Cuticular protein gene LmACP8 is involved in wing morphogenesis in the migratory locust,Locusta migratoria
2021-05-23ZHAOXiaomingYANGJiapengGOUXinLlUWeiminZHANGJianzhen
ZHAO Xiao-ming,YANG Jia-peng ,GOU Xin ,LlU Wei-minZHANG Jian-zhen
1 Institute of Applied Biology,Shanxi University,Taiyuan 030006,P.R.China
2 College of Life Science,Shanxi University,Taiyuan 030006,P.R.China
Abstract Cuticular proteins (CPs) are major components of the insect cuticle-associated organs such as integument and wings,although the importance of CPs for wing development and function in hemimetabolous insects remains understudied. In the present study,a wing cuticular protein LmACP8 was identified from Locusta migratoria,which belongs to the RR-2 subfamily of cuticular protein R&R consensus (CPR) chitin-binding proteins. LmACP8 was mainly expressed in the wing pads and showed high expression levels before ecdysis of third-,fourth-,and fifth-instar nymphs,with its encoded protein located in the procuticle of wing pads and adult wings. Depletion of LmACP8 by RNA interference markedly reduced the amount of its protein,which consequently caused abnormal wing morphogenesis in the transition from nymph to adult of L.migratoria. We further demonstrated that the abnormal morphogenesis was caused by severe damage of the endocuticle in the wings. LmACP8 was suppressed by 20-hydroxyecdysone (20E) in vivo,however,its expression was significantly up-regulated after knocking down the hormone receptor gene LmHR39. Thus,the LmACP8 that is negatively regulated by the LmHR39-mediated 20E signaling pathway is involved in wing development during the nymph to adult transition.
Keywords:cuticular proteins,LmACP8,20-hydroxyecdysone,wing development
1.lntroduction
The exoskeleton of arthropods is formed by multiple layered cuticles that are mainly composed of chitin and associated proteins in the form of chito-protein matrices,and it provides good protection against microorganisms,parasitism,predation,and chemicals (Vincent and Wegst 2004).Insect cuticle consists of three horizontal layers,epicuticle,exocuticle and endocuticle from outside to inside,that are secreted by epidermal cells (Appelet al.2015). Cuticular proteins (CPs) are the principal structural constituents of insect cuticle and play a major role in determining the physical properties of the cuticle (Arakaneet al.2009). In recent years,a very large number of insect CPs and CPlike proteins have been identified and divided into thirteen different families as defined by their unique amino acid sequence motifs,such as cuticular protein Rebers and Riddiford (R&R) consensus (CPR) family,cuticular protein Tweedle motif (CPT) family,cuticular protein hypothetical(CPH) family,etc (Zhouet al.2016). CPs in different families may have distinct functions during insect development.
The largest CP family is the CPR family,and its CPs contain an R&R consensus of 35 amino acid residues(Rebers and Riddiford 1988),including RR-1,RR-2,and RR-3 CPRs (Willis 2010). The CPRs with the RR-1 and RR-2 consensus sequences might be associated with soft (flexible) or rigid (hard) cuticles (Willis 2010),or might predominantly form endocuticle or exocuticle,respectively(Andersen 2000). For example,inBombyxmori,a deletion mutation ofBmCPR2encoding an RR-1 protein was found to be responsible for the stony mutant that leads to significantly decreased cuticular chitin content,abnormal distribution of internodes,and intersegmental folds in larvae (Qiaoet al.2014). InTriboliumcastaneum,two major structural RR-2 proteins,TcCPR27 and TcCPR18,are essential for maintaining elytra rigidity in the beetle(Arakaneet al.2012). In addition,CP genes are essential for the survival of insects. InNilaparvatalugens,silencing of 15 CPR genes led to lethal phenotypes,indicating that these genes play essential roles in the integument structure and normal development (Panet al.2018). In the migratory locustLocustamigratoria,a destructive agricultural pest with powerful fore-and hindwings for long-distance migration (Liuet al.2020),several CPs were purified from the wing cuticle of pharate adult locusts and their amino acid sequences were determined by the combined use of mass spectrometry and automated Edman degradation (Kroghet al.1995).In our previous work,we identified and confirmed the sequences of the respective open reading frames in the transcriptome ofL.migratoria(Zhaoet al.2017). Among these genes,we found an adult cuticular protein (LmACP7)gene that encodes for a CPR family protein with an RR-2 motif,which occurred in the exocuticle of adult wings and was an essential structural component required for normal morphological and functional maintenance of the adult wing cuticle (Zhaoet al.2019a). However,the functions of CPs in the development of wings are still not well established.
