;an extra elongation at 72°C for 10 min. The purified PCR product was used for sequencing. Homology alignment of the nucleotide sequences was performed in GenBank with the BLAST program.
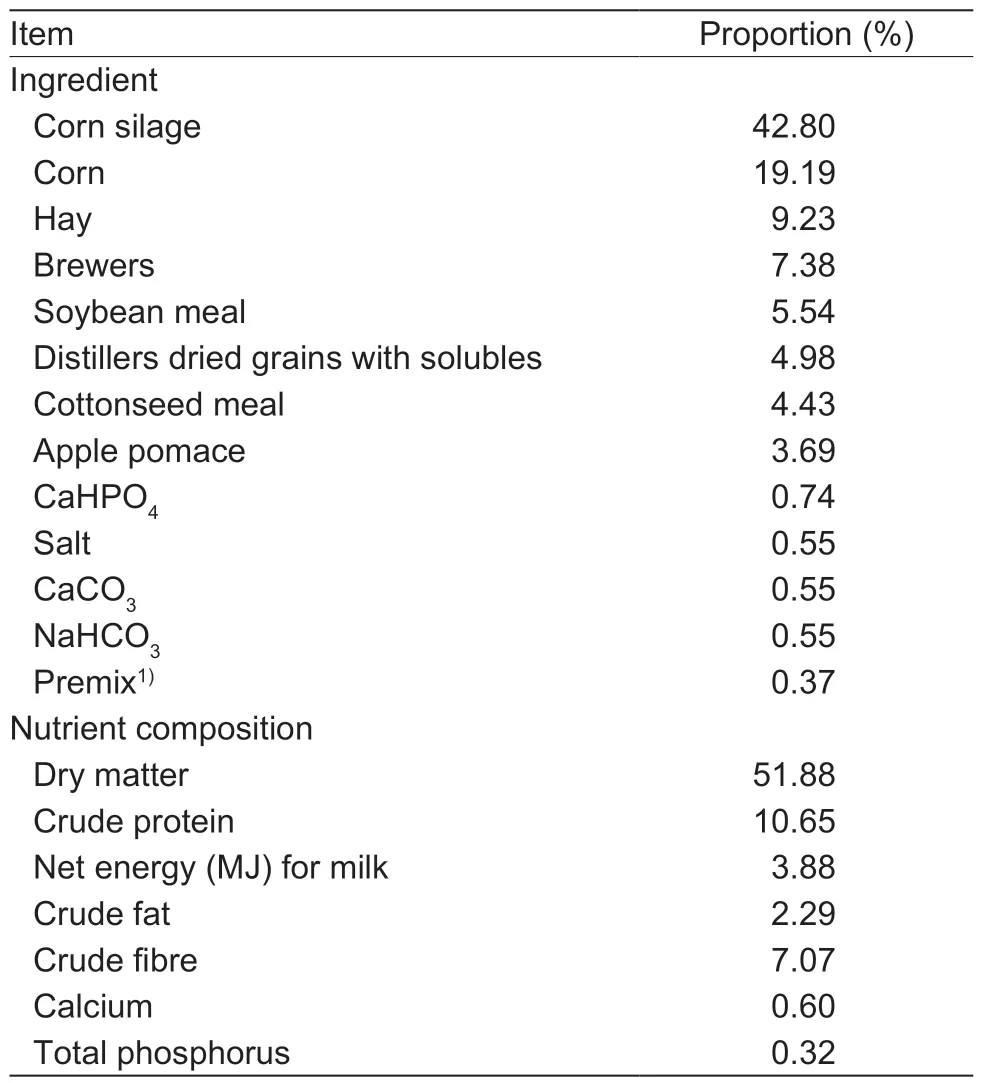
Table 1 Composition and nutrient levels of the TMR diet for dairy cows (as-fed basis)
2.11.Clinical mastitis diagnosis (experiments 3 and 4)
Clinical mastitis was diagnosed when udder clinical symptoms occurred (i.e.,redness,pyrexia,swelling) and/or when abnormal milk (i.e.,watery appearance,flakes,clots,blood or pus) was observed before milking. Over the course of the experiment,any teat with clinical mastitis was sampled aseptically for bacterial culture before treatment was applied.
