Molecular characterization of the ryanodine receptor from Adoxophyes orana and its response to lethal and sublethal doses of chlorantraniliprole
2021-05-23
Research Institute of Pomology,Chinese Academy of Agricultural Sciences,Xingcheng 125100,P.R.China
Abstract The insect ryanodine receptor (RyR) is a novel target of the anthranilic and phthalic insecticides,which have high activity against lepidopteran insects. Several diamide insecticides have been used to control pests in orchards in China. To enhance our understanding of the effects of diamides on RyRs,full-length cDNAs were isolated and characterized from the summer fruit tortrix moth,Adoxophyes orana,which is the most severe pest of stone and pome trees worldwide. In addition,the modulation of AoRyR mRNA expression by diamide insecticides was investigated. The AoRyR mRNA obtained had an open reading frame (ORF) of 15 402 bp nucleotides encoding 5 113 amino acids,and shared high and low identity with its orthologs in other insects and mammals of 77–92 and 45–47% identity,respectively. One alternative splice site with two exclusive exons was revealed in AoRyR (a/b). The usage of exon was more frequent in eggs and larvae than in pupae and adults. Quantitative real-time reverse transcription PCR (qRT-PCR) showed that AoRyR mRNA was expressed at all developmental stages,especially in eggs,male pupae and male adults. The expression levels of AoRyR mRNA in the whole body were up-regulated markedly after 3rd instar larvae were treated with chlorantraniliprole at LC10 ,LC20 and LC50 dosages. The results could provide the basis for further functional studies of AoRyR and for the development of new chemicals with selective activity against insects.
Keywords:ryanodine receptor,Adoxophyes orana,chlorantraniliprole,mRNA expression
1.Introduction
The insect ryanodine receptor (RyR) is the target of diamide insecticides. RyRs are the largest known calcium channel proteins,and are composed of homomeric tetramers with a large hydrophilic N-terminal domain and a small membrane-spanning C-terminal domain. Each monomer of approximately 5 000 amino acids has a molecular weight of approximately 560 kDa. RyRs are located in various excitable tissues,such as muscles and neurons,and in many different cell types (Ogawa and Murayama 1998;Fill and Copella 2002;Hamilton 2005;Sattelleet al.2008).RyRs play an important role in regulating the release of calcium from the lumen of the sarcoplasmic/endoplasmic reticulum to the cytosol of muscle and non-muscle cells and causing muscle contraction (Ogawa 1998). Three RyR isoforms (RyR1,RyR2 and RyR3) exist in mammals,and two isoforms (RyRα and RyRβ) have been cloned in fish,amphibians,and birds (Ottiniet al.1996;Ogawaet al.1999).However,only one RyR,which is encoded by two mutually exclusive exons,exists in insects (Wang J Jet al.2012).Each pair of mutually exclusive exons of approximately 99 bp in each RyR shares approximately 54.5% identity.
Diamide insecticides,a class of insecticides that target insect RyRs,were developed in the 1990s (Lahmet al.2005;Teixeiraet al.2012). To date,several commercialized diamide insecticides are widely applied in China,such as flubendiamide,chlorantraniliprole and cyantraniliprole (which are also applied worldwide),in addition to tetrachloroamide(Sunet al.2015b). This class of insecticides has high activity against lepidopterans and is relatively safe for natural enemies and mammals (Tohnishiet al.2005;Gopal and Mishra 2008;Dinteret al.2009;Ioriattiet al.2009;Tiwari and Stelinski 2012). This differential toxicity may be due to the different isoforms of RyRs which exist between lepidopteran insects and mammals (Sattelleet al.2008).
Currently,diamides are widely used to control insects that attack food crops and vegetables (Ioriattiet al.2009;Hanet al.2012;Roditakiset al.2013;Camposet al.2015).Some insects have become highly resistant to diamide insecticides because of overuse (Troczkaet al.2012;Linet al.2013;Wanget al.2013;Guoet al.2014a,b;Yanet al.2014). Diamide insecticides are seldom used for controlling pests of tree fruits,however,chlorantraniliprole is reported to have excellent activity against the summer fruit tortrix moth (Adoxophyesorana) (Zhanget al.2011).Adoxophyesoranais one of the most damaging pests of the leaves of stone and pome fruit trees,such as apple,peach,pear,and apricot trees. At present,this pest is mainly controlled by insecticides and is resistant to tebufenozide,methoxyfenozide and lambda-cyhalothrin (Sunet al.2014). So far,resistance to diamide insecticides has not been reported in the summer fruit tortrix moth. However,with the increasing application of diamide insecticides forA.orana,resistance can develop in the future. Here,we identified the full-length RyR mRNA fromA.orana(AoRyR)and investigated theAoRyRmRNA expression pattern and its response to chlorantraniliprole treatments.
2.Materials and methods
2.1.Insect rearing
A laboratory colony ofA.oranawas originally collected in 2016 from Xingcheng (40.61°N,120.73°E) in Liaoning Province,China. The larvae were reared on peach tree leaves without pesticides in a laboratory for two generations under an 16 h L:8 h D photoperiod at (25±1)°C and 70–80%relative humidity (Sunet al.2015 a).