In the process of insect molting,CPs are periodically synthesized (Charles 2010). Many CP genes have been identified from pre-ecdysial and post-ecdysial cuticles in different insect species and are strictly regulated at the transcriptional level during insect growth and development (Shahinet al.2018;Zhao 2020). CP genes in different tissues and developmental stages are regulated by 20-hydroxyecdysone (20E) (Charles 2010).In holometabolous insects,BmWCP1-9(wing disc cuticle protein gene),identified from wing discs ofB.mori,have been reported to be ecdysone-pulse responsive (Nojiet al.2003;Denget al.2011,2012). Among them,BmWCP2,BmWCP4,BmWCP5andBmWCP10were regulated by the nuclear transcription factor βFTZ-F1-mediated 20E signaling pathway (Nitaet al.2009;Wanget al.2009a,b;Denget al.2011). InDrosophilamelanogasterandManducasexta,the transcription of larval (larval cuticle protein-14,LCP-14)or pupal (ecdysone-dependent gene 78,DEG78) cuticle protein genes were negatively regulated by 20E (Apple and Fristrom 1991;Hirumaet al.1991). In the hemimetabolous insectL.migratoria,LmAbd-9,an endocuticle structural glycoprotein gene from the abdominal cuticle involved in the formation of the endocuticle,was negatively regulated by the hormone receptor 39 (HR39)-mediated 20E signaling pathway (Zhaoet al.2019c). The function of transcription factors that respond to 20E is generally the transmission of upstream signals by binding to the special areas of the downstream genes to activate or inhibit transcription.However,the detailed role of CPs which are regulated by transcription factor-mediated 20E signaling pathway in wing development and wing cuticle formation remain to be elucidated in hemimetabolous insects.
In the present study,another adult cuticular protein gene,LmACP8,was characterized based on our previous transcriptome data (Zhaoet al.2017). We then revealed its developmental stage and tissue-specific expression profiles,determined the localization of its encoded protein in the wing pads of fifth-instar nymphs and adult wings by immunohistochemistry,and precisely localized the protein in adult wing cuticle by immunogold labeling. RNA interference (RNAi) was utilized to investigate its roles in the formation of adult wing cuticle. Based on our previous RNA-seq data,we confirmed thatLmACP8is negatively regulated by the LmHR39-mediated 20E signaling pathway.Our results indicated thatLmACP8,which is negatively regulated by 20E signaling pathway,is required for normal wing morphogenesis as an important structural component in the wing cuticle.
2.Materials and methods
2.1.lnsects rearing
The eggs ofL.migratoriawere purchased from a locust breeding center in Hebei Province of China. The eggs were incubated at (30±1)°C,50% relative humidity with a light/dark (14 h/10 h) photoperiod in our laboratory. After the eggs hatched,the nymphs were fed with fresh wheat sprouts under the same conditions. The third-,fourth-,fifth-instar nymphs and adults were collected for total RNA isolation.
2.2.Bioinformatics analysis of LmACP8
The cDNA sequences of the target gene were found by searching the transcriptomic data from the dissected nymphal wing discs (GenBank accession no.GBDZ00000000) (Liuet al.2014) and the genome ofL.migratoria(LocustBase)(Yanget al.2019). Conceptual translation of cDNA sequences was carried out with translation tools at ExPASy(http://www.expasy.org/tools/dna.html). The deduced protein domains were determined by SMART (http://smart.embl.de/). Analyses of the deduced amino acid sequences,including molecular mass and isoelectric point (pI),were carried out using the EXPASY proteomics server (http://www.expasy.org). Multiple amino acid sequence alignments were performed using GENEDOC software with the default parameters.
2.3.Tissue-specific and developmental expression analysis of LmACP8
Total RNA was extracted using RNAiso™ Plus (TaKaRa Bio,Kusatsu,Japan) from each of ten different tissues,including the wing pads,integument,foregut,midgut,hindgut,Malpighian tubules,gastric cecum,fat body,ovary,and testes,from the 7-day-old fifth-instar nymphs. For expression analysis ofLmACP8at different developmental stages,we dissected the wing pads from third-instar nymphs(days 1,3,and 5),fourth-instar nymphs (days 1,3,and 5),fifth-instar nymphs (days 1,3,5,7,and pre-ecdysis),and adult wings (ecdysis,6 h,and 12 h). The evaluation of total RNA,synthesis of cDNA and quantitative reversetranscription PCR (qRT-PCR) analysis were performed as described in detail previously (Zhaoet al.2018).Relative mRNA levels of target genes were normalized to the expression of the internal markerRPL32(accession no.HQ388820),which is expression stable at different development stages and different tissues (Yanget al.2014),and were calculated with the 2–ΔΔCTmethod (Livak and Schmittgen 2001). The primers are list in Table 1.
2.4.Polyclonal antibody preparation
The partial coding region ofLmACP8(360 bp) was amplified with specific primers (Table 1),and subcloned into pET32a vector (Novagen,Germany). The recombinant pET32a vector was used to transformEscherichiacolistrain BL21(DE3) cells (TransGen,Beijing,China) and induced by isopropylthio-galactoside (IPTG) for expressing LmACP8 fusion protein with thioredoxin tag in the N-terminal according to our previous method (Zhaoet al.2019a). The fusion protein was purified using Ni-sepharose as described previously (Zhaoet al.2014). Purified protein (purity>85%)was injected into New Zealand White rabbits for polyclonal antibody preparation,which was performed by Beijing Protein Innovation Co.,Ltd.,China.