2.12.Statistical analysis
In this study,the data of MIC (experiment 1) and inhibition zone diameter (experiment 2) were analyzed by one-way ANOVA using SPSS 17.0 (SPSS,Inc.,Chicago,IL,USA).The replicate served as the experimental unit. Multiple comparisons among treatment means were performed using Duncan’s test. Orthogonal polynomial contrasts were applied for linear and quadratic responses of dependent variables to independent variables in experiment 2. In experiments 3 and 4,the data of milk quality were analyzed by two-way ANOVA using the above SPSS system,and the model included the time,treatment and their interaction.A Chi-square test was used to analyze differences in subclinical mastitis infection prevalence among different times. Somatic cell score (SCS) was calculated using the formula:SCS=log2(SCC/100 000)+3. The replicate cow or teat served as the experimental unit. Differences were considered statistically significant whenP<0.05 and considered a trend when 0.05<P<0.1.
3.Results
3.1.MIC evaluation of chitosans with different molecular weights (experiment 1)
The MIC test was performed for water solutions of chitosans with different molecular weights (Table 2). Low MIC values indicate higher bacterial susceptibility and antibacterial activity. There was no difference (P>0.05) in MICs between 50 and 150 kDa chitosans after incubation with different pathogenic bacterial strains tested in this study. Both 50 and 150 kDa chitosans showed significantly lower (P<0.05)MICs than 5 and 350 kDa for all tested strains exceptSta.aureus,and the MIC obtained with 50 kDa chitosan againstSta.aureuswas 2.0 mg mL–1,which was lower (P<0.05)than with 5 and 350 kDa. These results showed that 50 kDa chitosan had the maximum antibacterial activity against mastitis-causing bacteria compared with 5,150 and 350 kDa chitosans. So the 50 kDa chitosan was used for the teat dip preparation.
3.2.Antibacterial activity assessment of barrier teat dip (experiment 2)