2.2.Molecular cloning and bioinformatic analysis of AoRyR profiles
Total RNA was isolated from a mixture of eggs,larvae,pupae,and adults using Trizol as described previously (Sunet al.2012). First-strand cDNA was synthesized from 1 μg of total RNA using a TaKaRaTMcDNA Synthesis Kit (TaKaRa,China). TheAoRyRwas assembled from 13 fragments(Fig.1),and the primers for these fragments are shown in Table 1. Six pairs of degenerate primers (S1–S6) were designed according to the alignment between the amino acid residues of RyR isoforms from the lepidopteran insects and other species. The fragments of S7 to S11 were then amplified using the gene specific primers that were designed according to the results from S1 to S6. Based on S1 and S6,the S12 and S13 fragments were obtained by rapid amplification of cDNA ends (RACE). Amplification of each fragment was performed with the following steps:an initial denaturing step at 94°C for 1 min;30 cycles of 98°C for 10 s,48 to 65 °C (determined by the Tm of the primers) for 30 s,and 72°C for 1 to 3 min (depending on the length of the amplified fragments);and a final additional polymerization step at 72°C for 5 min. The PCR products were purified and cloned into a pMD19-T vector (TaKaRa). The cloned cDNAs were then sequenced at BGI (Beijing,China),and the whole cDNA ofAoRyRwas assembled by overlapping all the amplified fragments. The sequences were analyzed by the same methods as used in our previous research,and alternative splicing (AS) was identified (Sunet al.2012,2016).
Diagnostic PCR (the ASP-a primers were 5´-TTTGAGATAC TTACTGCCGG and GGCGCCGA AGCTGTCGTTGGA-3´;the ASP-b primers were 5´-TTTGAGATACTTACTGCCGG and CTTGCCGAACGATTCTGAACT-3´) was used to determine the usage of each putative alternative fragment ofAoRyRmRNA from eggs,larvae,pupae,and adults.Data were collected from sets of 30 positive clones for each fragment and at each developmental stage.
2.3.Lethal and sublethal experimental design
Solutions of chlorantraniliprole (98%;Shandong Weifang Rainbow Chemical Industry Co.,Ltd.,China) dissolved in dimethyl sulfoxide were prepared and then diluted with a 0.1% Tween 80 solution to final concentrations of 0–5 mg L-1. Peach tree leaves were immersed into the above insecticide solutions for 10 s and then allowed to air dry before being placed in sealed Petri dishes for a dilution series with each insecticide dosage level. Third instarA.oranalarvae (n=20) were enclosed within each sealed Petri dish.Mortality was then evaluated 72 h after being raised on leaves treated with chlorantraniliprole. The LC10,LC20and LC50values were then calculated by using a regression log-probit model analysis (Zhanget al.2015).
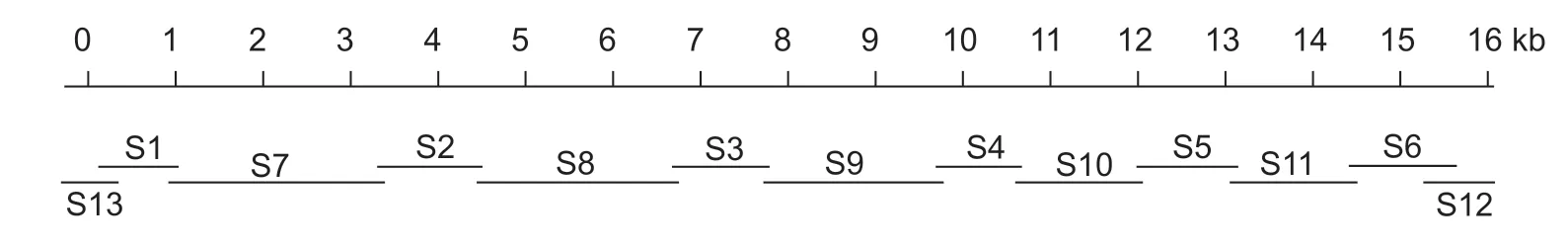
Fig.1 PCR amplification and cloning of AoRyR cDNA. Degenerate and unique primers were used to generate 13 PCR fragments(S1–S13). The full-length AoRyR cDNA sequence was obtained by overlapping all amplified fragment sequences.
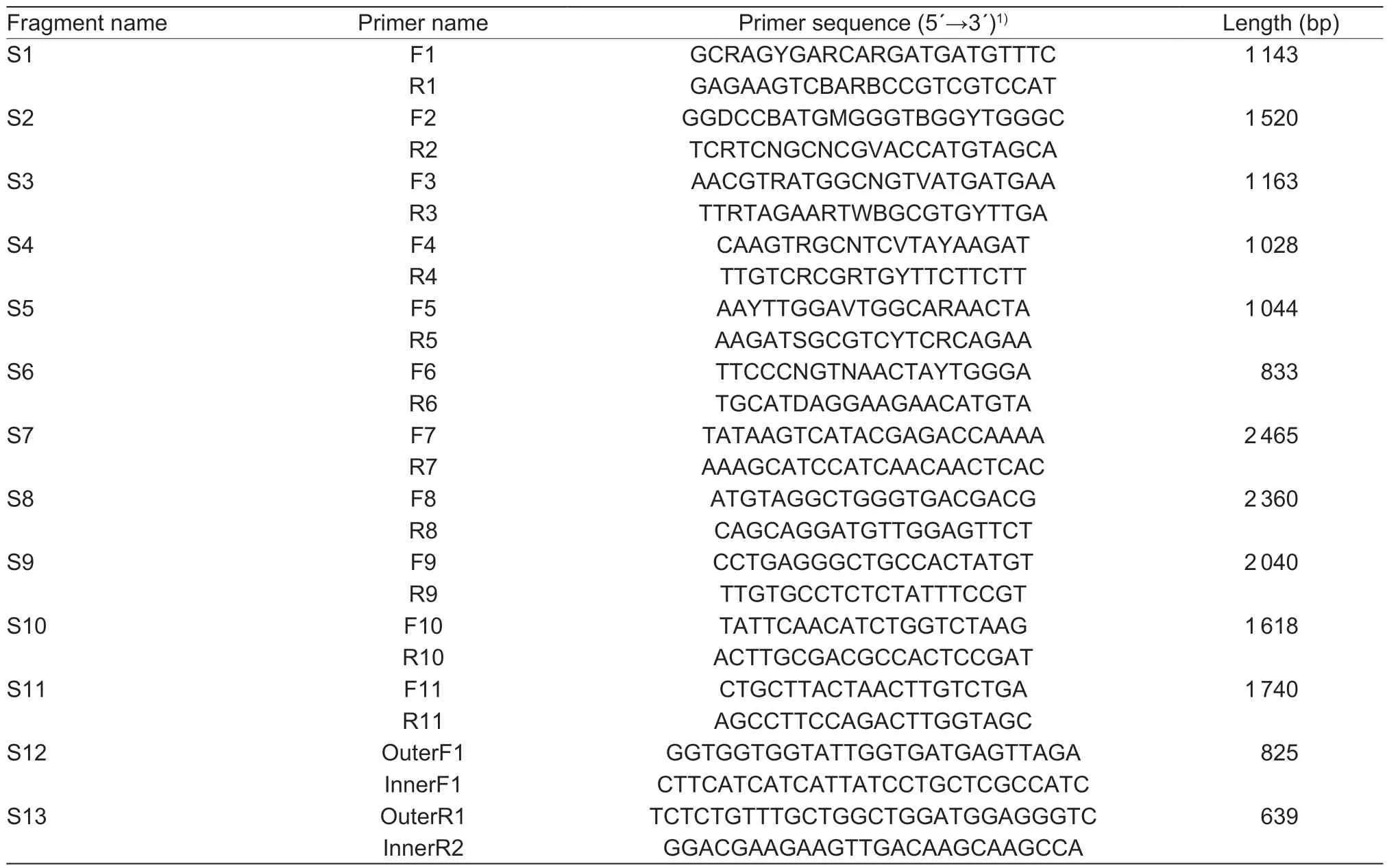
Table 1 Primers used in cloning AoRyR cDNA