2.5.lmmunohistochemistry and immunogold staining
To localize LmACP8 protein in the wing pads and wing cuticle,immunostaining was performed as described previously (Liuet al.2009;Zhaoet al.2019a). In brief,paraffin sections (5 μm) of the wing pads from day 2 fifthinstar nymphs and the wings from 2-day-old adults were prepared. The LmACP8 protein was detected in paraffin sections by incubation with the LmACP8 rabbit antiserum(1:200) as a primary antibody at 4°C overnight followed by washing with PBS three times for 5 min each. The tissues were then incubated with Cy3-Affinipure Donkey Anti-Rabbit(Jackson ImmunoResearch,USA) secondary antibody for 1 h at room temperature. After washing the tissues three times with PBS,the specimens were incubated with Fluorescent Brightener 28 (FB28) (Sigma,USA) (1 mg mL–1) for 5 s to detect chitin (Topraket al.2010). SYTOXR Green nucleic acid stain (Life Technologies,USA) was added at a dilution of 1:50 000 to label nuclei. The stained tissues were observed and imaged using an LSM 880 confocal laser-scanning microscope (Zeiss,Oberkochen,Germany) at 20× or 60× magnification. All the images in each staining experiment were collected under exactly the same conditions.
To determine the precise location of the LmACP8 proteinin the wing cuticle of adults,wing was treated and ultrathin sectioned samples (~90 nm) were prepared for immunogold labeling with anti-LmACP8 rabbit antibody (1:20),followed by the secondary antibody conjugated with 10 nm gold particles (1:20) (Gold conjugated Goat anti-rabbit IgG,BBI)as described in detail previously (Zhaoet al.2019a). The images were observed and captured with a JEM-1200 EX transmission electron microscope (TEM;JEOL,Japan).
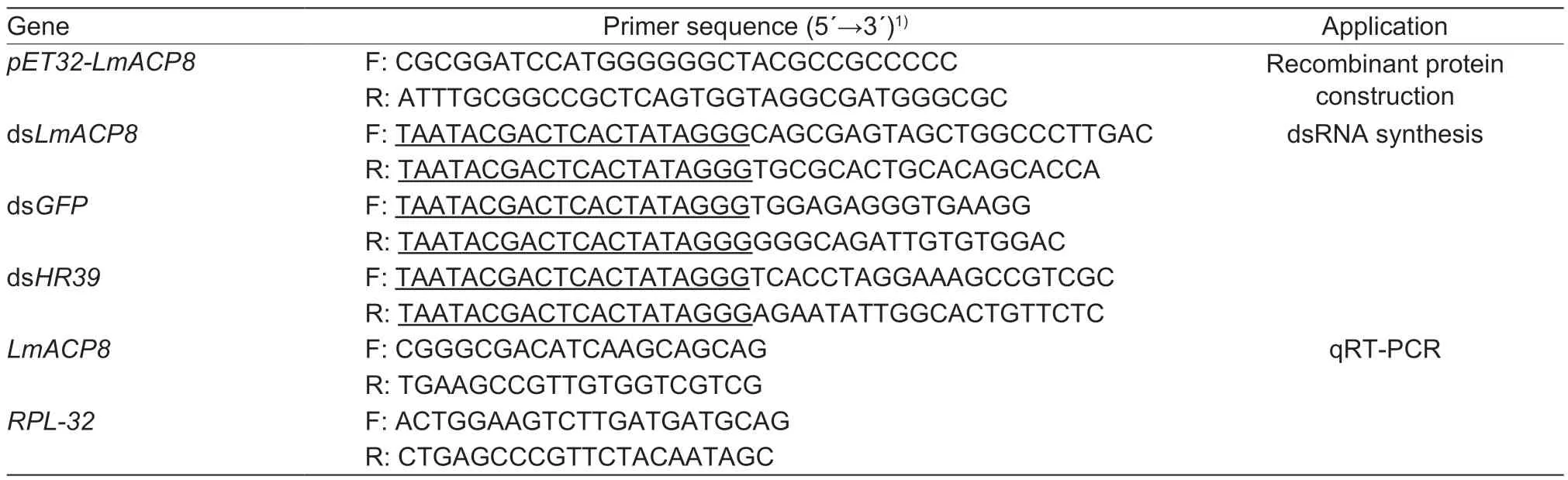
Table 1 Primer sequences used in this study
2.6.RNA interference (RNAi) and transmission electron microscopy (TEM)
The forward and reverse primers harboring the T7 RNA polymerase promoter sequences for synthesizing doublestranded RNA (dsRNA) ofLmACP8(dsLmACP8) andGFP(dsGFP,control) genes were designed as shown in Table 1.The dsRNAs ofLmACP8andGFPwere synthesized using T7 RiboMAX™ Express RNAi System (Promega,Madison,WI,USA) as described previously (Zhaoet al.2018). The synthesized dsLmACP8and dsGFPwere dissolved in appropriate volumes of deionized water,and the concentration was determined and adjusted to 2 μg μL–1. The integrity of dsLmACP8and dsGFPwas confirmed using agarose gel (2%) electrophoresis analysis. The 2-dayold fifth-instar nymphs were randomly divided into two groups (each with 30 nymphs) for injection of each dsRNA sample. Aliquots of 4 μL (10 μg) dsLmACP8or dsGFPwere injected into the hemocoel between the second and third abdominal segments by using a microsyringe. The level of LmACP8 protein was detected at four days after the injection of dsRNA by western blotting as described previously (Zhaoet al.2019a). The remaining nymphs were maintained under the same conditions as described above. The visible phenotype changes were recorded when the nymphs molted into adults.