After plates were incubated at 37°C for 24 h,the diameter of the inhibition zone was measured,including the diameter of the hole (Table 3). The average diameters of inhibition zones forSta.aureus,Str.agalactiae,andB.cereusin teat dips containing 1.0 and 1.5% chitosan were higher(P<0.05) than the 0.0 and 0.5% groups. However,no differences (P>0.05) were observed among the different chitosan contents in the antibacterial activity againstPse.aeruginosaorK.pneumonia.As chitosan content increased,the antibacterial activity of teat dip againstE.coli,Sta.aureus,Str.agalactiaeandB.cereusincreasedlinearly or quadratically (P<0.05),and antibacterial activity of teat dip againstPse.aeruginosaincreased linearly (P<0.05).However,there was no difference (P>0.05) in diameters between teat dips containing 1.0 and 1.5% chitosan for all bacteria species evaluated. Thus,these results indicated the teat dip with 4.0% povidone-iodine and 1.0% chitosan had higher (P<0.05)in vitroantibacterial efficacy than other teat dip formulations against most tested strains in this study.
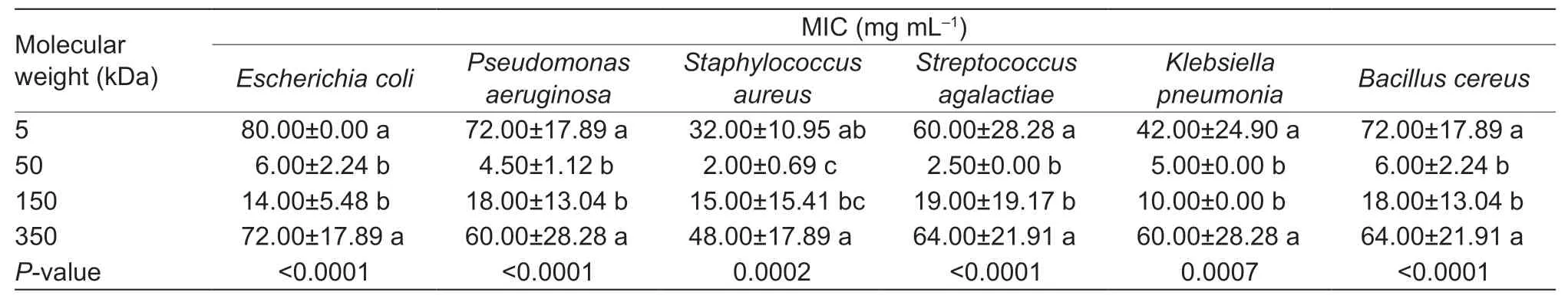
Table 2 Effect of chitosan molecular weight on the minimal inhibitory concentration (MIC) values against different bacteria strains in experiment 1
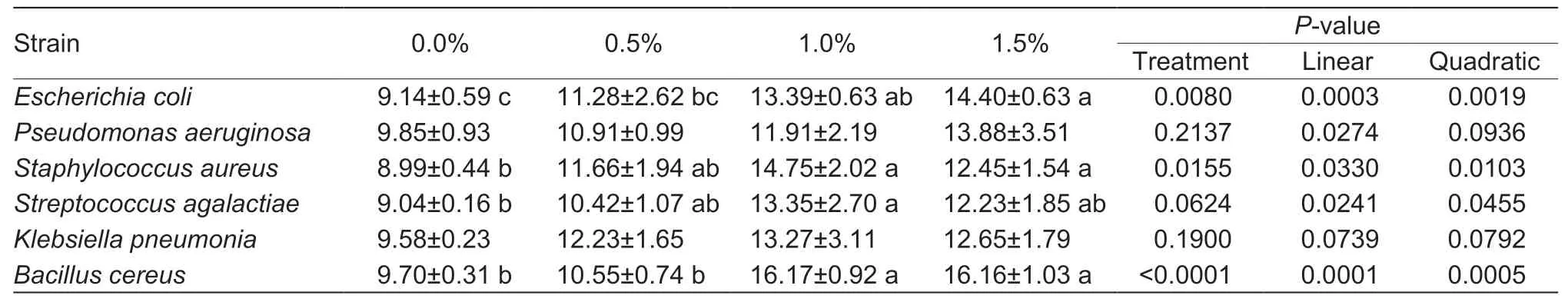
Table 3 The inhibition zone diameter (mm) of teat dips with different chitosan contents in experiment 2
3.3.Milk quality evaluation (experiments 3 and 4)
In experiment 3,a total of 450 quarter milk samples were collected during the 56-d experiment. The data on the effects of time,treatment and their interaction on milk quality are shown in Table 4. We found that time affected (P<0.0001)the milk quality indicators (except protein content). The milk quality indicators (except protein content) at the beginning of the experiment were higher (P<0.05) than those on 28 and 56 d in all teats,while no differences (P>0.05) were observed in any milk quality indicators between 28 and 56 d. No differences (P>0.05) in milk quality indicators were observed between nonbarrier and barrier teat dip treatment teats throughout the experiment,and no interaction (P>0.05)between time and treatment was observed. There were no clinical mastitis cases during the experiment.