The effects of the LC10,LC20and LC50doses of chlorantraniliprole on the expression ofAoRyRwere examined. Third instar larvae were raised for 48 h on leaves treated with chlorantraniliprole (LC10,LC20or LC50) and then transferred to peach tree leaves without insecticides for an additional 48 h. Ten living larvae were collected,immediately frozen in liquid nitrogen,and then stored at–80°C. Each experiment consisted of four dishes per concentration.
2.4. Expression levels of RyR mRNA from A.orana and effects of chlorantraniliprole on expression of AoRyR
The relative expression levels ofAoRyRin the larvae treated with LC10,LC20and LC50doses of chlorantraniliprole and the abundances ofAoRyRduring different developmental stages (eggs,larvae,pupae and adults) were determined using real-time quantitative PCR with a Bio-Rad CFX96 PCR System (Hercules,CA,USA) together with SYBR®PremixEx Taq™ (TaKaRa) and the corresponding primer pairs(Table 2). The samples of 100 eggs,50 1st instar larvae,and 20 of either 2nd to 5th instar larvae,3-day old male and female pupae or 3-day old female and male adults were prepared. For the 3rd instar larvae,the same individuals used in the bioassays were used in the analysis of the effects of chlorantraniliprole on the expression ofAoRyRabundance. The amplification conditions were as follows:95°C for 30 s,followed by 45 cycles of 95°C for 5 s,55°C for 15 s and 72°C for 15 s. The amplification efficiency of each gene was estimated by using E=10–1/slope–1,where the slope was derived from the plot of the cycle threshold (Ct) value versus the log of the serially diluted template concentration,which was calculated to be 0.9500 to 0.9900. The data analysis model for quantifying the transcript level ofAoRyRwas calculated according the 2–ΔΔCTmethod (Schmittgen and Livak 2008). Each sample included at least three replications.

Table 2 Toxicity of chlorantraniliprole against 3rd-instar larvae of Adoxophyes orana at 72 h treatment
2.5.Data analysis
The bioassay experiment data were analyzed by probit using SPSS 20 (SPSS,Inc.,Chicago,IL,USA). TheAoRyRexpression data are shown as the mean±SD,and a statistical analysis was performed by Duncan’s multiple range test for significance (P<0.05) using SPSS 20.
3.Results
3.1.Isolation of AoRyR
AoRyRsequences,with either exon a (AoRyR-ASa) or b(AoRyR-ASb) (GenBank accession numbers:MG013970 and MG013971),were 16 071 bp long with an open reading frame (ORF) of 15 342 bp,as analyzed by overlaying all of the amplified sequenced fragments. The twoAoRyRisoforms encode proteins of 5 113 amino acid residues,with molecular weights of 577 755.99 and 577 674.97 Da,respectively,and predicted isoelectric points of 5.38 and 5.39,respectively.
A phylogenetic tree constructed using the twoAoRyRs and 28 other RyRs (Fig.2) shows that all RyR subunits clearly segregated from the RyRs of Nematoda and the RyRs of vertebrates and insects. The lepidopteran RyRs grouped together but are divided into seven branches depending on the family. The twoAoRyRs clustered withGmRyR from the oriental moth,suggesting that these two genes are closely related.