For TEM,wing subjected to RNAi was treated and analyzed on a JEM-1200 EX TEM (JEOL,Japan) as described in detail previously (Zhaoet al.2019a).
2.7.20E treatment and RNAi of LmHR39
According to our previous transcriptomic data,the transcript ofLmACP8was markedly up-regulated after suppression of the hormone receptor geneLmHR39(Zhaoet al.2019c). To determine whetherLmACP8is responsive to 20E signaling,20E powder (Sigma,St.Louis,MO,USA) was dissolved in 10% ethanol to a concentration of 1 μg μL–1according to our previous study (Liet al.2015). The 2-day-old fifth-instar nymphs were injected with 10 μg of 20E dissolved in 10%ethanol,whereas each nymph in the control group was injected with an equal volume of 10% ethanol. The wing pads of the nymphs were dissected at 12 h after treatment to extract total RNA,and the expression ofLmACP8was detected by qRT-PCR. Meanwhile,we performed another RNAi experiment using dsRNA forLmHR39,which was regulated by the LmEcR-LmHR3-mediated 20E signaling pathway (Zhaoet al.2019c). dsLmHR39and dsGFPwere synthesizedinvitroand 10 μg of dsLmHR39was injected into each fifth-instar nymph (2-day) as described above. The same amount of dsGFPwas injected as a control. After 48 h,the wing pads were dissected to detect the expression ofLmACP8.
2.8.Statistical analysis
All of the data were statistically analyzed by independent sample Student’st-test. Asterisks indicate significant differences (*,P<0.05;**,P<0.01;***,P<0.001).
3.Results
3.1.Sequence analysis of LmACP8
LmACP8was identified from the transcriptome database ofL.migratoria,and its cDNA sequence is 555 bp. The open reading frame (ORF) is 531 bp,encoding a protein of 173 amino acids which contains an RR-2 motif (amino acid 84-152) and multiple repeated motifs (AAPA and PVAVA)(Fig.1-A). The putative protein contains a chitin binding domain 4 (CBD4) (Fig.1-B),with a theoretical molecular weight of 18.52 kDa and pI of 6.48. Multiple sequence alignment analysis showed high similarities in the RR-2 motif and CBD4 with those of other insect species (Fig.1-C).
3.2.Tissue-specific and developmental expression characteristics of LmACP8
To investigate the tissue-specific expression inL.migratoria,we used qRT-PCR to analyze the mRNA expression levels in different tissues from day 7 fifth instar nymphs. The results showed thatLmACP8was highly expressed in the wing pads,followed by the integument and fat body,but showed low expression levels in other tested tissues (Fig.2-A). To explore stage-specific expression patterns ofLmACP8,the temporal expression patterns ofLmACP8mRNA in the wing pads from third-,fourth-,fifth-instar nymphs and the wings of adults were analyzed using qRT-PCR. The results showed thatLmACP8was expressed at a low level in the early and mid-stages of each instar of the nymphs,and then increased to reach a peak before ecdysis (Fig.2-B),followed by a decrease after adult emergence (Fig.2-C),which was the opposite of the 20E titers (Liuet al.2018).