A total of 1 532 quarter milk samples were collected during experiment 4. There were no differences (P>0.05) between nonbarrier and barrier teat dip groups in body weight,parity,lactation stage,or status of infection at the beginning of the experiment (Table 5). No differences (P>0.05) in milk quality indicators were observed between nonbarrier and barrier teat dip groups (Table 6). As for the milk yield,no interaction(P>0.05) between time and treatment was observed,but time affected (P<0.0001) the milk yield. The milk yield on 0 d was higher (P<0.05) than on 28 and 56 d. For SCS,an interaction (P<0.05) between time and treatment was observed,but time and treatment had no effects (P>0.05)on SCS. The fat content,protein content and fat-to-protein ratio in milk samples were affected (P<0.05) by time and time×treatment. The fat content and fat-to-protein ratio on28 d were lower (P<0.05) than those on 0 and 28 d,but the protein content on 28 d was higher (P<0.05) than other times. The SCC in milk was not affected (P>0.05) by time,treatment or their interaction.
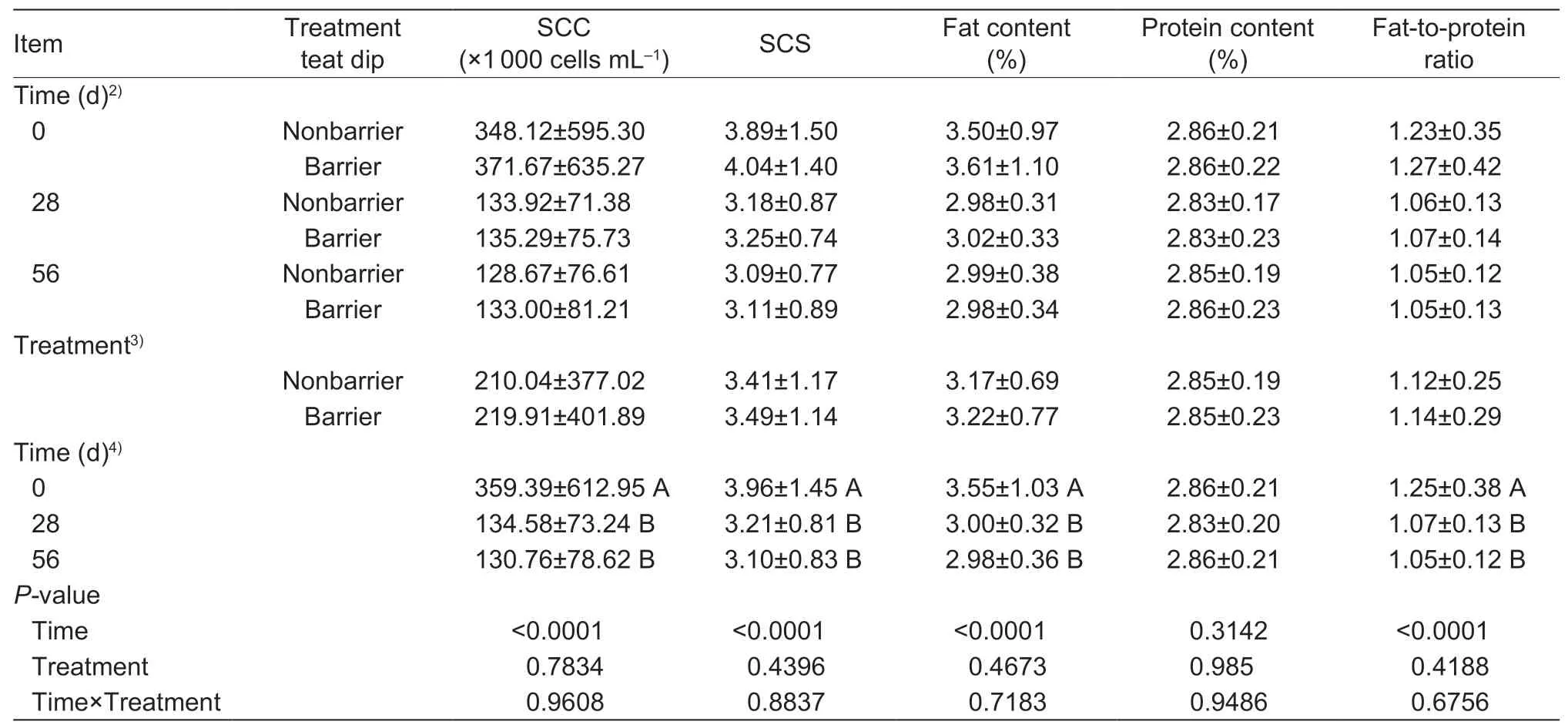
Table 4 Effect of different teat dips and times on the milk quality in experiment 31)

Table 5 Body weight,parity and days in milk (DIM) of dairy cows in nonbarrier and barrier teat dip groups on 0 d in experiment 4
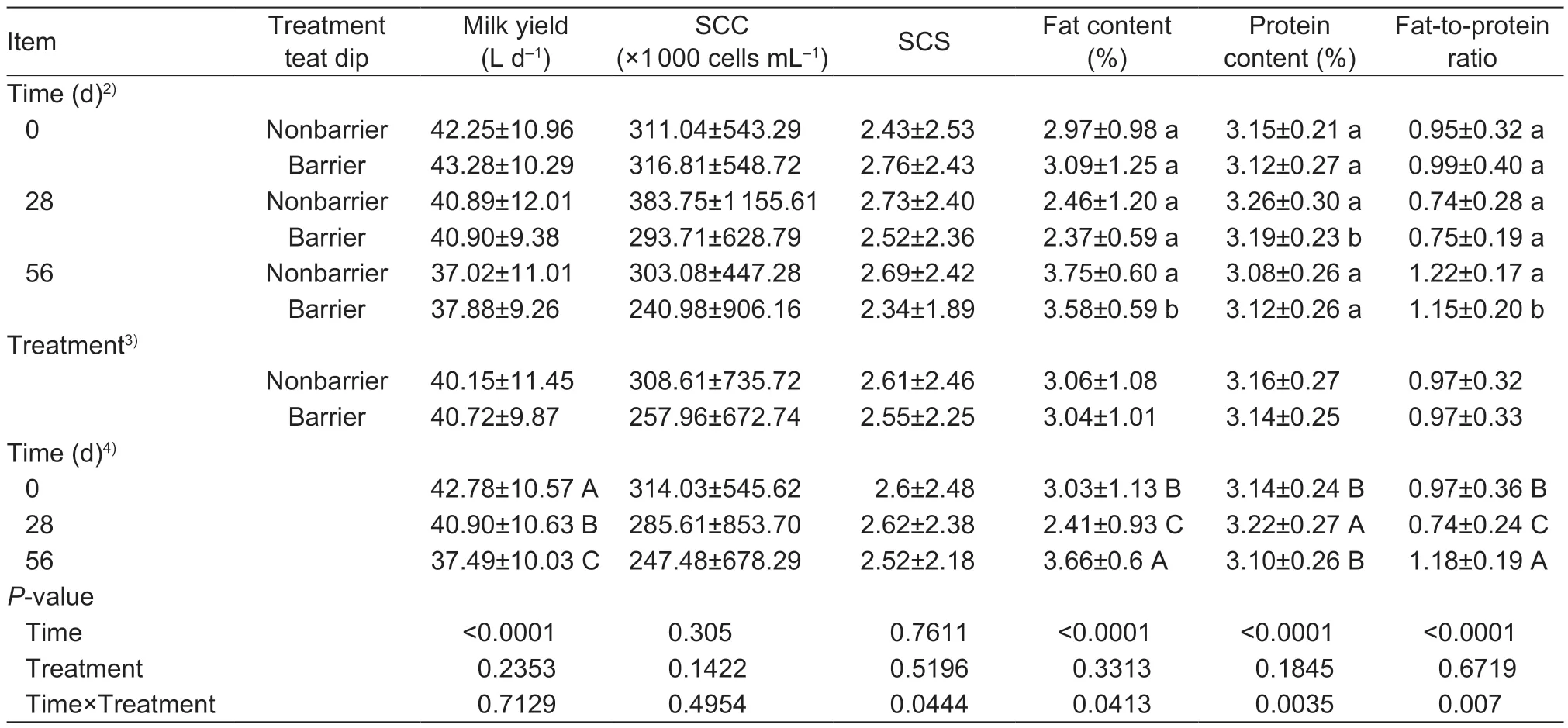
Table 6 Effect of different teat dips and times on the milk quality in experiment 41)
3.4.Bacteriological analysis (experiments 3 and 4)
Bacterial pathogens were identified in milk samples when SCC≥200 000 cells mL–1. During experiment 3,a total of 116 milk samples exceeded the SCC threshold,therefore,they were submitted for bacteriological culture (Table 7).Of all samples submitted for culture,39 (nonbarrier) and 43 (barrier) quarter milk samples had bacterial growth.During the 56-d experiment,all teats had a reduction(P<0.01) in the subclinical mastitis infection prevalence.The most-frequently isolated microorganism in all teats wasK.pneumoniae(6.89% of total teats),followed byB.subtilis(2.44%),B.cereus(2.22%) andPse.aeruginosa(2.22%).At the beginning of the experiment,36 and 38 quarter milk samples had SCC≥200 000 cells mL–1in nonbarrier and barrier teat dip