Seven standard features ofAoRyRwere predicted(Fig.3). Six conserved and essential hydrophobic transmembrane segments,TM1 (F4463–L4485),TM2 (N4644–Y4666),TM3 (V4723–L4745),TM4 (F4865–A4887),TM5 (L4913–F4935)and TM6 (V4993–I5012),were predicted in the C-terminal region using the TMHMM server. Moreover,an RIH-associated domain (residues 4 011–4 124) and an EF-hand domain pair (Ca2+-binding sites,residues 4 208–4 263) were also predicted within the C-terminal region. A MIR domain (217–398),two RIH domains (447–647 and 2 230–2 459),three SPRY_RyR domains (653–805,1 085–1 217 and 1 536–1 688) and four repeated RyR domains (860–949,973–1 063,2 830–2 920 and 2 956–3 040) were also found.
One putative alternative splice site inAoRyRwas revealed by the alignment of multiple cDNA clone sequences and the sequences of the PCR products. This site was located between nucleotides 3 421 and 3 519 (a/b) (Fig.4),which constituted one pair of mutually exclusive exons(Wang J Jet al.2012). The identity shared between a and b is 55.6%,and the identity shared by the amino acid motifs encoded by a and b is 54.5%. The results of diagnostic PCR for the frequencies of avs.b (Fig.5-A) show that the usage of exon a was more frequent in eggs and larvae than in pupae and adults.
To examine the relative expression levels ofAoRyRmRNA in different developmental stages and sexes,Ao18Swas chosen as the reference gene (Fig.5-B).qRT-PCR analysis showed thatAoRyRmRNA exists in all developmental stages,especially in eggs,pupae and adults.While it was detected at relatively low levels in the larvae,the highest expression level was detected in the eggs. The relative expression levels in female and male pupae were 1.42-and 3.25-fold higher than those in 3rd instar larvae,respectively. In addition,the expression level ofAoRyRin male pupae was 2.30 times higher than in female pupae,and it was 2.46 times higher in male adults than in female adults.
3.2.Effects of LC10 ,LC20 and LC50 chlorantraniliprole treatments on mRNA expression in A.orana
The toxicity results for the effects of chlorantraniliprole on 3rd instarA.oranaare shown in Table 2. These results showed that at 72 h after exposure,the mortality ofA.oranain the control group was 5%,and the LC50value of chlorantraniliprole forA.orana3rd instar larvae was calculated to be 0.430 mg L-1,while the corresponding LC10and LC20values were estimated to be 0.060 and 0.152 mg L-1,respectively.
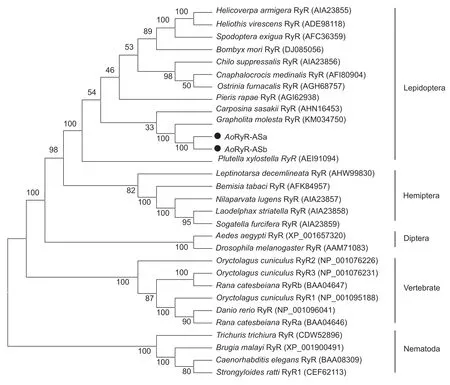
Fig.2 Phylogenetic analysis of AoRyRs and other RyR isoforms from different invertebrate and vertebrate species. The AoRyR amino acid sequence was aligned to the sequences of the 28 RyR isoforms used for phylogenetic analysis. A neighbor-joining tree with 1 000 bootstrap replicates was generated by MEGA6.
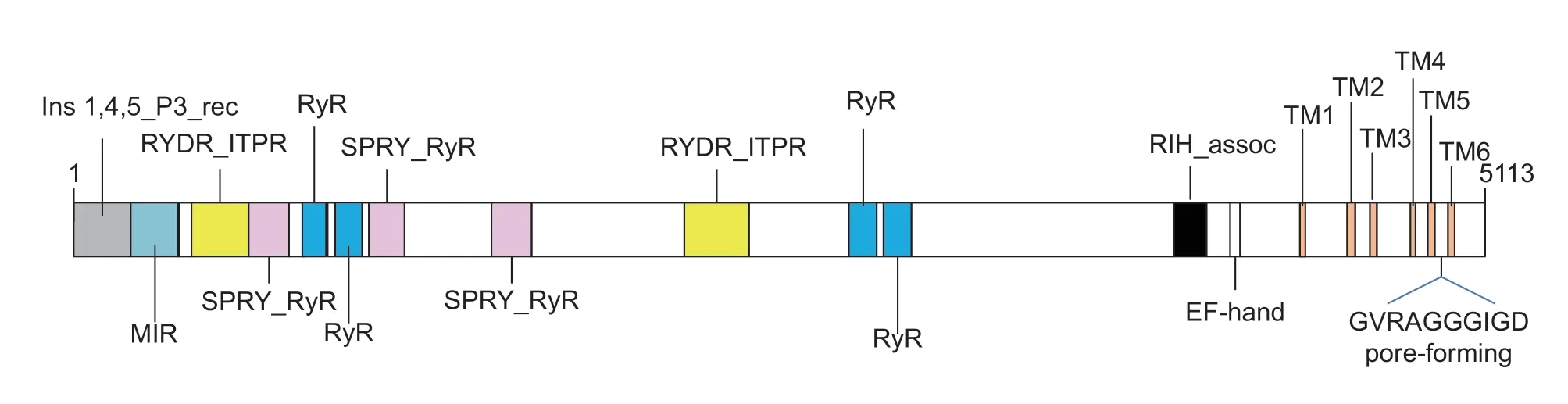
Fig.3 Analysis of AoRyR structure. Schematic showing the locations of putative domains identified using the Conserved Domains Database in NCBI and TMHMM server,including MIR domains,RIH domains,SPRY_RyR domains,RyR domains,RIH-associated domains,EF hands,and TM domains.