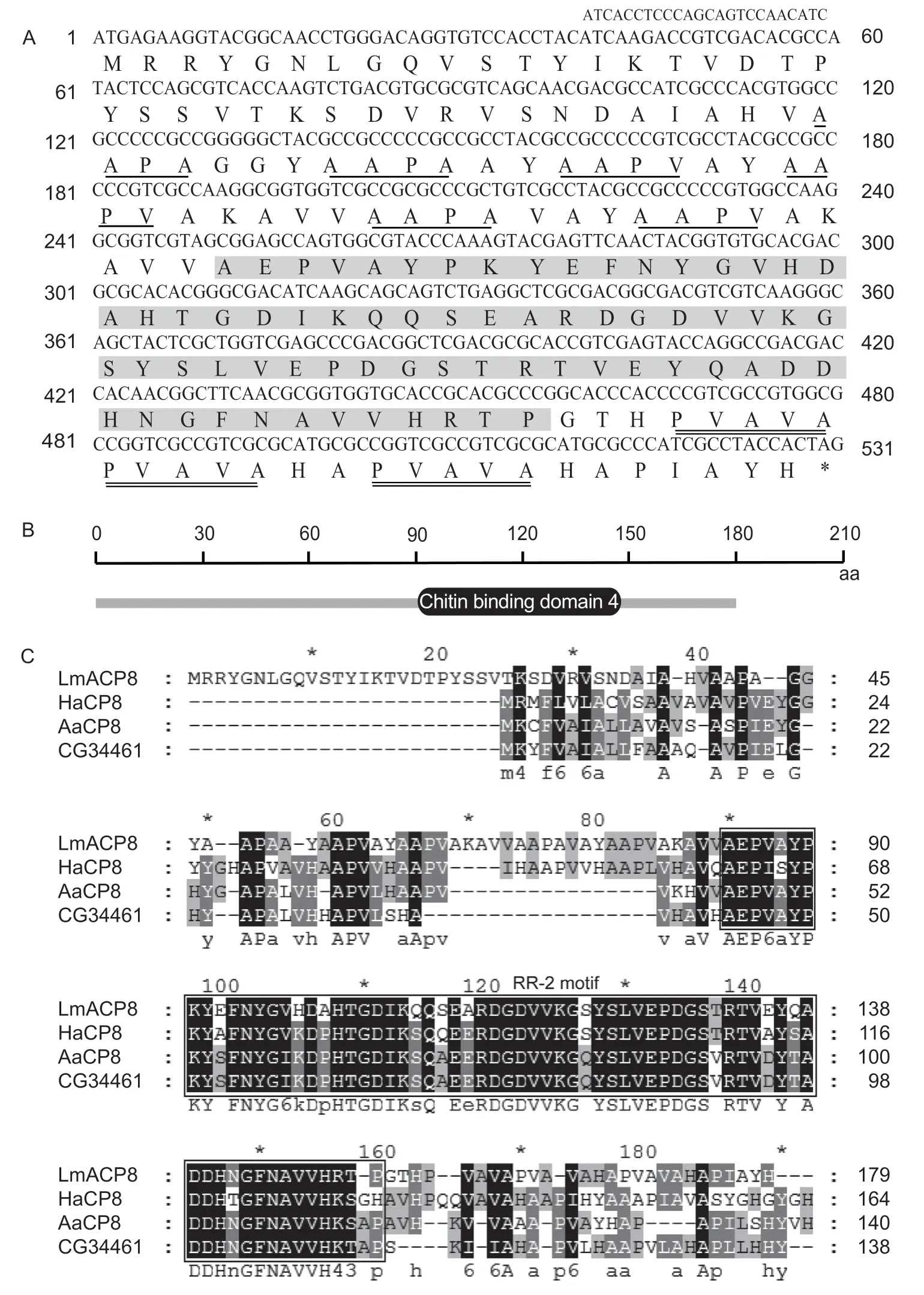
Fig.1 Bioinformatics analysis of LmACP8 in Locusta migratoria. A,the sequence of LmACP8. The single underlines denote AAPA repeat motif;the double underlines denote PVAVA repeat motif;the gray denotes RR-2 motif. B,the chitin binding domain of LmACP8 predicted by SMART. C,multiple sequence alignments of the deduced CP8 proteins in four insect species. The RR-2 motif is enclosed in black boxes. HaCP8,Helicoverpa armigera CP8 protein (XP_021188416.1);AaCP8,Aedes aegypti CP8 protein (XP_001655400.1);CG34461,Drosophila melanogaster CP8 protein (NP_001097547.1).
3.3.LmACP8 localized in the procuticle of wing pads and adult wings
To analyze the localization of LmACP8 in locust wing,we performed immunohistochemistry experiments on paraffin sections of wing pads from 2-day-old fifth-instar nymphs after injection of dsLmACP8and dsGFPat day 2 in the fourth instar nymphs. Compared to that of the control,LmACP8 was specifically detected with a strong signal that co-localized with chitin in the procuticle of the wing pads,but little signal was found in epidermal cells (Fig.3-A–D and E–H). Similar localization and distribution of LmACP8 was also observed in the wings from 2-day-old adults(Fig.3-I–K). To locate the LmACP8 more precisely in the adult wing,we used immunogold labeling to examine the protein localization combined with TEM analysis. In the wing veins,LmACP8 was detected throughout the procuticle,and the signal of LmACP8 in the exocuticle was greater than in the endocuticle (Fig.3-L).