groups,respectively. At the conclusion of the experiment 3,nonbarrier and barrier teat dip treatment teats each had 11 quarter milk samples exceeding the threshold,thus,the subclinical mastitis infection prevalence was similar in the two treatments. Nonbarrier teat dip treatment teats had reductions (P<0.05) inSta.aureusandB.subtilisinfections during the 56-d experiment. In addition,barrier teat dip treatment teats had reductions (P<0.05) inK.pneumoniaeandPse.aeruginosainfections during the 56-d experiment.
During experiment 4,a total of 1 532 milk samples were collected from individual teats for analysis of milk SCC.Around 69.71% of milk samples had SCC<200 000 cells mL–1,thus,were not cultured. At the beginning of the experiment,84 and 96 quarter milk samples had SCC≥200 000 cells mL–1in nonbarrier and barrier teat dip groups,respectively. At the conclusion of the experiment,84(nonbarrier teat dip group) and 56 (barrier teat dip group)quarter milk samples had SCC≥200 000 cells mL–1,and a reduction (P<0.05) of 29% was observed for the subclinical mastitis infection prevalence for barrier teat dip group when compared with nonbarrier teat dip group. During the 56-d experiment,barrier teat dip group had a reduction(P<0.05) in the subclinical mastitis infection prevalence,but there was no difference (P>0.05) in nonbarrier teat dip group. The most-frequently isolated microorganism in both groups wasPse.aeruginosa(9.2% of total teats),followed byE.fergusonii(2.81%),B.cereus(1.89%),Sta.sciuri(1.83%),andK.pneumoniae(1.7%).Staphylococcus saprophyticus,Str.agalactiae,Sta.aureus,andProteus vulgariswere isolated with low frequencies.Escherichia coliwas not isolated in nonbarrier teat dip group (Table 8). The barrier teat dip group had a lower(P<0.05) bacterial infection rate (15.16%) as compared with nonbarrier teat dip group (23.85%) at 56 d. Nonbarrier teat dip group had a reduction (P<0.05) inB.cereusinfection during the 56-d experiment. Barrier teat dip group had a reduction (P<0.05) inE.fergusoniiinfection during the 56-d experiment,but the infection rate with this bacterium increased (P<0.05) in nonbarrier teat dip group during experiment 4.