The 3rd-instar larvae were raised continuously for 48 h on peach leaves that had been treated with these three different concentrations of chlorantraniliprole. The results showed that the relative expression levels ofAoRyRmRNA were upregulated 1.42-,1.59-,and 2.22-fold after 48 h of treatment with LC10,LC20and LC50doses of chlorantraniliprole,respectively (Fig.6-A). To clearly demonstrate the effects of the insecticide on the expression ofAoRyRmRNA,groups of larvae were first allowed to feed for 48 h on leaves treated with chlorantraniliprole and were then allowed to feed on leaves without chlorantraniliprole for an additional 48 h (Fig.6-B). Interestingly,compared with the levels of the control group,the relative expression levels ofAoRyRmRNA from the larvae that fed on leaves treated with the three lethal and sublethal doses of chlorantraniliprole were significantly downregulated after an additional 48 h without chlorantraniliprole,as the levels exhibited 0.91-,1.29-and 1.86-fold changes at the LC10,LC20and LC50doses,respectively.
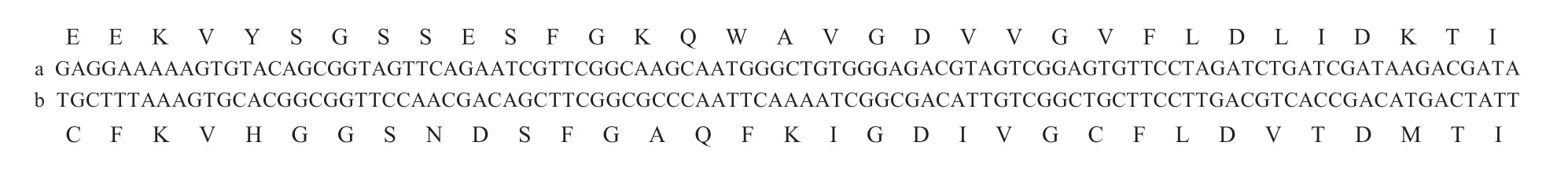
Fig.4 Nucleotide and putative amino acid sequences of the two alternatively spliced exons (a and b) in the AoRyR gene.
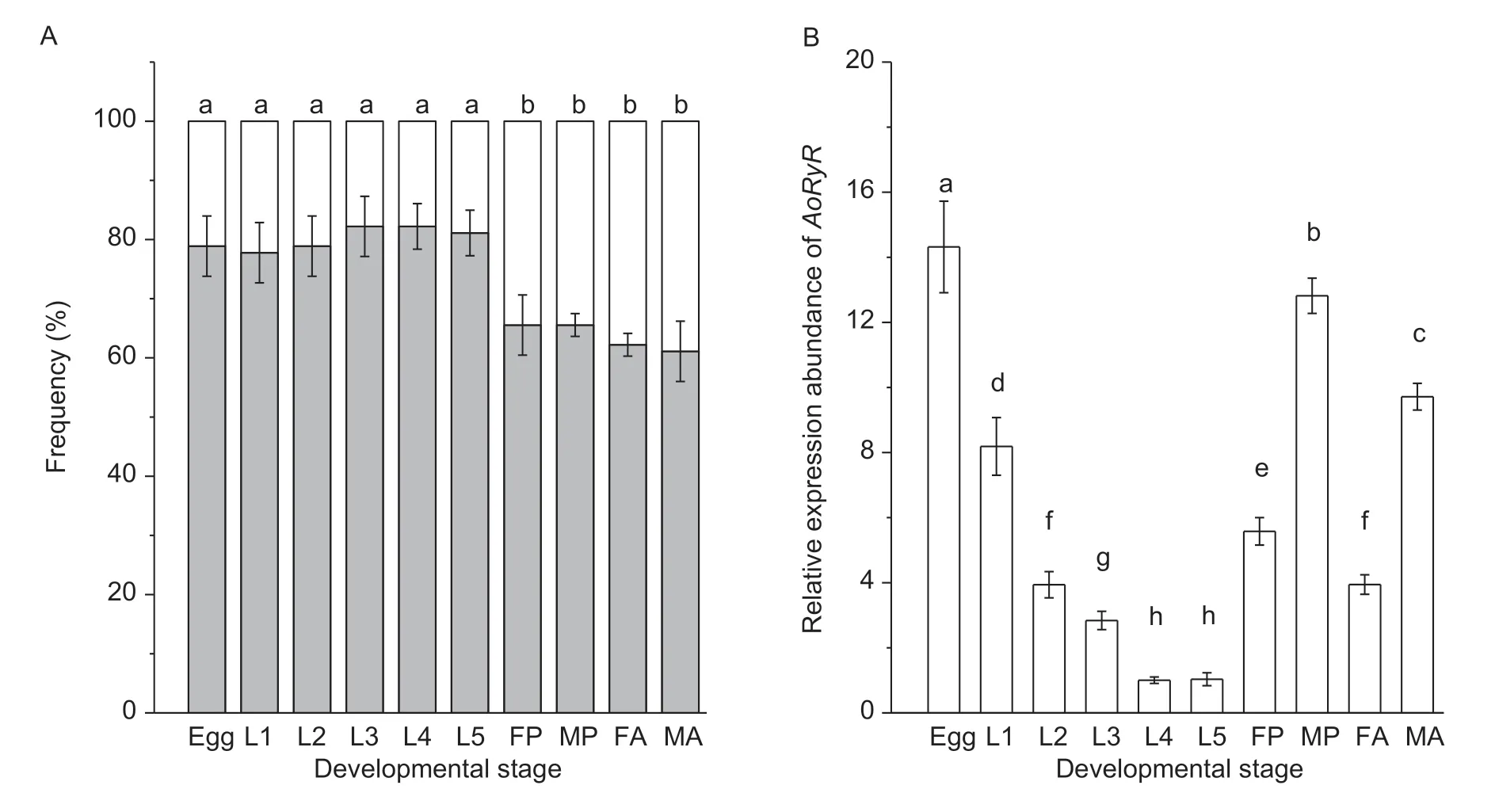
Fig.5 Relative frequency of individual AoRyR alternatively spliced exon usages (A) and the relative expression levels of AoRyR (B)in different development stages. L1–L5,1st-,2nd-,3rd-,4th-and 5th-instar larva,respectively;FP,female pupae;MP,male pupae;FA,female adult;MA,male adult. The error bars represent the standard deviations of the means of four replicates. The different letters indicate (P<0.05) significant differences between the results of the different treatments,as determined using Duncan’s test.