3.4.LmACP8 is required for the development of locust wings
To investigate the function ofLmACP8in the development of the adult wing,the morphology of the wings in adults was examined after reducing the LmACP8 protein level by RNAi.Compared to the control,the level of LmACP8 protein was markedly decreased in 2-old-day adults which subsequently resulted in wing defects (in about 46.6% of injected insects)during the nymph-adult transition. The wings were wrinkled and did not elongate fully,failing to extend far enough to cover the entire abdomen (Fig.4-A and B). To further explore the ultrastructure of wing cuticle,TEM analysis was performed on the dsRNA-treated animals. As shown in Fig.4-C,the wing cuticle consists of the normal epicuticle and the procuticle that contains chitin lamellae subdivided into an exocuticle and an endocuticle in the dsGFP-treated control. However,in the dsLmACP8-treated animals,it was found that the lamellae in both the exocuticle and the region connecting the endocuticle and the apical plasma membrane of epidermal cells was disordered,and the microvilli and cell junction were degraded compared to dsGFP-treated control animals (Fig.4-D). Although the exocuticle had a normal thickness and structure including pore canals,the thickness of the endocuticle was dramatically decreased in the dsLmACP8-treated animals (Fig.4-D). These results indicated thatLmACP8is needed for the structural integrity of the wing cuticle during the nymph to adult molt.
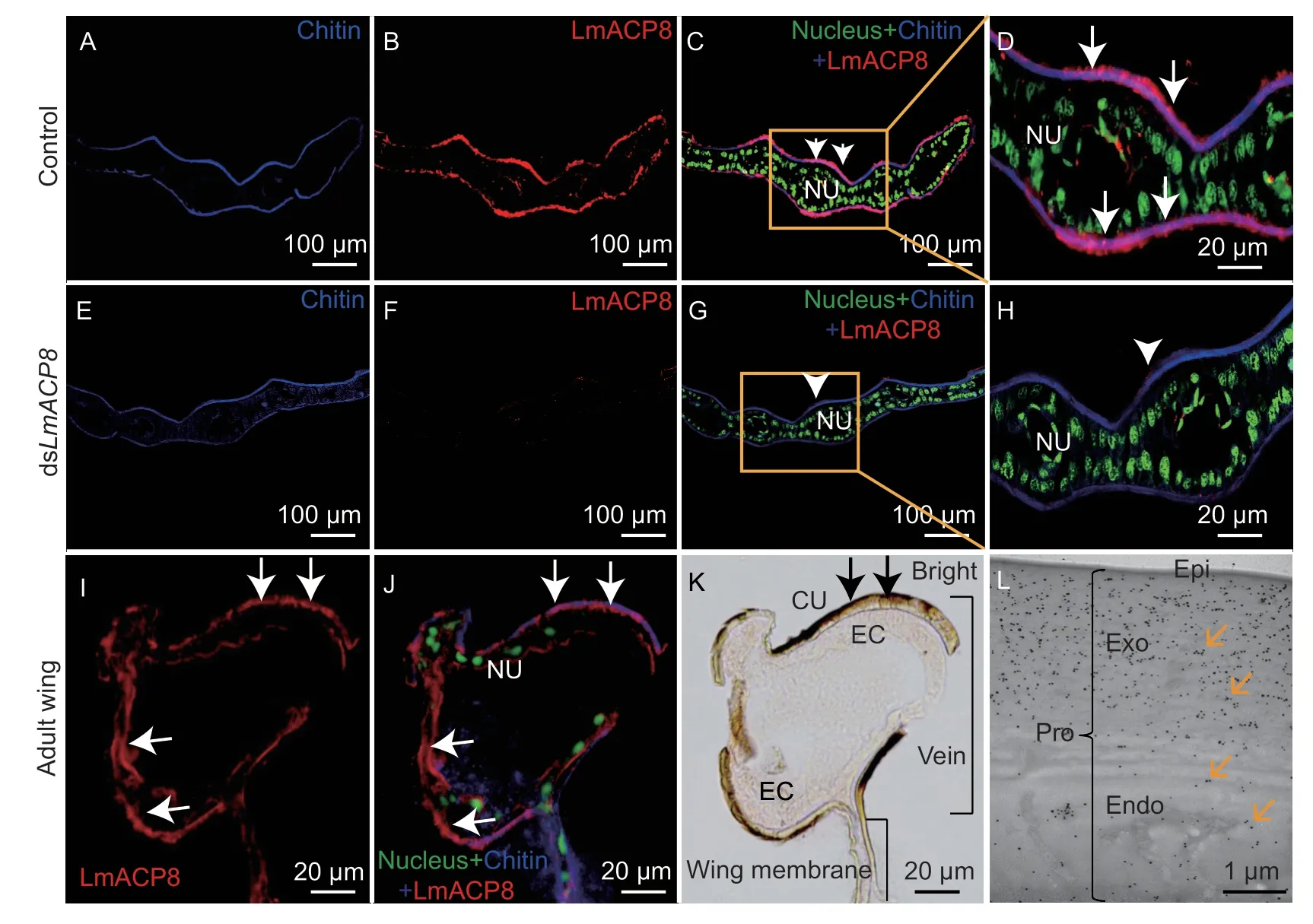
Fig.3 Localization of LmACP8 in wing pads and wings. A–C,localization of LmACP8 in normal wing pads of fifth instar nymph (day 2) by immunochemistry. D represents an enlarged view of the indicated portion of C. E–G,localization of LmACP8 in LmACP8-RNAi wing pads of fifth-instar nymph (2-day old) by immunochemistry. H represents an enlarged portion of G. All the images for each staining experiment were collected under the same conditions. I–K,localization of LmACP8 in adult wing by immunochemistry.White arrows indicate LmACP8 signal in cuticle,black arrows indicate wing cuticle. CU,cuticle;EC,epidermal cell. L,precise localization of LmACP8 in adult wing of Locusta migratoria by immunogold labeling. Epi,epicuticle;Endo,endocuticle;Exo,exocuticle. Orange arrows indicate gold particles.