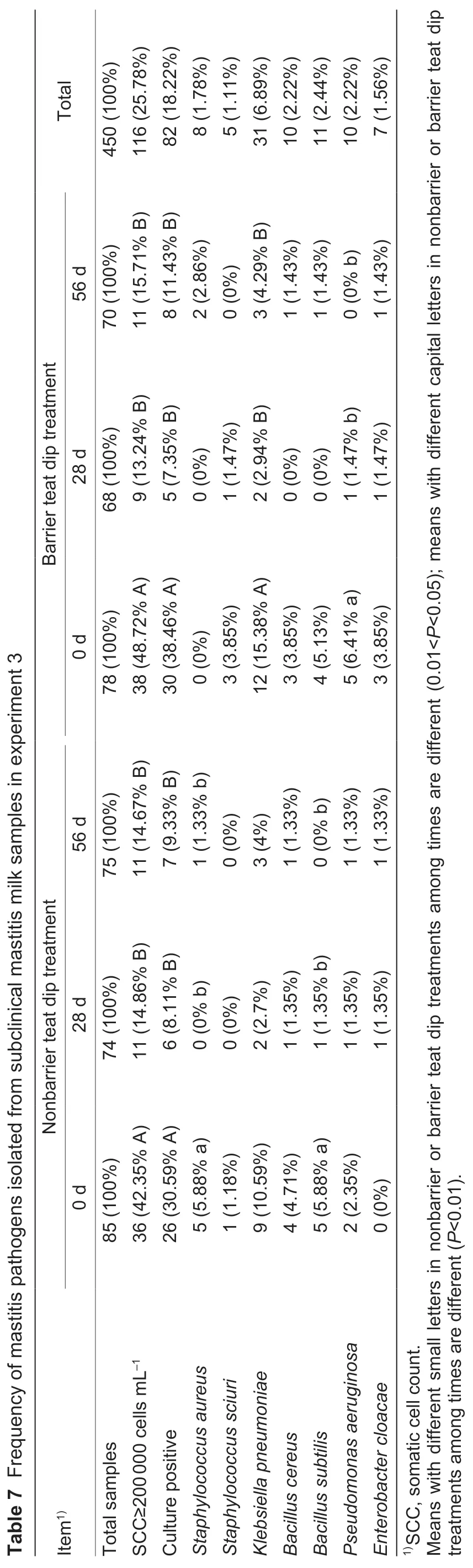
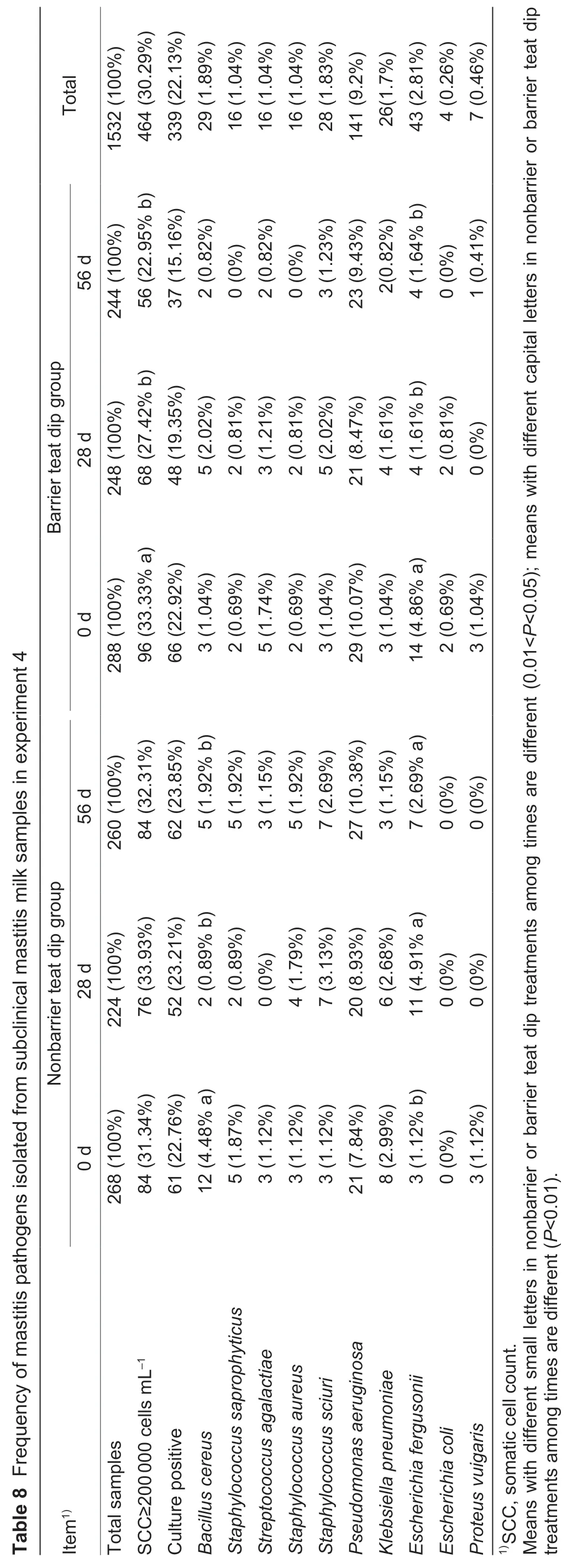
The clinical mastitis incidence during experiment 4 was low,with only five cows (nonbarrier teat dip group=3,barrier teat dip group=2) developing clinical mastitis. Thus,the incidences and relative risks of clinical mastitis were similar in the two groups. Bacteriological culture results indicated thatK.pneumoniae,Sta.aureus,andPse.aeruginosawere detected in nonbarrier teat dip group. The clinical mastitis in barrier teat dip group was due toSta.aureusandEnterobacter cloacae.