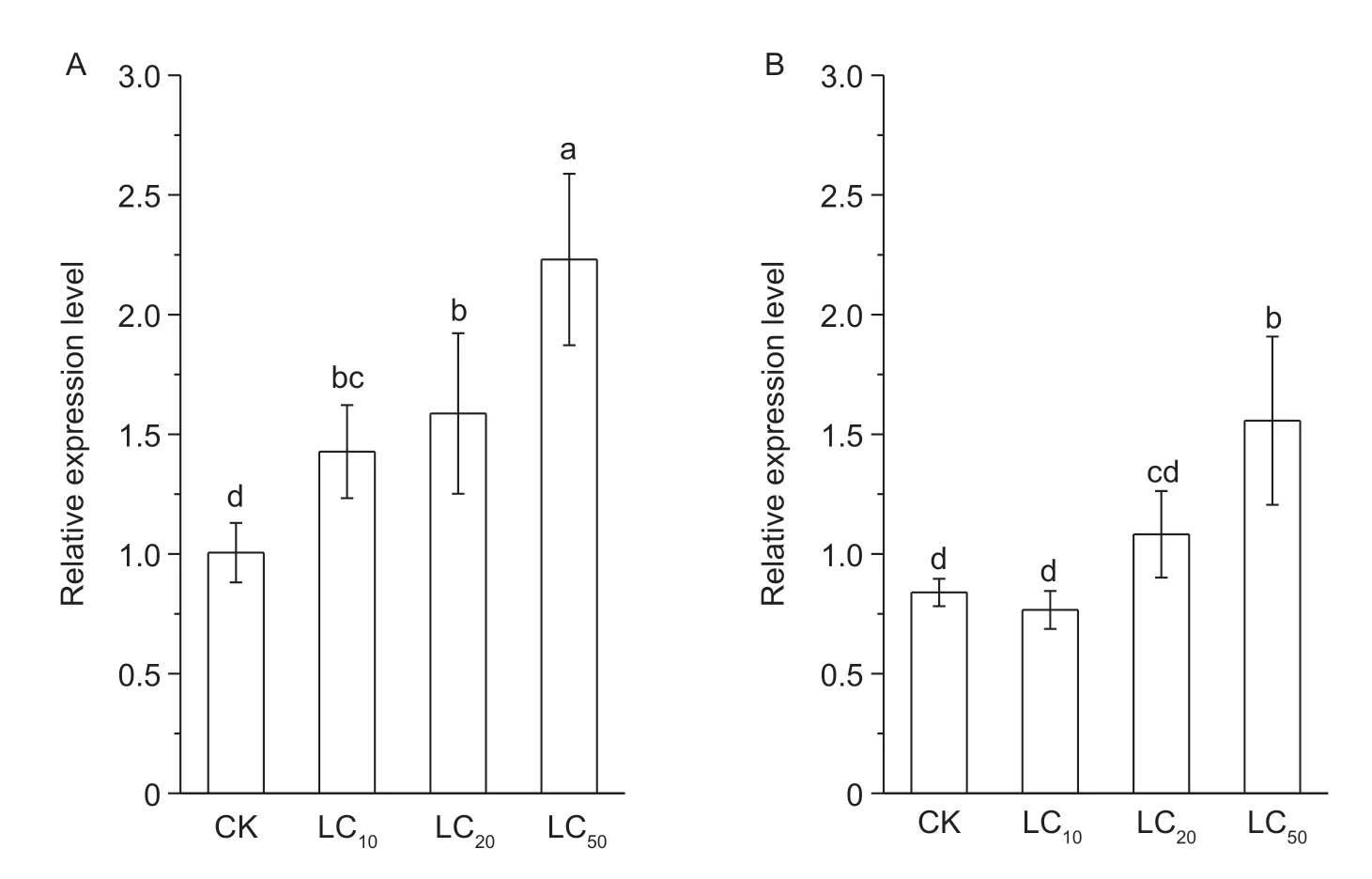
Fig.6 The relative expression levels of AoRyR in 3rd larvae treated with sublethal concentrations of chlorantraniliprole. Third instar larvae were raised for 48 h on leaves treated with chlorantraniliprole (A) and then transferred to peach tree leaves without insecticides for an additional 48 h (B). The error bars represent the standard deviations of the means of four replicates. The different letters indicate (P<0.05) significant differences between the results of the different treatments,as determined using Duncan’s test.