3.5.LmACP8 is negatively regulated by the LmHR39-mediated 20E signal pathway
According to our previous result of transcriptome data from the wing pads of fifth-instar nymphs after treatment with dsGFPor dsLmHR39,the transcript ofLmACP8was significantly up-regulated approximately 86.8% (t=–28.4,df=4,P=0.00009) in dsLmHR39-treated nymphs compared to that of dsGFP-treated control (Fig.5-A),andLmHR39responded to the LmEcR-LmHR3-mediated 20E signaling pathway,implying thatLmACP8may be negatively regulated by the LmHR39-mediated 20E signal pathway. To test this hypothesis,the 2-day-old fifth-instar nymphs were treated with 20E for 12 h. qRT-PCR results showed that the expression ofLmACP8was down-regulated approximately 94% (t=13.7,df=4,P=0.0002) after treatment with 20E compared with that in the control (Fig.5-B). By contrast,theLmACP8mRNA level was up-regulated approximately 83.5% (t=–4.255,df=8,P=0.003) after suppressing the expression ofLmHR39by RNAi (Fig.5-C). These results indicated thatLmACP8is negatively regulated by the LmHR39-mediated 20E signal pathway.
4.Discussion
In arthropods,structural CPs play important roles in determining the diverse physical properties of the cuticle depending on developmental stages as well as different body regions. In the present study,we identified a cuticular protein geneLmACP8from locust wing pads,which encodes a member of subgroup RR-2. The members of subgroup RR-2 have mainly been found in cuticle-associated body parts which tend to be stiff and sclerotized,such as the elytra and hindwing cuticle ofT.castaneum(Dittmeret al.2012;Nohet al.2017). We thus proposed a hypothesis that a correlation exists between the types of these proteins and mechanical properties of the locust wing cuticle.
In our previous work,a wing-specific cuticular protein gene encoding an RR-2 protein,LmACP7,was identified and found to be distributed in the exocuticle of wings and involved in the maintenance of the structural integrity of adult wings (Zhaoet al.2019a). Similar toLmACP7,LmACP8is also mainly expressed in the wing pads and has a high expression level at each pre-ecdysial stage (Fig.2),and its encoded protein is mainly located in the exocuticle of wings(Fig.4-D). WhenLmACP8expression was suppressed by RNAi,the resulting adult wings were not fully elongated but remained wrinkled,implying thatLmACP8may have functions similar toLmACP7. Alternatively,because of the small amount of post-ecdysial expression (Fig.2-B),LmACP8 is also distributed in the endocuticle of wings and has a co-localization with chitin that exists in the procuticle of the wing pads and wings. Dysfunction ofLmACP8resulted in disordered lamellae of the exocuticle and the thickness of the endocuticle was dramatically decreased,indicating thatLmACP8is needed for structural integrity of the wing cuticle.
As reported previously,in the ventral epidermis ofDrosophilaembryos,cells either lose their adhesiveness and fall out of the epidermis or undergo apoptosis (Bloor and Kiehart 2002). InB.mori,a cuticular protein gene,BmorCPH24,was involved in the synthesis of endocuticle and its disruption induced apoptosis of epidermal cells (Xionget al.2017). In this work,we found the region connecting the endocuticle and the apical plasma membrane of epidermal cells was disordered and the microvilli were degraded in dsLmACP8-treated insects,findings which are similar to the results ofLmACP7-deficiency that induced cell apoptosis or a similar process (Zhaoet al.2019a). Additionally,we also found the epidermal cells of wings from dsLmACP8-injected insects exhibited cell debris and cell junctions which were degraded. Taken together,a deficiency ofLmACP8may result in a cell apoptosis-like process during molting.However,determining how a structural protein induces a cell apoptosis-like process needs further investigation.