4.Discussion
Both povidone-iodine and chitosan have shown excellent antibacterial effects on mastitis-causing bacteria. Thus,it would be very useful to identify the effects of povidoneiodine and chitosan combinations in the teat dip of dairy cows in the prevention of mastitis. Our hypothesis that chitosan with a specific molecular weight combined with lower concentrations of povidone-iodine might be more effective than higher concentrations of povidone-iodine alone in preventing mastitis has been supported by the results of this study. We found that 50 kDa chitosan had the maximuminvitroantibacterial activity compared with 5,150 and 350 kDa chitosans,and the barrier teat dip reduced subclinical mastitis infection prevalence by 29% compared with 10% povidone-iodine. In addition,the povidone-iodine and chitosan combination was useful for prevention ofK.pneumoniae,Pse.aeruginosa,andE.fergusoniiinfections. Our results provided scientific experimental bases for the application of chitosan in the teat dip of dairy cows.
Previous studies reported that molecular weight is an important factor modulating the antibacterial activity of chitosan (Silva-Diaset al.2014;Asliet al.2017). However,many studies reported contradictory results for antibacterial activity of chitosan due to the use of materials of diverse provenance. It was reported that chitosan with high molecular weight yielded low antibacterial activity againstE.coli(Zheng and Zhu 2003),while another study indicated that larger chitosan molecules exhibited greater antibacterial activity than smaller ones (Tokuraet al.1996). In experiment 1,we used well-defined forms of chitosan with a similar degree of deacetylation to evaluate the effect of chitosan molecular weight on thein vitroantibacterial activity against several mastitis-causing bacteria. MIC test results indicated that the antibacterial activities of 5 and 350 kDa chitosan were much lower (P<0.05) than 50 and 150 kDa chitosan for the six mastitis-causing bacteria strains,and 50 kDa chitosan had the maximumin vitroantibacterial activity. Hence,we showed here that molecular weight is critical for the antibacterial activity of chitosan.
Some studies (Penget al.2017;Davoodbashaet al.2018) suggested that the low molecular weight chitosan(LMWC) with molecular masses of 50–190 kDa elicited more favorable bactericidal activity against pathogenic bacteria than high molecular weight chitosan,which was in agreement with the present study. It was demonstrated that the antibacterial activity of LMWC was mainly due to the difference in zeta potential between the surface of LMWC and the bacteria (Chenet al.2010). In this study,most Grampositive bacteria (especiallySta.aureusandStr.agalactiae)were more sensitive to LMWC than Gram-negative bacteria,which is in agreement with a previous report (Raafat and Sahl 2009). Due to the unique polycationic nature of chitosan,it can interact with the negatively charged teichoic acids in the cell wall of Gram-positive bacteria,and eventually disrupt the cell membrane to kill them (Raafatet al.2008).Regarding Gram-negative bacteria,chitosan can interact with lipopolysaccharides in the cell membrane of bacteria,which would not significantly influence the dynamics of the cell envelope,since lipopolysaccharides are confined to the outer membrane (Raafat and Sahl 2009).
In experiment 2,the barrier teat dip with 4.0% povidoneiodine and 1.0% chitosan had higher (P<0.05)in vitroantibacterial efficacy against mastitis-causing bacteria than the barrier teat dip with 4.0% povidone-iodine. Thus,chitosan can enhance the antibacterial activity of the teat dip. In addition to its antibacterial action,chitosan is a biodegradable molecule that has been used as a drug carrier(Saberet al.2010). Chitosan can act as a reservoir for iodine,which may effectively provide time/mass-controlled release of iodine,thus enhancing the antibacterial activity(Chenet al.2016). Recently,combining chitosan with iodine has created a delivery system for iodine,and the results indicated that the chitosan-iodine complex had better antimicrobial properties than iodine alone (Zemljicet al.2018).