4.Discussion
RyR was named after the alkaloid ryanodine,which was isolated from extracts ofRyaniaspeciosaVahl,a widespread flowering plant in South America. This alkaloid was reported to induce muscle contraction by regulating Ca2+release and to have high killing activity towards insects and mammals (Rogerset al.1948;Pessahet al.1985).Thus,agricultural use of the chemical has been banned for years. Diamide insecticides were first developed in the 1990s,and with the specific insect RyRs as targets of this class of insecticides,they have become popular in the field of agricultural chemicals because they do not target mammalian RyRs. To elucidate the mechanism of diamide insecticides and the mechanism of insect resistance to these insecticides,full-length RyR mRNA has been isolated from various insects,mainly lepidopterous insects (Sunet al.2012,2015c;Wang X Let al.2012;Wang Jet al.2013;Yanget al.2014;Yuanet al.2014). Chlorantraniliprole was reported to control the summer fruit tortrix moth in orchards in 2010 (Zhanget al.2011). The isolatedAoRyRs revealed a deduced amino acid sequence that was 5 113 residues long,and the sequence was similar to those reported for other lepidopterans. According to the phylogenetic tree,AoRyRs clustered withCsRyR andGmRyR,which belong to two other species that inflict damage similar to that done by the summer fruit tortrix moth to fruit tree species including apple,pear,peach,and others. Different diamide insecticides have different insecticidal spectra:flubendiamide has high activity against most Lepidoptera insects,while chlorantraniliprole and cyantraniliprole have toxic effects on Homoptera,Coleoptera,Diptera,and Thysanoptera in addition to Lepidoptera insects (Teixeira and Andaloro 2012).Therefore,the selectivity of diamide insecticides towards insect RyRs might be related to the differences between species depending on the phylogenetic tree analysis.
Conserved and essential hydrophobic transmembrane segments are typical characteristics of RyRs. The TM segments share high homology among the known insect RyR isoforms (Sunet al.2016). The pore-forming segments of the RyR Ca2+release channel,GVRAGGGIGD,located from residues 4 985 to 4 994 between TM5 and TM6 in theAoRyR sequenced here,have also been found in all other RyRs (Zhaoet al.1999). All RyR isoforms have this motif.Moreover,the residues that enable Ca2+sensitivity and conductance of the RyR Ca2+release channelOcRyR1(E4032,I4897,R4913,and D4917) were also detected inAoRyR (E4170,I4992,R5008,and D5012) (Zorzatoet al.1990).
Several binding regions critical to ryanodine sensitivity have been predicted. Two of them are located near the N-terminus (residues 183–290 from BmRyR) and the C-terminal TM domain (residues 4 610–4 655 fromDmRyR or residues 4 111–5 084 fromBmRyR) (Xuet al.2000). In addition,the RyR fromPlutellaxylostellawith resistance to diamide insecticides showed changes at 3–7 locations,which reduced the sensitivity of the receptor to chlorantraniliprole (Katoet al.2009;Taoet al.2013;Wang X Xet al.2013). The amino acid residues G4895,I4738 and Q4542 were also found to alter sensitivity inAoRyR,which were the same as the amino acid mutations G4946E,I4790M and Q4594L from theP.xylostellawhich was resistant to chlorantraniliprole. Interestingly,residue D1339 was found inAoRyR,which corresponds to the mutation site E1338D fromP.xylostellawith resistance to chlorantraniliprole. However,that residue was an S only in the RyRs from nematodes (Wang X Let al.2012;Wang X Xet al.2013;Guoet al.2014b;Yanet al.2014).Moreover,homology modeling and docking studies of the diamondback moth RyR revealed that the binding sites for three activators (Ca2+,ATP and caffeine) and the regulator ryanodine are conserved across insect and mammalian RyRs,but the binding site for diamide insecticides is species specific (Linet al.2018). The docking results showed that in the C-terminus,four residues (K4700,S4919,F4925 and V4945) in the DMB RyR might be responsible for the sensitivity of the DMB RyR,which were sites K4649,S4868,F4872 and V4894 inAoRyR. In addition,each residue can differ between the insect,nematode,and other invertebrate RyRs. We speculate that these eight residues might be involved in Ca2+release from channels among the insect,nematode,and other invertebrate RyRs. However,six residues (I4738,M4821,N4948,N4950,N4961 and L4976) in the TM ofAoRyR are unique in lepidopteran RyRs compared with non-lepidopteran RyRs. Linet al.(2019) also found that the binding site for diamide insecticides is species specific. Moreover,the binding sites of diamide insecticides were distinct from the ryanodine binding site in the insect RyRs,which suggested that this class of insecticides may bind to several sites in the RyRs (Linet al.2019).
AS is a key event regulating transcription in eukaryotic cells. A less abundant subtype of AS is represented by mutually exclusive exon (MXE) splicing (Mandalet al.2019).In MXE,the splicing site is defined by one of two or more mutually exclusive splicing mechanisms,in which either exon a or exon b can be alternatively included in theAoRyRmRNA. These two exons share 55.6% identity,suggesting that they may have been derived from the duplication of an ancestral element. Interestingly,the alternative splicing site has been found in other RyRs fromB.dorsalis,C.medinalis,G.melore,O.furnacalis,and other species. The analysis of alternatively spliced regions revealed that region a/b was located in the central part of the predicted SPRY domain.The distribution of the alternatively splicedAoRyRmRNAs during development revealed that while no differential expression was observed from the egg to the larval stage,exon a is more prevalent in the larvae than in the pupae and adults. Some researchers have reported various differential expression patterns of the mutually exclusive splicing sites during different stages of development,including in the head,thorax and abdomen (Sunet al.2014;Zhanget al.2015). However,the biological function of MEXs from insect RyRs has not been reported,and MEXs are not found in vertebrate RyRs.