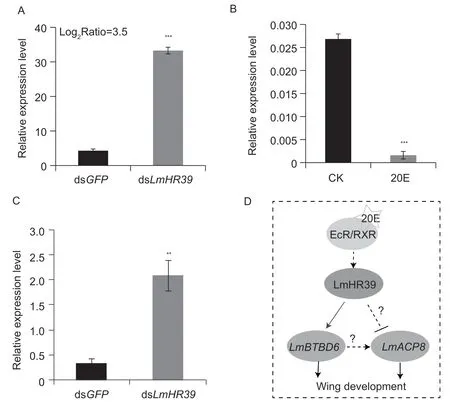
Fig.5 The expression of LmACP8 as regulated by the LmHR39-mediated 20E signaling pathway. A,the FPKM of LmACP8 from our previous transcriptome data. B,the expression level of LmACP8 after 20E induction detected by qRT-PCR. C,the expression level of LmACP8 detected by qRT-PCR after injection of dsGFP or dsLmHR39. RPL-32 was used as the reference control. All data are reported as mean±SD of at least three independent biological replications. **,P<0.01;***,P<0.001. D,20E binds to its receptors EcR and RXR,triggering the transcription of the nuclear receptor factor gene LmHR39,and then the LmHR39-mediated 20E signaling pathway negatively regulates the expression of LmACP8,whereas the LmHR39-mediated 20E signaling pathway prompts LmBTBD6 to directly or indirectly regulate the expression of LmACP8 that is involved in the development of wings during molting.
In arthropods,the molting process is regulated by steroid hormones actingvianuclear receptor proteins (Nakagawa and Henrich 2009). In holometabolous insects such asB.mori,the structure of wing pads undergoes significant changes to form the pupal wing during larval-pupal transition,which is mainly controlled by 20E signaling (Denget al.2012). During this process,the expression of the wing cuticle protein genes is regulated by 20E signaling (Nojiet al.2003). However,the regulatory mechanism of wing development in heterometabolous insects is still unclear.In our previous work,the nuclear receptor geneLmHR39,which is involved in the 20E signaling pathway,was required for locust molting and wing development,and many genes involved in this process were revealed through RNA-seq analysis (Zhaoet al.2019c). Interestingly,the expression ofLmACP8is dramatically increased in the dsLmHR39-treated group based on transcriptome data,and this result was further confirmed byLmHR39-RNAi (Fig.5-A and C).By contrast,the expression level ofLmACP8is decreased significantly after 20E induction compared to that of control(Fig.5-B),suggesting thatLmACP8is down-regulated by the LmHR39-mediated 20E signaling pathway. According to our recent work,a BTB domain-containing protein 6 (LmBTBD6)gene was identified,mainly expressed in the wing pads,and found to be regulated by the LmEcR-LmHR39-mediated 20E signaling pathway and involved in wing development during the nymph to adult transition (Zhaoet al.2019b). After suppressing the expression ofLmBTBD6,the transcription ofLmACP8was significantly decreased (Zhaoet al.2019b),but whetherLmACP8is directly or indirectly regulated by LmBTBD6 needs further investigation. In addition,during the development of insect wings,it is believed that multiple proteins and related factors are required for the formation and construction of the wing cuticle structure,and are crosslinked;however,the specific mechanism of action remains understudied.
Based on our results and previous studies,we propose a hypothesis that 20E binds to a heterodimer formed by its receptors EcR and RXR,triggering transcription of the hormone receptor geneLmHR39,after which the LmHR39-mediated 20E signaling pathway negatively regulates the expression ofLmACP8;whereasLmBTBD6,regulated by the LmHR39-mediated 20E signaling pathway,promptsLmACP8expression that is involved in the maintenance of the structural integrity of adult wings during the nymph to adult transition (Fig.5-D).Locusta migratoriais one of the most destructive agricultural pests;therefore,this work might provide potential targets for pest control.
5.Conclusion
Based on our previous transcriptome data,a wing cuticular protein geneLmACP8was identified and the deficiency of this gene caused abnormal wing morphogenesis with severe damage of the endocuticle during nymph to adult transition.We further demonstrated thatLmACP8was negatively regulated by the LmHR39-mediated 20E signaling pathway.Thus,we concluded thatLmACP8,as negatively regulated by the LmHR39-mediated 20E signaling pathway,is involved in the structural integrity of the wing cuticle,which provides a new insight into our understanding of the molecular mechanism of insect wing development.
Acknowledgements
This work was supported by the National Key R&D Program of China (2017YFD0200900),the National Natural Science Foundation of China (31702067 and 31970469),the National Natural Science Foundation of China–Deutsche Forschungsgemeinschaft of Germany (31761133021),and the Scientific and Technological Innovation Programs of Higher Education Institutions in Shanxi,China (2019L0033).
Declaration of competing interest
The authors declare that they have no conflict of interest.
杂志排行
Journal of Integrative Agriculture的其它文章
- Low glycemic index:The next target for rice production in China?
- Do cooperatives participation and technology adoption improve farmers’ welfare in China? A joint analysis accounting for selection bias
- Impacts of household income on beef at-home consumption:Evidence from urban China
- The water-saving potential of using micro-sprinkling irrigation for winter wheat production on the North China Plain
- Changes in bacterial community and abundance of functional genes in paddy soil with cry1Ab transgenic rice
- Synergistic effect of Si and K in improving the growth,ion distribution and partitioning of Lolium perenne L.under saline-alkali stress