In experiment 3,compared with 0 d,a reduction (P<0.01)in subclinical mastitis infection prevalence was observed in all teats on 28 and 56 d. There were no differences (P>0.05)in SCC,SCS,or other milk quality indicators between nonbarrier teat dip and barrier teat dip treatments throughout the experiment. Thus,it could be concluded that the effect of the barrier teat dip containing 1.0% chitosan and 4.0%povidone-iodine in the prevention of mastitis is similar to 10% povidone-iodine (nonbarrier teat dip) in experiment 3.
During experiment 4,no differences (P>0.05) were observed in milk yield,SCC,fat content,or protein content between these two treatments. Thus,we conclude that inclusion of chitosan and other compounds used in barrier teat dip had no detrimental effects on milk yield or quality.In addition,we detected a 29% reduction (P<0.05) of subclinical mastitis in barrier teat dip group compared with nonbarrier teat dip group on 56 d. In barrier teat dip group,the subclinical mastitis infection prevalence on 56 d(22.95%) was lower (P<0.05) than on 0 d (33.33%). In contrast,the subclinical mastitis infection prevalence in nonbarrier teat dip group did not vary (P>0.05) significantly throughout the experiment. These results indicated that barrier teat dip containing povidone-iodine and chitosan was effective in preventing subclinical mastitis.
Compared with the nonbarrier product,under the natural exposure conditions in experiments 3 and 4,the barrier product containing povidone-iodine and chitosan reduced the subclinical mastitis infection prevalence induced by mastitis pathogens. This difference was mainly attributed to the reductions inK.pneumoniae,Pse.aeruginosa,andE.fergusoniiinfections. Mastitis caused byK.pneumoniaeis mostly attributed to opportunistic infection resulting from direct fecal exposure or ingestion of contaminated drinking water (Fuenzalida and Ruegg 2020). It was reported that a single predominant strain ofK.pneumoniaewas responsible for an outbreak of clinical mastitis in a dairy herd in New York State (Munoz et al.2007).Pseudomonas aeruginosawas the most common infection observed in the present study. A study conducted in South Bengal reported that approximately 5.4% of subclinical mastitis cases were caused byPse.aeruginosastrains (Banerjeeet al.2017).Pseudomonas aeruginosais an opportunistic pathogen causing subclinical mastitis in cattle when the environment is contaminated and when cattle defenses are reduced by stress,concomitant disease,or nutritional imbalances (Kelly and Wilson 2016). In cows,E.fergusoniihas been reported as the pathogen causing various clinical conditions such as abortion,diarrhea and mastitis (Bain and Green 1999).In general,a barrier product can form a protective film that inhibits growth of challenge organisms at the teat end,thereby decreasing the opportunity for invasion into the teat canal. Overall reduction in the infection rate demonstrated the advantage of this barrier teat dip containing povidoneiodine and chitosan,which was significantly effective for controllingK.pneumoniae,Pse.aeruginosa,andE.fergusoniiinfections.
5.Conclusion
The evaluation ofin vitroantibacterial activity highlighted the potency of the 50 kDa chitosan against several mastitiscausing bacteria. The barrier teat dip with 4.0% povidoneiodine and 1.0% chitosan also had higherin vitroantibacterial efficacy against mastitis-causing bacteria than the barrier teat dip with 4.0% povidone-iodine. Results of natural exposure experiments demonstrated that the barrier teat dip containing povidone-iodine and chitosan was effective in preventing subclinical mastitis and especially useful for prevention ofK.pneumoniae,Pse.aeruginosa,andE.fergusoniiinfections.These results underscored the potential of chitosan as an alternative component of teat disinfectant formulations for the prevention of bovine mastitis.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (31872324,31702142) and the Jiangsu Modern Dairy Industry Technology System,China (JATS (2018) 300).
Declaration of competing interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable internaltional,national and institutional guidelines for the care and use of animals were followed.
杂志排行
Journal of Integrative Agriculture的其它文章
- Low glycemic index:The next target for rice production in China?
- Effect of side deep placement of nitrogen on yield and nitrogen use efficiency of single season late japonica rice
- Advancements in plant regeneration and genetic transformation of grapevine (Vitis spp.)
- Indica rice restorer lines with large sink potential exhibit improved nutrient transportation to the panicle,which enhances both yield and nitrogen-use efficiency
- Effects of nitrogen management on the ratoon crop yield and head rice yield in South USA
- Response of grain-filling rate and grain quality of mid-season indica rice to nitrogen application