The expression profiles of RyR mRNA differ between invertebrates and vertebrates. The results of real-time quantitative PCR showed that theAoRyRmRNAs were most abundantly expressed in eggs,male pupae and male adults,whereas they were detected at relatively low levels in larvae. The expression of RyRs in other types of insects presented significant differences. The expression ofNlRyRin macropterous adults was reported to be significantly higher than in brachypterous adults,as well as in macropterous and brachypterous male adults(Wanget al.2014). The expression ofPxRyRin second instar larvae and adults was considerably higher than in prepupae and pupae (Guoet al.2012;Wang X Let al.2012). However,Cuiet al.(2013) demonstrated that the expression ofOfRyRmRNA was gradually up-regulated as development progressed. The lowest expression levels ofAoRyRwere detected in 4th and 5th instar larvae,and the highest level was in the eggs,with these results differing from those of other types of insects. However,the typical characteristics of expression in the sexes ofA.oranawere the same as those ofGmRyRandSiRyR(Sunet al.2016;Wuet al.2018). The varying expression patterns of RyR mRNA from different insects imply that their functions are different depending on developmental stages and species.
In this study,the effects of chlorantraniliprole onAoRyRmRNA expression profiles were also assessed by using qPCR. These results could provide additional biochemical information for determining the correlation between expression levels ofAoRyRmRNA and chlorantraniliprole dose. When the concentrations of chlorantraniliprole were increased,severe effects on performance were observed in the larvae that were fed leaves treated with chlorantraniliprole,specifically the expression ofAoRyR.This finding was similar to the results for the expression levels of whole-body RyR mRNA fromChilosuppressalis4th instar larvae after injection with chlorantraniliprole at 0.004 to 0.4 μg g–1and forPxRyRof insects that fed on leaves treated with diamide chemicals (Penget al.2017).The LC10dose of chlorantraniliprole had no marked effect onAoRyRmRNA expression after a period of 48 h free from the insecticide. This may indicate that the LC10dose of chlorantraniliprole was too low forA.oranaduring the first 48 h,so that the growth of the larvae could recover when they escaped from chlorantraniliprole exposure. However,higher doses of chlorantraniliprole notably up-regulated the relative expression ofAoRyRmRNA in the susceptible larvae. This phenomenon could have possibly resulted from the mode of action of this class of diamide insecticides.Ca2+is induced by diamide insecticides through insect RyRs but not mammalian RyRs (Cordovaet al.2006),which results in diamides having high insecticidal activity against lepidopteran insects but no effects on vertebrates (Schmittet al.1996;Ebbinghaus-Kintscheret al.2007;Ioriattiet al.2009;Taoet al.2013).
The binding of diamides and their targets is complex,with both high-and low-affinity binding sites (Laiet al.1989;Ebbinghaus-Kintscheret al.2007). With respect to the concentrations,the highest-affinity binding site requires only 1–4 nmol L–1ryanodine,whereas 30 nmol L–1to 4 μmol L–1is required for the low-affinity sites (Pessah and Zimanyi 1991). Furthermore,the opening and closing of the RyR channel are regulated by the concentrations of both cytosolic Ca2+and diamide (Ebbinghaus-Kintscheret al.2007). For example,the gut muscle responds immediately with a persistent contraction when exposed to a final concentration of 1 μmol L–1flubendiamide,and the contractility does not recover even after the removal of flubendiamide(Ebbinghaus-Kintscheret al.2007). As shown in our results,changes in the transcription ofAoRyRmRNA were induced by chlorantraniliprole and were likely related to changes in the concentration of calcium ([Ca2+]c). Ebbinghaus-Kintscheret al.(2006) verified that flubendiamide caused a rapid increase in the free cytosolic [Ca2+]c in neuronal cells isolated from the neurons ofHeliothisviriplaca. Typically,the [Ca2+]c value peaked instantly after diamide application but then slowly returned to the basal level (Lahmet al.2007). However,the whole-body relative RyR expression levels in insects were not up-regulated instantaneously after treatment with diamides in our study. We deduced that the application of diamides induced only a slight secondary Ca2+response,indicating the long-lasting effect of these chemicals.
5.Conclusion
The ORF ofAoRyRencode 5 113 amino acids,with molecular weights of about 578 kDa. One alternative splice site with two exclusive exons was revealed inAoRyR(a/b).PCR showed that the usage of exon was more frequent in eggs and larvae than in pupae and adults. The results of the qRT-PCR showed thatAoRyRmRNA was expressed at all developmental stages,especially in eggs,male pupae and male adults. And the expression levels ofAoRyRmRNA in the whole body were up-regulated markedly after 3rd instar larvae were treated with chlorantraniliprole.
Acknowledgements
This research was supported by the National Key R&D Program of China (2017YFD0200300),and the Fundamental Research Funds for Central Non-profit Scientific Institution,China (1610182019011).
Declaration of competing interest
The authors declare that they have no conflict of interest.
杂志排行
Journal of Integrative Agriculture的其它文章
- Low glycemic index:The next target for rice production in China?
- Effect of side deep placement of nitrogen on yield and nitrogen use efficiency of single season late japonica rice
- Advancements in plant regeneration and genetic transformation of grapevine (Vitis spp.)
- Indica rice restorer lines with large sink potential exhibit improved nutrient transportation to the panicle,which enhances both yield and nitrogen-use efficiency
- Effects of nitrogen management on the ratoon crop yield and head rice yield in South USA
- Response of grain-filling rate and grain quality of mid-season indica rice to nitrogen application
